Clinical Medical
Reviews and Case Reports
Gel Bleed and Rupture of Silicone Breast Implants Investigated by Light-, Electron Microscopy and Energy Dispersive X-ray Analysis of Internal Organs and Nervous Tissue
R.M. Kappel1, L.L. Boer2 and H. Dijkman2*
1Plastic & reconstructive surgeon, Dr. Kappel Institute, Zwolle, The Netherlands
2Department of Pathology, Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands
*Corresponding author: H.B.P.M Dijkman, Department of Pathology, Radboud University Nijmegen Medical Centre, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands, Tel: 31-24-3655297, E-mail: henry.dijkman@radboudumc.nl
Clin Med Rev Case Rep, CMRCR-3-087, (Volume 3, Issue 1), Research Article; ISSN: 2378-3656
Received: December 29, 2015 | Accepted: January 27, 2016 | Published: January 29, 2016
Citation: Kappel RM, Boer LL, Dijkman H (2016) Gel Bleed and Rupture of Silicone Breast Implants Investigated by Light-, Electron Microscopy and Energy Dispersive X-ray Analysis of Internal Organs and Nervous Tissue. Clin Med Rev Case Rep 3:087. 10.23937/2378-3656/1410087
Copyright: © 2016 Kappel RM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: We studied a patient who died in 2008 at the age of 56 and had been exposed to gel bleed from her silicone breast implants for 17 years. Tissue samples and nervous tissue could be obtained for analysis.
Design: During autopsy, a wide range of different tissue samples were collected, frozen and embedded in paraffin and plastic (Epon). The paraffin samples were stained with Hematoxylin and Eosin (HE) as well as with Modified Oil O Red (MORO). Tissues embedded in plastic (Epon) were sectioned and prepared for light microscopy using toluïdin blue staining for Transmission electron microscopy (TEM) and Energy Dispersive X-ray microanalysis (EDX) to measure elemental Silicon (Si).
Results: We found 2 types of silicone material in multiple tissue and brain samples of this patient. The first is a droplet-like form. EDX measurements demonstrated that the droplets are composed of elemental Si. The second is a plaque-like form; these structures are comprised of elemental Si and Ti (Titanium). Occasionally we found that these plaques were located inside the tissue without a lining and sometimes they were located inside the lumen of blood vessels.
Conclusion: The use of EDX analysis over light microscopic examination only, is now a contributing factor for the establishment of silicone bleeding and migration throughout the whole body in high amounts.
Keywords
Silicone breast implants, Silicone gel bleed, PDMS, Si, TEM, EDX
Introduction
Some women, who have received silicone breast implants either for breast augmentation or breast reconstruction, develop health problems in different gradations over the years and a thorough explanation for this has yet to be given [1-3] this is because this kind of research can only be performed on living humans. In addition, as the existence of associated complaints is still largely denied and neglected by the various medical disciplines, they are not recorded in medical histories of the involved women and thus do not appear in meta analyses [4] So, only combining various pieces of scientific information can give us insight. It is already known that explantation of the implants can up to a certain point and especially in the early stages cause an improvement of these complaints [5,6] With regard to the silicone issue, EDX analysis has been performed only once before, in excised lymph nodes [7]. In this study, with a novel approach, the light microscopic presence of silicone droplets and plaques in the various tissues (Table 1), are subjected to TEM and EDX analysis for measuring Si-counts. This adds a new element to the already existing knowledge with regard to silicone gel bleed from, and rupture of silicone breast implants.
![]()
Table 1: A summary of positive silicone holding material of the patient. The plaques are comprised of elemental silicon and titanium. The vacuoles (droplets) are comprised of elemental silicon. Several techniques are included in this table, illustrating the amount of silicon in Frozen sections, paraffin sections and plastic sections.
View Table 1
Materials and Methods
Patient
During autopsy, tissue samples of multiple organs and different sections of brain and spinal cord areas were collected. The patient received the silicone breast implants in 1985. In her medical files her general practitioner reported in 1997 that she had developed “adverse reactions to her implants”, without specifying what these reactions were. In 2001 they were replaced by new silicone breast implants together with a capsulectomy. At this operation the implants appeared to be ruptured, thus leaving a near empty elastomer shell. Still in 2001 she developed capsular contracture of the left breast and had a capsulectomy. At that time the medical health complaints she had enumerated, such as painful breasts, a burning sensation of the breasts, lymph packages in the left armpit, severe memory function disorder, walking function disorder, sleeping disturbances, complaints about bowel function and skin disorders. She described an overall feeling of chronic illness and complained of sudden numbness of the legs. In 2002 both her one-year-old implants were removed. In June 2003 she developed a subcutaneous swelling in her left armpit and subsequently axillary lymph nodes were removed for histological examination. The pathologist reported that he found extensive histiocytic reaction on small needle-like particles, in concurrence with a reaction on silicon. Two weeks later the patient developed enlarged lymph nodes in her left groin area. In 2004 she developed an invasive ductal carcinoma of the left breast; however, this might be coincidental and not linked to the silicone implants. Post mortem, the same ductal carcinoma has been histologically found in the liver, ribs, vertebrae and hypothalamus. In the end, this caused her death. For orientation purposes in this new type of study, a second case was introduced, acting as a ‘positive control’. This patient had silicone implants for 14 years and upon removal both implants were ruptured. The peri-prosthetic capsule only could be harvested. Of both patients in this study the samples were processed (HE, MORO and Toluidine blue staining) and assessed by light microscopy. Subsequently, EDX measurements were performed to quantify the amounts of silicone as found in the TEM.
Light microscopy
The modified Oil Red O (MORO) is a histological staining, used to visualize silicone. This was initially performed on frozen sections to maximize detection as paraffin preparation can dissolve some of the silicone. Paraffin sections were deparaffinized before staining with MORO. This was done with xylene for 15 minutes and 3 dips into absolute alcohol. Next, the slides were rinses in demineralized water. For the MORO staining an oversaturated colouring solution of Oil Red O (0.5 g In 100 ml 100% 1,2-propanediol) was used, which acts like a solvent. The silicone polymers, which likely have fatty rest groups, are stained by the oil red O, because the Oil Red O is better resolved in the silicone polymers than in the 1,2-propanediol. The oil Red O moves from the relatively polar solvent to the non-aquatic polymers of the silicone. The principle of this staining thus is the physical binding between the Oil Red O molecules and the silicone polymers. After 5 days, the slides were agitated and differentiated in 85% 1,2-propanediol. After rinsing in tap water, the slides were stained with Hematoxylin Mayer to counter stain the nuclei in the surrounding tissue. After 10 minutes of rinsing in tap water, the slides were dipped in a 1% acetous alcoholic solution for further differentiation. After this, the slides were rinsed for 4 minutes and dipped in saturated lithium carbonate solution (4 g lithium carbonate in 100 ml demineralized water). In the next and final step the slides were rinsed for 4 minutes in tap water and covered with gelatine-glycerin, a water based cover medium. Positive staining is seen as bright and deep red staining.
Electron microscopy
The plastic (Epon) embedded tissues were fixated in a 2% glutaraldehyde 0.1 M sodium cacodylate buffer solution with a pH of 7.4 for a minimum of 4 to a maximum of 24 hours [8]. Removal of free non-reacted aldehydes was done by a washing step in 0.1 M sodium-cacodylate buffer solution with pH range of 5.0-7.4. All tissues were dehydrated in an ethanol-propylene oxide series and manually imbedded in EPON, an epoxy resin. EPON polymerizes for 1 night at 45°C and for 2 days at 67°C in an incubator [9]. Semi- and ultra thin sections were obtained with a Reichert-Jung ultramicrotome by using a Histo diamond knife for 1 μm semi thin sections and an Ultra diamond knife for the 90-200 nm ultrathin sections. Semi thin 1 μm sections were stained with toluïdin blue. This staining gives a clear overview of the semi thin sections and can be used to visualize areas of interest to be viewed in the ultrathin TEM sections or measured with EDX. Ultra thin 90-200 nm sections were collected on membrane coated (polyvinyl formal in 1% ethylene dichloride) 3.05 mm 100 mesh copper grids. The 90 nm ultrathin cuts were additionally contrasted with 4% uranyl acetate solution for 30 minutes and lead citrate for 6 minutes so they obtained the desired contrast for morphological examination. The uncontrasted 200 nm ultrathin cuts were used for EDX measurements. Specimen were next studied with a Jeol (JEM-1200 EX II TEM/STEM) Transmission/Scanning Electron Microscope operating at 64 kV with Energy Dispersive X-ray (EDX) equipment.
Results
Droplets and plaques containing silicone were found in the random parts of the sampled tissues. They could be seen in the HE, MORO and Toluïdin blue through light microscopy. An overview of silicone containing organs and tissues of our patient and the findings in TEM/EDX analysis is presented in table 1. Table 1 is a summary of positive silicone containing material. The plaques are comprised of elemental silicon and titanium. The vacuoles (droplets) are comprised of elemental silicon. Thus we identified two variants of silicone containing material. These were also found in macrophages. Different stainings are the MORO silicone staining and Sudan Black/Normal ORO fat stains which have been performed on a silicone positive paraffin control, consisting of a peri prosthetic capsule. Also electron microscopical investigation and EDX analysis was done on this positive control. Questions about the specificity of the MORO staining for silicone in freeze and paraffin cuts could thereby be established (figure 1).
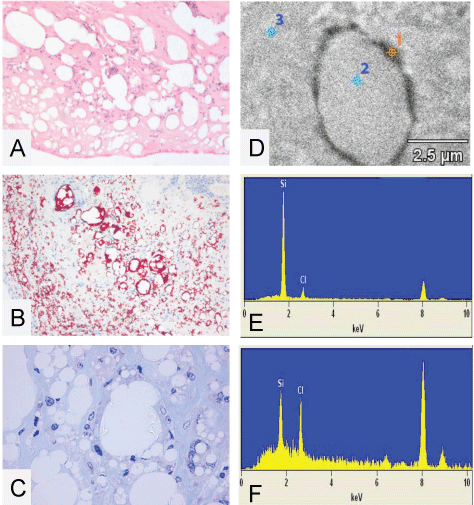
.
Figure 1: (A) A histological 4 μm paraffin cut of the capsule surrounding the silicone implants from patient 2 with bleeding and complete rupture of the implant. As can be seen there are numerous vacuoles which are, as viewed by a higher magnification, filled with threadlike structures of translucent amorphous refractile non-polarizing material suggestive of silicone deposition (HE staining); (B) A histological 4 μm paraffin cut from the capsule surrounding the silicone implant from patient 2 stained with the MORO to demonstrate if the translucent amorphous thread like structures are stained. As can be seen strong positive stained material is present, particularly located perivacuolar; (C) A higher magnification of the semi thin toluïdine blue stained cut from the capsule surrounding the silicone implants of patient 2. As can be seen clearly there is a vacuole partially filled with threadlike structures of translucent material. Also here the vacuoles are not completely filled with the material, suggesting there is material lost in the processing steps. The embedding of the tissue in epon makes it inappropriate to visualize (stain) the silicone with the MORO staining. EDX analysis is performed to conclude if high concentrations of silicone are measurable; (D-F) EDX spectra and EDX analysis of a non contrasted 200 nm EPON section from the capsule surrounding the implant from patient 2. Top right image shows three measuring points. Point 1 is on the electron dense perivacuolar material, point 2 and 3 are in and around the vacuole; As can be seen in spectra 1 there is a high peak of elemental silicon at 1.74 KeV (E); In measuring points 2 and 3 there are considerable less amounts of elemental silicon found and stay under 500 silicon net counts (F). With this in mind we have set a standard: net counts lower than 500 are negligible and counts over 1000 are of silicon stacking and of pathological interest. As can be seen in measure point 1 there are 6626 Si- counts and thus suggestive for silicone stacking. Chlorine peaks found at approximately 2.6 KeV are originating from the EPON, peaks at resp. 8 and 9 KeV are due to copper, used as material for the grid holder. Both peaks can be ignored. Picture E EDX on dense perivacuolar material (point 1). Si count 6.626, Inside the vacuole (point 2). Si count 302 and picture F EDX on the surrounding tissue (point 3). Si count 447. Original microscopic magnification A-B 50x, C 400x.
View Figure 1
The Modified Oil Red O (MORO) staining was initially performed on 4 μm freeze cuts from frozen material of our patient, acquired in body autopsy. This first staining was performed to check if any positive material is present and stainable. Results of this first MORO staining show large quantities of positive stained material in a large range of tissues. (Table 1 and Figure 2).
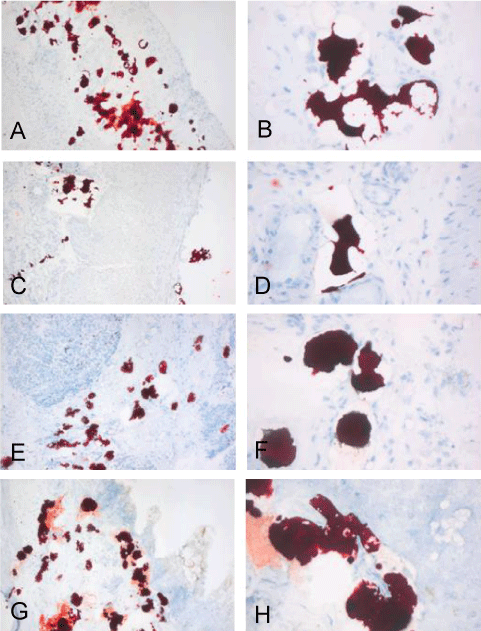
.
Figure 2: Pictures of the MORO stained 4 μm cryosections, are a representative selection from the positive stained tissues (table 1). Images clearly show strong positive stained material in; diaphragm (A-B); small intestine (C-D); pancreas (E-F) and colon (G-H), of patient 1. The positive material is often presented in vacuoles but often this material is not contained in the vacuoles and appears to be dragged or washed out. Original magnifications left 50x and right 200x.
View Figure 2
To ensure that the frozen material from patient 1 also contains the silicone after treatment with different fixatives we performed MORO staining on different samples. As can be seen in all panels there is a significant amount of positive material present (Figure 3).
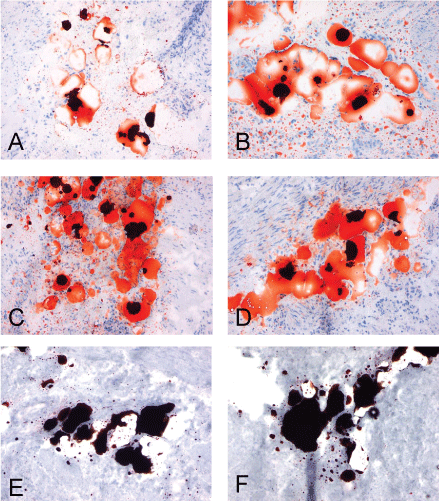
.
Figure 3: Pictures all show the same freeze cut of the frozen urinary bladder from patient 1 fixated with different fixatives previous to MORO staining. All left images are 4 μm thick, all right images are 9 μm thick. Panel A/B 15 minutes 4 % buffered formalin fixation. Panel C/D shows 15 minutes 2% glutaraldehyde fixation. Vacuoles are both in the 4 and 9 μm cuts completely filled with positive stained material. Panel E/F shows 15 minutes 2% glutaraldehyde + 15 minutes 2% osmium tetroxide fixation. As can be seen in all panels there is a significant amount of positive material present. Original magnification: all 100x.
View Figure 3
Also different lymph nodes were excised for histological examination and showed extensive foreign body reaction in the presence of many epithelioid multinucleated giant histiocytes and the presence of translucent material, suggestive for silicone. MORO positive stained material is located inside vacuoles and histiocytes (Figure 4). Samples are cut approximately 100 and 200 nm thick for assessment in TEM and analysis by EDX. EDX analysis is performed on this lymph node material to see if large amounts of elemental silicon is measurable. (Figure 5).
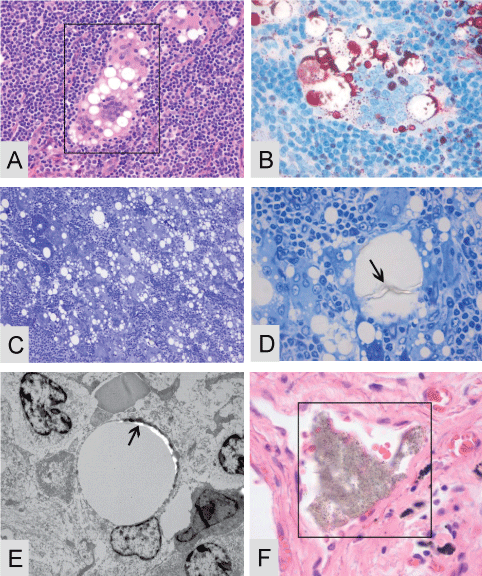
.
Figure 4: Multiple lymph nodes of patient 1 were filled with large quantities of multinucleated giant histiocytes in presence of possible exogenous material, suggestive of silicone A-E. (A) A 4 μm paraffin cut stained with Hematoxylin and Eosin in presence with a giant histiocyte and cytoplasmatic vacuoles filled with, when viewed with higher magnification, translucent amorphous material; (B) A 4 μm paraffin cut stained with the MORO, positive stained material is present inside the giant histiocytes; (C) A 1 μm toluïdin blue stain where multiple giant histiocytes are seen in presence of multiple vacuoles; (D) A higher magnification of the toluïdin blue stain were threadlike translucent material is present inside the vacuoles; (E) A TEM micrographs of a vacuole with dens perivacuolar material, on this dens material EDX analysis is performed, see details Figure 5 A-B. Often material is clearly located inside a bloodvessel, this event can be found throughout all tissues (F). Original microscopic magnification; A/B and D/F; 400x, figure C; 200x and figure E 5000x.
View Figure 4
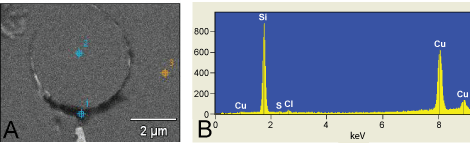
.
Figure 5: TEM micrograph of the EDX measuring points performed on the lymph nodes of patient 1. Point 1 is on the dens perivacuolar material. Point 2 is inside the vacuole and point 3 is on the surrounding tissue (A); EDX analysis of patient 1 lymph nodes from the subcutaneous swelling in the left armpit. Point 1 (spectra 1) = 10759 Si-counts (B). As can be seen in measuring point 1 there is a large peak of elemental silicon found which corresponds with the electron dens peri-vacuolar material.
View Figure 5
EDX analysis reveals the true nature of the plaque like structures found in the toluïdin blue stain, elemental silicon and titanium were found in all the plaque like structures. The found component of elemental titanium represents the dens particles located inside the plaques. Also loose droplets of elemental silicon were found, situated partially in vacuoles, for instance in the thyroid gland, figure 6.
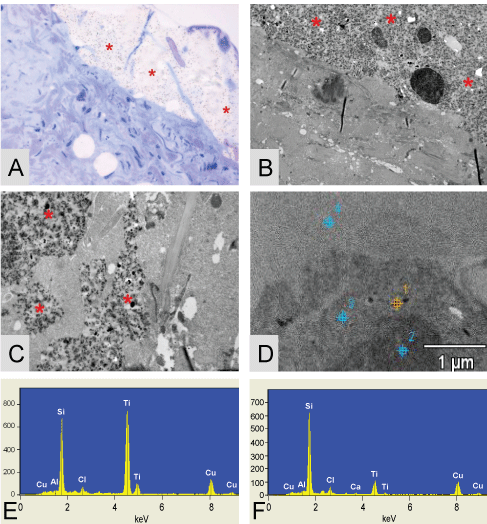
.
Figure 6: Figures show the plaque like structures * found in the thyroid of patient 1. (A) The toluïdin blue stained eponsection, the plaque is located alongside the tissue, suggesting that is has no connection with the tissue; (B) A TEM micrograph where it is nicely demonstrated that the plaque like structure is situated around collagen; (C) A higher magnification of a TEM micrograph were it is nicely demonstrated that the plaques are completely surrounded by thyroid tissue. Dens small particles inside the plaque are clearly visible. EDX analysis is performed on these structures; (D) The TEM micrograph of the EDX measuring points performed on the thyroid of patient 1; (E) Depicted EDX on a dens particle found inside the plaque (spectra 1) = 7200 Si-counts/11652 Ti-counts; (F) EDX on the plaque itself (spectra 2) = 7252 Si-counts/1579 Ti-counts and point 3 is on the surrounding tissue (spectra 3) = 545 Si-counts (not shown). Original microscopic magnifications figure A/B and C; resp. 400x, 3K and 7.5K.
View Figure 6
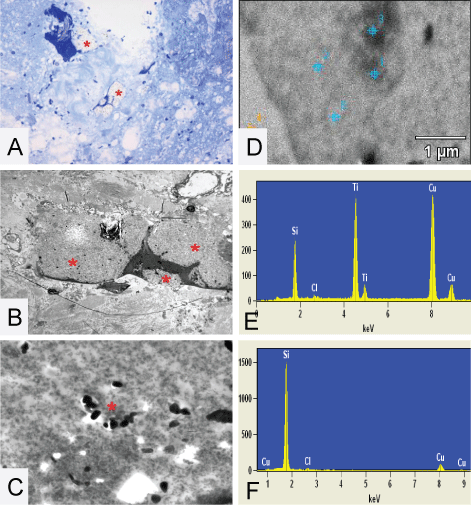
.
Figure 7: (A,B and C) The plaque like structures * found in the high cervical spinal cord of patient 1. (A) The toluïdin blue stained eponsection, the plaque is located inside the surrounding spinal cord tissue; (B) A TEM micrograph where it is nicely demonstrated that the plaque like structure is situated around collagen and in close proximity of nerve tissue; (C) A higher magnification of a TEM micrograph were small particles inside the plaque are clearly visible around the asterisk. EDX analysis is performed on these structures and detects Si and Ti, (E); (D) The TEM micrograph of the EDX measuring points performed on the high cervical spinal cord of patient 1 and demonstrates inside the plaque (spectra 1) = 16854 Si-counts (F). Original microscopic magnifications figure A/B and C; resp. 200 x, 1.5K and 12K.
View Figure 7
The plaque like structures found in the spinal cord of patient 1 are located inside the surrounding spinal cord tissue. The TEM micrographs demonstrate the plaque like structure and a higher magnification of a TEM micrograph show small particles inside the plaque, clearly visible around the asterisk. EDX analysis is performed on these structures (Figure 7 and Figure 8). Measurements are done using EDX-spot-analysis, measuring a surface in the range of 0.1-0.15 square microns. A summary of the EDX–measured Si counts is given in table 2.
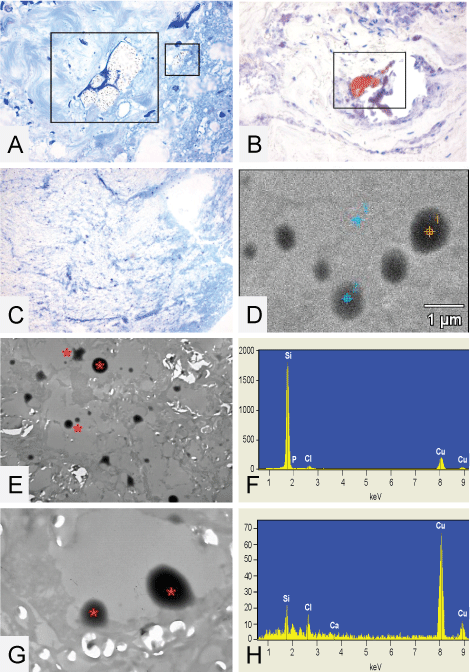
.
Figure 8: (A) Toluïdine blue of the spinal cord high cervical, clearly visible is a structure located around collagen, same structures often are positive with MORO staining, reddish plaque is visible (B); (C, D and E) The droplets * found in the thoracic spinal cord of patient 1; (C) The toluïdin blue stained epon section, no visible droplets are detectable; (E) A TEM micrograph where it is nicely demonstrated that there are actually droplets present located inside vacuoles which appear to be partially washed out; (G) A higher magnification of a TEM micrograph were it is clearly seen that the droplets actually are located inside a vacuole and are part of the tissue. EDX analysis is performed on these droplets, figure D shows a TEM micrograph of the EDX measuring points performed on the thoracic spinal cord of patient 1; (F) Demonstrates EDX analysis Point 1 on a droplet found inside vacuolated spaces (spectra 1) = 22211 Si-counts. Point 3 is on the surrounding tissue (spectra 3) = 195 Si-counts (H). Original microscopic magnifications figure A and B 400x, resp. 200x, C-E and G; resp. 50 x, 2.5K and 10K.
View Figure 8
![]()
Table 2: A summary of the EDX-measured Si counts in different samples.
View Table 2
Discussion
Gel bleed is a phenomenon that is inherent to all types or models of silicone breast implants, regardless whether they are soft and round or cohesive anatomically shaped [10-14]. The bleed retardation layer in the late models, retards the bleeding, but does not abolish it. The bleeding silicone polymers behave like softeners and eventually weaken the silicone elastomeric shell of silicone breast implants, regardless of the brand [15]. This could be the reason why spontaneous ruptures occur. It goes without saying that implant rupture is an intensified form of gel bleed. Table 1 gives a good impression of the dissemination of the silicone polymers found throughout the body. This also gives an impression of the Si-presence, although the amount can be variable, depending on which part of the organ analysis had been performed. As a starting point, EDX-measurement was done on pure silicone gel. The average Si-net counts of 4 measurements was approximately 345,500 counts (data not shown). In contrast, EDX measurements in control tissues of patients without silicone breast implants, always levelled below 500 net counts. With that in mind we maintained a safe threshold of 1000 silicon net counts in the tissues examined. Counts below 1000 were considered negligible and can act as a negative control, whereas net counts beyond 1000 represent Silicon stacking and thus are of pathological interest. Epidemiological studies have not been able to show a statistical significant connection between silicone breast implants and “silicone Implant Incompatibility Syndrome” [3]. The common conception seems to be that if the connection cannot be demonstrated epidemiologically, it has to be absent. Studies to elucidate the silicone issue, should at least document the health complaints that surface in some women with silicone breast implants and follow them for many years. However, women with health complaints are present and when the transplants were explanted they often report an improvement of their condition. What is also remarkable, is that hours after explantation surgery, the pre-operative complaints can first increase tremendously for days to weeks, before they subside. These two experiences with this group of patients clearly suggest that such a link exists.
In this study agglomerates of silicone in the form of plaques together with titanium or in the form of droplets are detected in several organs and nervous tissue. Titanium was frequently used in the sealing patches of the silicone breast implants and that could possibly be the source of the titanium in the plaques. In the lymph nodes there are signs of chronic inflammation, but this does not seem to be the case in the organs and nervous tissue, where the silicone material is either located within vessels or encapsulated in collagen. However, the presence of silicone embolism or more fibrosis within internal organs could interfere with proper functioning of its cell systems. In nervous tissue it could interfere with conduction of nerve impulses. Many of the patients with silicone related complaints demonstrate a more neurological form of the disease with ultimately loss of control of the lower extremities and walking difficulties. This phenomenon has sporadically been coined as pre-MS by neurologists. The patient in this study had those walking difficulties and became wheelchair bound. But other neurological phenomena also exist, like loss of clear thinking, tremors and psychological disturbances. This has to be investigated further.
Although strict Si-counts are not absolute in number, the samples that we have seen by electron microscopy, do reveal that the disseminated silicone material is everywhere in the body. The larger silicone polymer molecules can be detected in agglomerates. The smaller molecules of siloxane monomers and oligomers, may infiltrate on a cellular level and at random work their way through the multitude of biochemical intracellular pathways, stressing the cells and creating a variable syndrome of various health complaints.
In this way this syndrome may appear to be elusive and parade over a long period of time as no more than the accelerated aging process, too subliminal for any epidemiological study to be detected, but which will in due course surface in susceptible women.
This hypothesis can only be tested through further research.
Conclusion
Our study using light microscopy and EDX-analysis, clearly demonstrates that silicone material is present in patients who have been exposed to gel bleed from silicone breast implants for longer time, in all organs and nervous tissue in great amounts.
Acknowledgments
Our patient had silicone breast implants and health complaints. She decided to donate her body for collecting tissues samples and nervous tissue, to further investigate the science of the pathogenesis of “Silicone Implant Incompatibility Syndrome” (SIIS).
References
-
Borenstein D (1994) Siliconosis: a spectrum of illness. Semin Arthritis Rheum 24: 1-7.
-
Vermeulen RC, Scholte HR (2003) Rupture of silicone gel breast implants and symptoms of pain and fatigue. J Rheumatol 30: 2263-2267.
-
Cohen Tervaert JW, Kappel RM (2013) Silicone implant incompatibility syndrome (SIIS): a frequent cause of ASIA (Shoenfeld's syndrome). Immunol Res 56: 293-298.
-
Janowsky EC, Kupper LL, Hulka BS (2000) Meta-analyses of the relation between silicone breast implants and the risk of connective-tissue diseases. N Engl J Med 342: 781-790.
-
Melmed EP (1998) A review of explantation in 240 symptomatic women: a description of explantation and capsulectomy with reconstruction using a periareolar technique. Plast Reconstr Surg 101: 1364-1373.
-
Kappel RM, Pruijn GJ (2012) The monobloc hydrogel breast implant, experiences and ideas. Eur J Plast Surg 35: 229-233.
-
Truong LD, Cartwright J, Goodman MD, Woznicki D (1988) Silicone lymphadenopathy associated with augmentation mammaplasty. Morphologic features of nine cases. Am J Surg Pathol 12: 484-491.
-
Sabatini DD, Bensch K, Barrnett RJ (1963) Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol 17: 19-58.
-
Glauert AM, Glauert RH, Rogers GE (1956) A new embedding medium for electron microscopy. Nature 178: 803.
-
Barker DE, Retsky MI, Schultz S (1978) "Bleeding" of silicone from bag-gel breast implants, and its clinical relation to fibrous capsule reaction. Plast Reconstr Surg 61: 836-841.
-
Beekman WH, Feitz R, van Diest PJ, Hage JJ (1997) Migration of silicone through the fibrous capsules of mammary prostheses. Ann Plast Surg 38: 441-445.
-
Pfleiderer B, Ackerman JL, Garrido L (1993) Migration and biodegradation of free silicone from silicone gel-filled implants after long-term implantation. Magn Reson Med 30: 534-543.
-
Garrido L, Pfleiderer B, Jenkins BG, Hulka CA, Kopans DB (1994) Migration and chemical modification of silicone in women with breast prostheses. Magn Reson Med 31: 328-330.
-
Hardt NS, Yu L, LaTorre G, Steinbach B (1995) Complications related to retained breast implant capsules. Plast Reconstr Surg 95: 364-371.
-
Reams BD (1992) Silicone gel-filled breast implants [transcript]. Panel meeting FDA, General and Plastic Surgery Devices Panel. William S. Hein & Co, 68-91.





