Clinical Medical
Reviews and Case Reports
A Rare Case of Primary Mucosal Melanoma of the Palate
Elzbieta Sierko1, Aleksander Nobis1, Dominika Hempel2,3, Marek Z Wojtukiewicz2,4 and Ewa Sierko2,3*
1Students' Scientific Association affiliated with the Department of Oncology Medical University of Bialystok, Poland
2Department of Oncology, Medical University of Bialystok, Poland
3Department of Radiotherapy, Comprehensive Cancer Center in Bialystok, Poland
4Department of Clinical Oncology, Comprehensive Cancer Center in Bialystok, Poland
*Corresponding author: Ewa Sierko, Department of Oncology, Medical University of Bialystok, 12 Ogrodowa St., 15-025 Bialystok, Poland, Tel: 48-85-6646734, E-mail: ewa.sierko@iq.pl
Clin Med Rev Case Rep, CMRCR-3-121, (Volume 3, Issue 8), Case Report; ISSN: 2378-3656
Received: June 29, 2016 | Accepted: August 01, 2016 | Published: August 03, 2016
Citation: Sierko E, Hempel D, Sierko E, Wojtukiewicz MZ, Nobis A, (2016) A Rare Case of Primary Mucosal Melanoma of the Palate. Clin Med Rev Case Rep 3:121. 10.23937/2378-3656/1410121
Copyright: © 2016 Sierko E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Melanoma usually occurs on the skin. Only 1% of all melanomas affect mucosal membrane of the head and neck with 1951 cases reported from 1945 to 2011 in the world [1]. The article describes the case of the 65-year-old patient suffered from the palate melanoma that was irradiated on tumor area. The other therapeutic options for melanoma patients were discussed.
Keywords
Mucosal melanoma, Palate, Radiotherapy
Introduction
Primary melanomas located in the oral cavity constitute as low as 0,2-8,0% of all melanomas [1] predominantly affected palate and maxillary gingiva [2]. Less frequently oral melanoma (OM) can be found in mucosal epithelium, stria vascularis of inner ears, retina, and uveal tract. Oral melanoma (OM) has been reported in patients aged 20 to 80 years and has a male predilection. The etiology of OM is unknown. Possible risk factors of these tumors may be tobacco use, chronic irritation from ill-fitting dentures as well as factors associated with environmental pollution [3]. It was suggested that mucosal melanomas may arise from both pigment-producing cells and Schwann cells found in the mucous membrane [4]. In case of OM genetic aberrations are less common, than in cutaneous melanoma. The most often occurred aberrations are those, concerning cKIT gene (20%), NRAS gene (5%) and BRAF gene (3%) [5]. Even 15.6-39% of OM patients have KIT aberration or more copies of KIT gene [6,7]. The clinical presentation of this neoplasm can vary widely if it comes to intensity of black saturation, proliferation rate and metastatic potential [8].
OM is characterized by frequently delayed diagnosis and beginning of subsequent treatment, because most mucosal melanotic lesions are painless in their early stages. These facts worsen prognosis of patients suffered from OM [8]. 5-year survival rate of OM patients amounts 17%, although early recognition and treatment significantly improve the prognosis [9,10].
Case Report
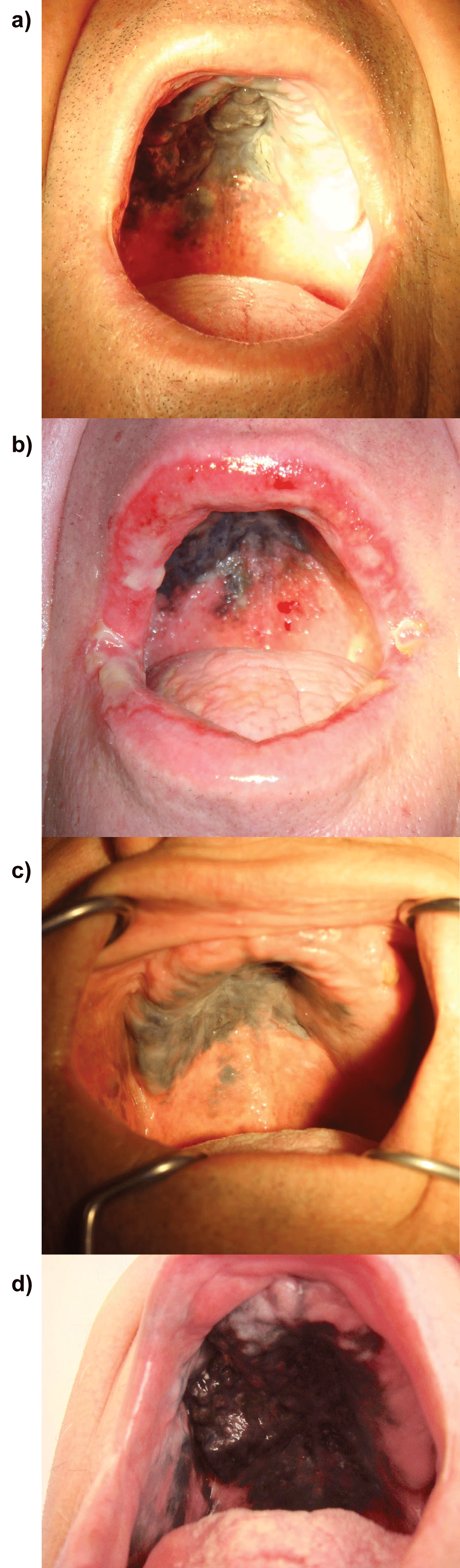
A 65-year-old man treated from skin psoriasis was referred to the oncology department by dermatologist with the suspicion of palate melanoma. Physical examination showed painless pigmented lesion present at mucous at the palate durum, palate mole, right buccal mucosa and right upper alveolus (Figure 1a). No enlarged regional lymphatic nodes were detected. Excisional biopsy was performed. Pathological examination proved mucosal lentiginous melanoma. Immunohistochemical analysis revealed that the neoplastic cells were positive for HMB-45, melan-A, S-100 and negative for AE1/AE3, confirming the diagnosis of melanoma.
.
Figure 1: Photographic documentation of the clinical status of the oral melanoma patient.
a) Pre-treatment clinical state of the patient (prior to radiotherapy) - melanoma of the palate; b) Acute radiation reaction of the mucous of the oral cavity;
c) Clinical status of the patient 5 months after radiotherapy cessation. Oral mucous melanoma decreased and stabilized, and was less saturated with the black color; d) Clinical status 1 year and 9 month after radiotherapy cessation. Progression of the oral mucosal melanoma: thick, dark tumor is seen on the palate.
View Figure 1
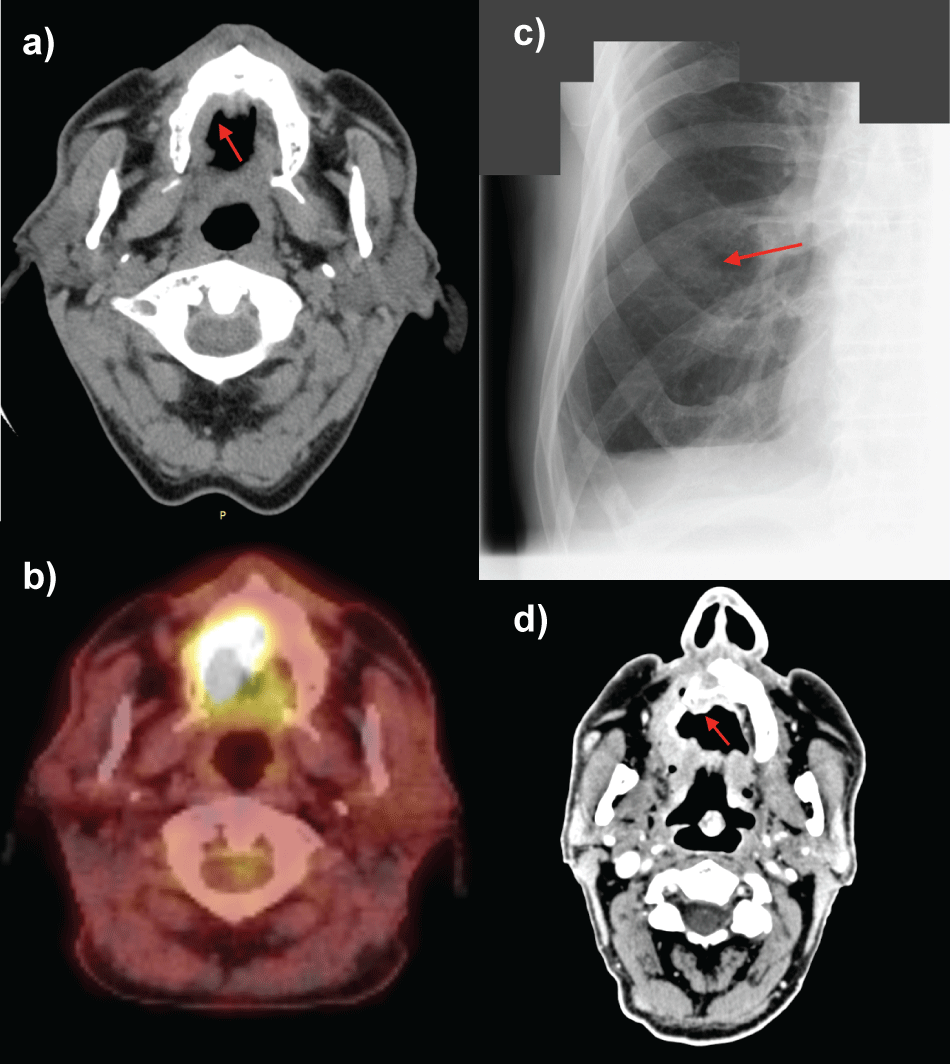
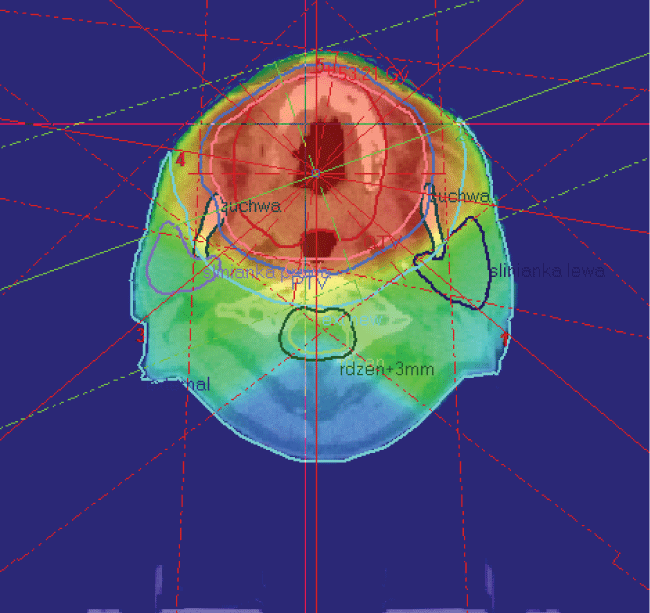
Computed tomography revealed a thickening of mucosal membrane in the area of the palate durum, especially on the right side (Figure 2a). PET-CT demonstrated active process mainly in the right side of the palate and confirmed absence of any active regional nodes (Figure 2b). Chest X-ray examination excluded metastases in the lungs. The patient refused major surgical treatment, so he underwent up-front conventional 3D RT (X 6 MV) to the tumor area at the dose of 50 Gy in 20 fractions (2,5 Gy per fraction); nodes were not irradiated (Figure 3). The radiation acute side effects were assessed according to Dische scale. The intensity of the skin rash and oral mucositis did not accede 13 points (Figure 1b).
.
Figure 2: Imaging studies (CT, PET-CT, RTG) of oral melanoma patient in pre-treatment period and during the treatment.
a) CT scan of patient's head performed prior to the beginning of the radiotherapy. Red arrow indicates mucosal melanoma of the palate; b) PET-CT scan. 18 F-FDG intake by oral mucosal melanoma indicates increased metabolic activity mainly in the right palate; c) Chest RTG. Metastatic lesion in the right lung indicated by red arrow; d) CT scan of patient's head, 2 years and 9 months after radiotherapy cessation. Red arrow indicates osteolysis of maxilla (local progression of the disease).
View Figure 2
.
Figure 3: Oral mucous melanoma patient underwent up-front conventional megavoltage 3D RT (X 6 MV) to the tumor area at the dose of 50 Gy in 20 fractions (2,5 Gy per fraction); nodes were not irradiated. Red isodoses indicate prescribed high dose of radiation.
View Figure 3
The physical examination performed soon after RT cessation, showed weaker saturation with the black color of the tumor, but no decrease in diameter was described. Two months after RT, the melanoma disappeared from the mucous of the right cheek and alveolus and infiltration was slightly reduced at the periphery of the palate. The extent of the lesion was stable also 3, 4, 5 months after RT, however intensity of the black color was further decreasing (Figure 1c). The melanoma lesion decreased by one third and stabilized one and a half year after RT. Unfortunately, 1 year and 7 months after RT cessation progression of the disease appeared on right alveolus causing two slightly painful, non-healing ulcers. Additionally, new intensive black spots appeared on the palate. Two months later the disease further progressed - a thick tumor was found on the palate, and more black spots on the mucous of the cheek and alveolus developed (Figure 1d). The patient sturdily refused chemotherapy because of a fear of adverse events. The recurrent melanoma was slowly, but constantly progressing, which was clinically observed and documented on CT examination. Nearly 3 years after RT cessation, the patient suffered from pain in the tumor area. Chest X-rays revealed suspicious lesions in the right lung (Figure 2c) while head and neck CT revealed enlargement of mucosa infiltration of right alveolus and palate. Tumor exceeded midline and caused osteolysis of right alveolus as well as palate durum (Figure 2d). This time patient signed informed consent to undergo monochemotherapy with dacarbazine and was referred to the regional chemotherapy center located nearest of his home.
Discussion
Mucosal melanoma of the oral cavity is unusual malignancy. There are no specific clinical symptoms of OM, so it may be difficult to distinguish on a clinical basis from solitary oral melanotic macules, labial lentigines, foreign bodies, or individual pigmentation. The OM cases misdiagnosed with racial pigmentation [11] or pyogenic granulomas [12] were also described. Furthermore it is not easy to differentiate mucosal melanoma from a metastatic melanoma [13]. Additionally the local inflammation may complicate the assessment of infiltrated areas [14]. There are some pathological disturbances that should be differentiated with OM, such as: other forms of pigmented oral diseases, including drug-, disease- or smoking-associated melanosis, oral melanotic macule, Kaposi's sarcoma, melanocytic nevus, melanoacanthoma, amalgam tattoo, vascular blood-related pigments, oral melanotic macule, Peutz-Jeghers syndrome, Addison disease, and drug-induced pigmentation [13].
OM occurs the most commonly in the palate, which is in close anatomic proximity to the most prevalent site for head and neck melanomas - the nasal cavity and maxillo-ethmoid massif [4,10]. The first symptoms of OM may not rivet patient's attention, because there may be some asymptomatic swelling and occasional bleeding. OM may be noticed by the way of treating different illnesses, because most of them are painless in their early stages. The diagnosis of OM is often unfortunately delayed until some symptoms resulting from ulceration, growth, or bleeding reveal. The pain is usually the late manifestation of melanoma infiltration [3].
Data regarding OM are scanty. Majority of information about OM comes from case reports, not from randomized clinical trials [2,8,12,13,15]. That is why the clear guidelines for treatment of melanoma in this location are lacking. Therapy of OM is mainly based on surgical excision of the primary tumor [2,8,10,12,13] which may be supplemented by adjuvant RT [2,15-17]. Adjuvant RT does not appear to affect survival but seems to improve locoregional control thus is recommended by majority of experts in OM patients [10,15]. There are limited data proving efficacy of chemotherapy and immunotherapy [18,19]. Unfortunately the OM is often inoperable, because of a large size of the lesion or localization which is unfavorable for the excision [20].
The described patient did not assent to any surgical treatment, thus was offered up-front RT. The physical examination as well as images did not indicate nodal spread that is why the irradiated fields were limited to the tumor area. The irradiation of the clinically negative neck is controversial [15]. Some authors recommend nodal irradiation because of the high risk of subclinical disease [15,20]. However, it is assumed that about 80% of OM patients have local disease, and only 5-10% - nodal spread at the time of diagnosis [20]. The poor prognosis of even early-staged OM patients results from occult metastases at presentation in the majority of patients [20]. The fractionation schedule varies between the authors from 1,5 Gy to even 13,8 Gy per fraction giving the total dose ranging from 32 to 76 Gy [2,10,15,16]. Taking into consideration the biology of melanoma and palate location 2,5 Gy per fraction was chosen. Radiation therapy is rarely used as a primary modality in OM patients - in patients with inoperable tumour, in medically compromised patients and in the older population [21]. Although cutaneous melanomas present weak radio sensitivity, patients may occasionally have good response to RT, especially in the case of early melanomas or in melanomas in situ [22]. The data from literature indicate that in some cases the definitive RT may result in survival rates comparable to that received by surgery, however, the efficacy of definitive RT in III and IV stage of the disease is poor reviewed in [2,15,16]. The possible late complications of the RT as mucosal ulcer, radiation necrosis or bone exposure are another limiting factors of definitive RT [15,16]. There are single reports that definitive particle radiation therapy promises to provide high rates of local control for OM inoperable patients. Unfortunately, overall survival rate does not extend significantly after particle radiotherapy treatment [23].
To date, separate small studies demonstrated, that adjuvant chemotherapy and immunotherapy may prevent distant metastases formation in the subset of OM patients improving also survival rates [19,23-25]. Recent molecular evidence suggests that proto-oncogene KIT aberrations determined in advanced head and neck melanoma patients may represent a potential diagnostic value and serve as a therapeutic target for tyrosine kinase inhibitors also in an adjuvant setting of OM patients [1].
Recurrences of the disease may occur even 10-15 years after primary therapy of OM patients. Distant metastases to the brain, liver, lungs, and bones are frequently observed as well. Unfortunately, most patients die because of the progressive disease in 2 years [24,25]. Systemic therapy is the management of choice in case of recurrent or metastatic OM. Dacarbazine treatment of cutaneous melanoma patients increased overall survival by 5,6-7,8 months, however in OM patients dacarbazine alone was not effective [24]. Combination of dacarbazine with interleukin-2 (IL-2) is unsatisfactory as well [24,25]. Increase of survival of melanoma patients occurred with the introduction of targeted therapies. Administering of ipilimumab for advanced melanoma patients increased overall survival from 6.4 months (melanoma peptide vaccine group) to 10.1 months [26,27]. This human IgG1 monoclonal antibody (MoAb), by inhibiting the activation of CTLA-4, enhances T-cell proliferation and T-lymphocytes' dependent antitumor response [26,27]. Based on results of clinical trials, in 2011, the Food and Drug Agency (FDA) approved ipilimumab for treatment of patients with inoperable or metastatic melanoma [26,27]. Unfortunately, at the time when the patient was treated due to progressive disease, there was no administrative possibility to qualify him to ipilimumab, which was only registered for cutaneous melanoma patients. Nowadays, in Poland, targeted therapy is refunded both for cutaneous and oral melanoma patients and is offered for them.
The review of literature indicates that to improve treatment efficacy of OM patients both early detection [28] as well as a lifelong follow-up [20] are of paramount importance in the management of this aggressive disease.
References
-
Lazarev S, Gupta V, Hu K, Harrison LB, Bakst R (2014) Mucosal melanoma of the head and neck: a systematic review of the literature. Int J Radiat Oncol Biol Phys 90: 1108-1118.
-
Kumar V, Vishnoi JR, Kori CG, Gupta S, Misra S, et al. (2015) Primary malignant melanoma of oral cavity: A tertiary care center experience. Natl J Maxillofac Surg 6: 167-171.
-
Bhullar RP, Bhullar A, Vanaki SS, Puranik RS, Sudhakara M, et al. (2012) Primary melanoma of oral mucosa: A case report and review of literature. Dent Res J (Isfahan) 9: 353-356.
-
Chan RC, Chan JY, Wei WI (2012) Mucosal melanoma of the head and neck: 32-year experience in a tertiary referral hospital. Laryngoscope 122: 2749-2753.
-
Kim CY, Kim DW, Kim K, Curry J, Torres-Cabala C, et al. (2008) GNAQ mutation in patient with metastatic mucosal melanoma. BMC Cancer Res 14: 6821-6828.
-
Curtin JA, Busam K, Pinkel D, Bastian BC (2006) Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol 24: 4340-4346.
-
Beadling C, Jacobson-Dunlop E, Hodi FS, Le C, Warrick A, et al. (2008) KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res 14: 6821-6828.
-
Gupta S, Tandon A, Ram H, Gupta OP (2015) Oral malignant melanoma: Report of three cases with literature review. Natl J Maxillofac Surg 6: 103-109.
-
Lengyel E, Gilde K, Remenár E, Esik O (2003) Malignant mucosal melanoma of the head and neck. Pathol Oncol Res 9: 7-12.
-
López F, Rodrigo JP, Cardesa A, Triantafyllou A, Devaney KO, et al. (2016) Update on primary head and neck mucosal melanoma. Head Neck 38: 147-155.
-
Martinelli-Kläy CP, Laporte ML, Martinelli CR, Martinelli C, Lombardi T (2016) Oral Malignant Melanoma Initially Misdiagnosed as a Racial Pigmentation: A Case Report. Dermatopathology (Basel) 3: 1-7.
-
van der Waal RI, Snow GB, Karim AB, van der Waal I (1994) Primary malignant melanoma of the oral cavity: a review of eight cases. Br Dent J 176: 185-188.
-
Femiano F, Lanza A, Buonaiuto C, Gombos F, Di Spirito F, et al. (2008) Oral malignant melanoma: a review of the literature. J Oral Pathol Med 37: 383-388.
-
Sedassari BT, Lascane NA, de Freitas AL, Mautoni MC, Sotto MN, et al. (2016) In Situ Melanoma of the Gingiva Associated with Dense Inflammation and Pigment Deposition: A Potential Diagnostic Pitfall in Evaluating Stromal Invasion. Head Neck Pathol.
-
Mendenhall WM, Amdur RJ, Hinerman RW, Werning JW, Villaret DB, et al. (2005) Head and neck mucosal melanoma. Am J Clin Oncol 28: 626-630.
-
Wada H, Nemoto K, Ogawa Y, Hareyama M, Yoshida H, et al. (2004) A multi-institutional retrospective analysis of external radiotherapy for mucosal melanoma of the head and neck in Northern Japan. Int J Radiat Oncol Biol Phys 59: 495-500.
-
Owens JM, Roberts DB, Myers JN (2003) The role of postoperative adjuvant radiation therapy in the treatment of mucosal melanomas of the head and neck region. Arch Otolaryngol Head Neck Surg 129: 864-868.
-
Meleti M, Leemans CR, Mooi WJ, Vescovi P, van der Waal I (2007) Oral malignant melanoma: a review of the literature. Oral Oncol 43: 116-121
-
Ahn HJ, Na II, Park YH, Cho SY, Lee BC, et al. (2010) Role of adjuvant chemotherapy in malignant mucosal melanoma of the head and neck. Oral Oncol 46: 607-611.
-
Aggarwal H, Kumar P, Alvi HA (2016) Significance of follow-up surveillance in oral malignant melanoma. South Asian J Cancer 5: 32.
-
Ulusal BG, Karatas O, Yildiz AC, Oztan Y (2003) Primary malignant melanoma of the maxillary gingiva. Dermatol Surg 29: 304-307
-
Hashemi Pour MS (2008) Malignant melanoma of the oral cavity: A review of literature. Indian J Dent Res 19: 47¬51.
-
Munde A, Juvekar MV, Karle RR, Wankhede P (2014) Malignant melanoma of the oral cavity: Report of two cases. Contemp Clin Dent 5: 227-230.
-
Umeda M, Komatsubara H, Shibuya Y, Yokoo S, Komori T (2002) Premalignant melanocytic dysplasia and malignant melanoma of the oral mucosa. Oral Oncol 38: 714-722.
-
Kowalski LP, Sanabria A (2007) Elective neck dissection in oral carcinoma: a critical review of the evidence. Acta Otorhinolaryngol Ital 27: 113-117.
-
Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711-723.
-
Fellner C (2012) Ipilimumab (yervoy) prolongs survival in advanced melanoma: serious side effects and a hefty price tag may limit its use. P&T 37: 503-530.
-
Kaul S, Kumar P (2013) Significance of early detection of oral malignant melanoma in improving prognosis. South Asian J Cancer 2: 199.





