Clinical Medical
Reviews and Case Reports
Pulmonary Lymphangitic Carcinomatosis due to Metastatic Adenocarcinoma of the Lung: A Case Report
Somaya AM Albhaisi* and Neama Luqman
Medicine Institute, Department of Internal Medicine, Sheikh Khalifa Medical City (SKMC), UAE
*Corresponding author: Somaya AM Albhaisi, Medicine Institute, Department of Internal Medicine, Sheikh Khalifa Medical City (SKMC), UAE, Tel: +971506837539, E-mail: somaya_90@hotmail.com
Clin Med Rev Case Rep, CMRCR-3-099, (Volume 3, Issue 3), Case Report; ISSN: 2378-3656
Received: February 27, 2016 | Accepted: March 29, 2016 | Published: March 31, 2016
Citation: Albhaisi SAM, Luqman N (2016) Pulmonary Lymphangitic Carcinomatosis due to Metastatic Adenocarcinoma of the Lung: A Case Report. Clin Med Rev Case Rep 3:099. 10.23937/2378-3656/1410099
Copyright: © 2016 Albhaisi SAM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Pulmonary lymphangitic carcinomatosis (PLC) is part of the spectrum of metastatic disease. Most cases result from dissemination of adenocarcinomas. Micro hematogenous spread to the periphery of the lung, with subsequent retrograde, centripetal lymphatic extension toward the hilar region, is the responsible mechanism in approximately 75% of patients. The remaining cases are due to retrograde extension from a hilar tumor or from an ipsilateral lung or breast carcinoma. In the latter settings, the lymphangitic spread is unilateral; in comparison, micro hematogenous seeding is frequently bilateral. HRCT can detect lymphangitic tumor in up to 50% of patients who are symptomatic but have normal appearing lungs on chest radiography. The imaging features of lymphangitic tumor are determined by the pattern of bronchovascular and lymphatic spread. The aim of this case report is to increase the awareness of health care professionals about Lymphangitis carcinomatosis (LC) as a differential diagnosis of interstitial lung disorders. The diagnosis may be difficult to make as the clinico-radiological picture is often confused with other interstitial lung disorders such as pulmonary congestion, interstitial pneumonitis, pulmonary fibrosis, bronchioloalveolar carcinoma and granulomas. This case emphasizes the importance of the osteopathic principle of treating the whole patient and evaluating all organ systems. Physicians should be aware of PLC from lung adenocarcinoma and consider it in patients with pulmonary symptoms who are unresponsive to antibiotics. In this case report, we review a male in his sixties, with long history of smoking, who presented with metastatic lung adenocarcinoma which was associated with the radiological finding of LC and a poor prognosis.
Keywords
Pulmonary lymphangitic carcinomatosis, Metastasis, Lymphatic
Introduction
Secondary neoplastic diseases of the lung frequently present as Lymphangitis Carcinomatosis (LC). It most commonly originates from primary malignancy in the breast, stomach, thyroid, cervix, colon, pleura and prostate [1,2] but may also originate from the lung itself. The most frequent cell types of lung cancer causing LC in patients with lung cancer include small cell carcinoma and adenocarcinoma. LC secondary to squamous cell lung carcinoma is rare. The spread of tumour cells to the pulmonary lymphatic system or the adjacent interstitial tissue results in thickening of the bronchovascular bundles and septa. Desmoplastic reaction due to proliferation of neoplastic cells, and lymphatic dilation by edema fluid or tumour secretions contribute to this interstitial thickening. Spread of the neoplasm outside the interstitium and lymphatic spaces into the adjacent parenchyma can result in a nodular pattern [3].
The pathologic features of PLC include infiltration of cancer cells and interstitial edema in and around lymphatic vessels as well as infiltration of inflammatory cells caused by lymph node metastasis in the lung. The metastatic cancer in the mediastinal and pulmonary hilar lymph nodes may obstruct lymphatic drainage, resulting in retrograde migration of cancer cells into terminal lung tissues via lymphatic vessels or anterograde migration of cancer cells in the pleura into the pulmonary hilar lymph nodes through intrapulmonary lymph vessels. In addition, a cancer embolus may form in the terminal vessels of the lung due to hematogenous metastasis, which can invade the surrounding lymphatic vessels. Thus, hilar and mediastinal lymph node metastasis may be present or absent in PLC, depending on the route of metastasis of the primary cancer.
PLC can present with dyspnoea and cough and, this may precede the diagnosis of the primary tumour. Even if histologically confirmed, the chest radiograph is normal in 30-50% of cases. A variety of chest radiographs and CT changes are reported and can mimic those of sarcoidosis [4]. The characteristic findings are thickening of the interlobular septa, with beading caused by perilymphatic nodules, polygon or polygonal arcade formation, and thickening of the central bronchovascular structures. In spite of the extensive involvement, the lung parenchyma is not distorted. The absent distortion distinguishes lymphangitic spread from sarcoidosis, which can otherwise produce similar findings but usually with less conspicuous thickening of interlobular septa. The prognosis for patients with PLC is extremely poor with less than half surviving past 3 months [5].
Case Report
A 64 years old Jordanian male presented to emergency department with 5 days history of shortness of breath exacerbated during his flight from Jordan to Abu Dhabi. It is associated with non-radiating pleuritic chest pain. No other symptoms were observed. Past medical history revealed that patient was found to have a hepatic mass however no further workup was done. Patient is a known heavy smoker, smoking around 1 pack per day for 45 years. On examination, patient was in moderate distress, afebrile, tachycardic, tachypnic with Oxygen saturation of 82%.
Chest examination noted equal bilateral air entry, abdominal examination showed Hepatomegaly. Significant blood tests results are summarized in table 1. Electrocardiograph showed sinus tachycardia, otherwise unremarkable. Chest radiograph showed Right hilar spiculated mass lesion.
![]()
Table 1: Significant Blood test results.
View Table 1
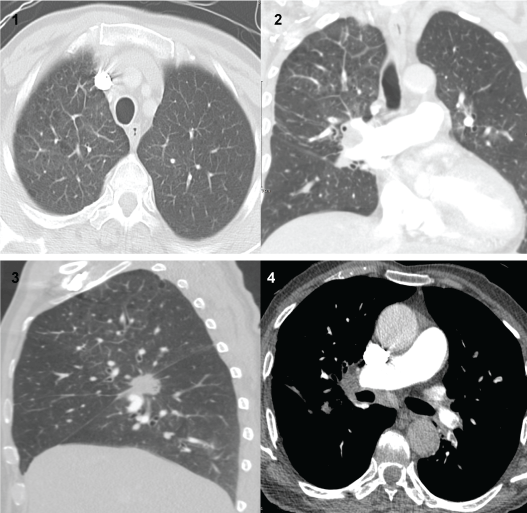
Patient was put on Oxygen and started on broad spectrum antibiotics. Due to the history of travel and high D-Dimers, a CT chest Angiograph was done which showed Segmental pulmonary embolism. No evidence of right cardiac strain was seen. A right lower lobe mass lesion most likely bronchogenic carcinoma with associated ipsilateral hilar lymphadenopathy and right upper lobe lymphangitis carcinomatosis, also an incidental left adrenal mass lesion suspected to be metastasis (Figure 1). So patient was started on low molecular weight heparin at therapeutic dose, antibiotics discontinued and a Multidisciplinary approach was followed in response to the findings mentioned.
.
Figure (1-4): Multiple filling defects could be seen at normal sized pulmonary vascular tree. Filling defects are distributed at bilateral upper and lower segmental pulmonary artery branches. The superior segment of right lower lung lobe was the seed of the spiculated mass lesion with central cavitation, measuring around 2.4 × 2.7 × 2.2 cm in its largest dimensions of the 3 orthogonal planes. Right upper lobe interlobular septal thickening was seen consistent with lymphangitis carcinomatosis. Right transverse fissure thickening was noted. Right hilar lymphadenopathy was noted measuring around 3.4 × 2.2 × 3.2 cm in its largest dimensions of the 3 orthogonal planes. The forementioned hilar lymphadenopathy was seen encasing the right lower segmental pulmonary artery branch. Also subcarinal lymph node enlargement was seen measuring around 1.9 cm in its largest short axis dimension. Upper abdomen sections revealed left adrenal mass lesion was noted measuring around 1.9 × 1.8 × 1.7 cm in its largest dimensions of the 3 orthogonal planes in the light view of the possible bronchogenic carcinoma metastatic origin is considered.
Impression: Segmental pulmonary embolism. Right lower lobe mass lesion likely bronchogenic carcinoma with associated ipsilateral hilar lymphadenopathy, right upper lobe lymphangitis carcinomatosis and left adrenal mass lesion likely metastasis.
View Figure 1
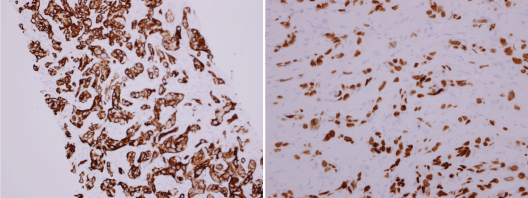
CT scan of the abdomen was done; it showed multiple large hypo dense lesions in the left lobe of the liver and caudate lobe suggesting liver deposits. Ill-defined sclerotic areas in the S1-S2 vertebra and T11 vertebra suggest vertebral body metastatic deposit. Furthermore, it was decided that Liver biopsy is needed to rule out Primary Hepatocellular carcinoma and the result was as following: First by immunohistochemistry, the tumor cells were positive for cytokeratin 7 and TTF -1 stains but they were negative for p63, Hep-Par-1 and CK20 markers (Figure 2). Second test was Mutational analysis which included EGFR, ALK-1 and ROSS-1 and all came out negative. So the conclusion of the report was that these features support the diagnosis of metastatic adenocarcinoma of the lung as the primary source.
.
Figure 2: By immunohistochemistry in our laboratory the tumor cells are positive for cytokeratin 7 and TTF-1 but negative for p63, Hep-Par-1 and CK 20. These features support the diagnosis of metastatic adenocarcinoma, primary lung.
View Figure 2
Patient's condition was improving gradually in a way that his tachycardia and tachypnea has resolved, He was still requiring Oxygen therapy (3 L/min via nasal cannula) and chest pain improved however after few days of admission, he started complaining of severe back pain. A Family meeting was conducted; it was decided to start the patient on chemotherapy though palliative therapy seemed more appropriate. Cycle I carboplatin and pemetrexed started with premedication of folic acid, B12 and dexamethasone in addition patient required different courses of pain management including a course of fentanyl. As a side effect of chemotherapy, the patient developed severe sepsis which improved after a course of antibiotics. However, he later on developed confusion with altered level of consciousness. It was thought to be due to the high doses of fentanyl that were used; so palliative care team was consulted to optimize his pain control. Patient's condition stabilized and he was able to tolerate the second cycle of chemotherapy. End-of-life care was explained to the family who agreed and requested to discharge the patient. The patient was discharged with follow up appointments as outpatient after 35 days of admission under the care of our oncology department. Patient's condition remains guarded but the prognosis is poor.
Discussion
Patients with PLC often present with breathlessness and a nonproductive cough. As with our patient, the onset of pulmonary symptoms may precede diagnosis of the primary tumor; however, the frequency of this presentation is unknown. Although chest radiographs appear normal for 30%-50% of patients with histologically proven disease [3] PLC has several characteristic changes that can be observed on radiographs.
PLC patients usually develop progressive dyspnea, cough, weight loss, fatigue and other symptoms, accompanied by hypoxemia, restrictive ventilatory dysfunction and diffusion dysfunction [6]. The history of cancer or surgery and characteristic features identified on lung CT scans can be used to diagnose PLC after exclusion of interstitial pneumonia, pulmonary fibrosis, sarcoidosis, pulmonary embolism, heart failure and hematogenous disseminated pulmonary tuberculosis. Biopsy and subsequent pathologic examination are not required for the diagnosis of PLC [7,8]. In the early stages, lung CT shows interstitial lesions, linear and reticular shadows and interlobar fissure thickening. Approximately one-third of PLC patients present with pleural effusion (unilateral or bilateral). Once patients develop dyspnea, other findings may be present, including irregular thickening of the tracheal vascular bundles and interlobular septa, as well as multiple beaded, small nodules of varying sizes (usually smaller than 3 mm in diameter) distributed along the interlobular septa and pleura.
Pulmonary lymphangitic carcinomatosis can mimic sarcoidosis radiologically. Nodular thickening and ground-glass attenuation are seen in 30%-60% of patients with sarcoidosis. The nodules in sarcoidosis mainly involve central regions of the middle and upper lobes of the lungs. In contrast, changes usually occur in the lower lobes in PLC. Although imaging studies may suggest sarcoidosis, the diagnosis should be confirmed by biopsy results indicating noncaseating granulomas and by the exclusion of other causes of granulomatous disease. Rapid onset and progression of symptoms, asymmetrically enlarged lymph nodes, predominant disease in the lower lobes of the lungs and lack of response to steroids within 2-4 weeks also should alert clinicians to a diagnosis other than sarcoidosis. Thickening of the interlobular septa and peribronchovascular interstitium without a nodular pattern may be seen in other conditions, such as pulmonary edema and idiopathic pulmonary fibrosis [3].
Although the diagnosis was delayed for our patient, an earlier diagnosis may not have altered the outcome because of the condition's extremely poor prognosis in most cases. Less than half of patients with PLC who present with respiratory symptoms survive for 3 months [9]. However, platinum-based chemotherapy has led to transient remissions in some cases [10]. Nebulization chemotherapy was introduced by Tatsumura et al. in the treatment of inoperable non-small lung cancer. Aerosolized particles of the chemotherapeutic drug, 5-Fluorouracil along with a solvent were delivered using an ultrasonic nebulizer through the mouth. 5FU administered by nebulization accumulates mainly in the trachea, bronchi and regional nodes. Nebulization allows direct and prolonged action of high concentration of 5-Fluorouracil on the tumor tissue. Tatsumura et al. have shown that more than 50% of particles (5-FU) with a 1$ micron diameter reach the alveoli. This study also demonstrated negligible systemic absorption of 5-FU. The nebulized drug is retained for a considerably longer period in tumor tissue than in normal tissue resulting in high antitumor activity with negligible or no systemic adverse effects. The presence of plc might prompt the use of diuretics and/or glucocorticoids. Although there is a lack of firm evidence to support benefit, systemic administration of loop diuretics may be beneficial to reduce lung congestion in dyspneic patients with plc. Glucocorticoids are not used for the palliation of dyspnea as a symptom. However, there are some settings in which they may effectively help to treat underlying causes of dyspnea such as plc.
Conclusion
Clinicians should exclude PLC when patients develop hypoxemia and interstitial pneumonia of unknown cause. PLC may cause significant deterioration of the patient's condition. Thus, only early identification, diagnosis and treatment can prolong the survival of lung carcinoma patients with PLC. Early identification, diagnosis and treatment are crucial to improving the survival of PLC patients. Combined use of CT, PET-CT and pathologic examinations may significantly increase the PLC detection rate. Despite employed strategies to improve the patient's lung edema and administered antitumor therapy, the efficacy of the treatment is still very poor. To date, no effective strategies have been developed for the treatment of PLC. Currently, antitumor therapy and antispasmodic therapy of the airway with theophylline or β2-adrenergic receptor agonists are used. However, these treatments usually have poor efficacy, and PLC is associated with a poor prognosis. Patients usually develop progressive dyspnea and die as a result of respiratory failure and/or heart failure. Nebulization chemotherapy might be effective in achieving rapid early response and along with systemic chemotherapy and supportive care can be used in treating PLC. Approximately 50% to 85% of PLC patients have a survival time between 3 and 6 months.
Acknowledgements
We would like to thank Dr. Mousa Al Abbadi from our histopathology lab and Dr. Tamer Elholiby from our radiology department for their help.
Competing Interests
Authors have declared that no conflict of interests exists.
Authors' Contributions
This work was carried out in collaboration between both authors. Both authors read and approved the final manuscript.
Consent
All authors declare that written informed consent was obtained from the patient (or other approved parties) for publication of this case report.
References
-
Janower ML, Blennerhassett JB (1971) Lymphangitic spread of metastatic cancer to the lung. A radiologic-pathologic classification. Radiology 101: 267-273.
-
Harold JT (1952) Lymphangitis carcinomatosa of the lungs. Q J Med 21: 353-360.
-
(1999) Section VI, Pulmonary neoplasms. In: Fraser RS, Muller NL, Colman N, Pare PD, Fraser and Pare's diagnosis of diseases of the chest. (4th edn), Elsevier Health Sciences, Philadelphia, 1390-1397.
-
Thomas A, Lenox R (2008) Pulmonary lymphangitic carcinomatosis as a primary manifestation of colon cancer in a young adult. CMAJ 179: 338-340.
-
Bruce DM, Heys SD, Eremin O (1996) Lymphangitis carcinomatosa: a literature review. J R Coll Surg Edinb 41: 7-13.
-
Wallach JB, McGarry T, Torres J (2011) Lymphangitic metastasis of recurrent renal cell carcinoma to the contralateral lung causing lymphangitic carcinomatosis and respiratory symptoms. Curr Oncol 18: e35-e37.
-
Babu S, B S, M G, Salih S (2011) A rare presentation of pulmonary lymphangitic carcinomatosis in cancer of lip: case report. World J Surg Oncol 9: 77.
-
Shin NY, Hong YJ, Kim AH, Shim HS, Nam JE, et al. (2011) Diffuse interstitial infiltrative lung metastasis of malignant melanoma: a case report. Korean J Radiol 12: 252-255.
-
Bruce DM, Heys SD, Eremin O (1996) Lymphangitis carcinomatosa: a literature review. J R Coll Surg Edinb 41: 7-13.
-
Kikuchi N, Shiozawa T, Ishii Y, Satoh H, Noguchi M, et al. (2007) A patient with pulmonary lymphangitic carcinomatosis successfully treated with TS-1 and cisplatin. Intern Med 46: 491-494.





