Clinical Medical
Reviews and Case Reports
Therapeutic Efficacy of Platelet-Rich Plasma Injections in Treating Chronic High Hamstring Tendinopathy
Jeffrey Krauss1*, Ryan Nugent2, Marko Bodor3,4,5 and Michael Fredericson1
1Department of Orthopaedic Surgery, Stanford University, California, USA
2Boston University School of Medicine, Massachusetts, USA
3Department of Physical Medicine and Rehabilitation, University of California Davis, California, USA
4Department of Neurological Surgery, University of California San Francisco, California, USA
5Interventional Spine and Sports Medicine, Napa, California, USA
*Corresponding author: Jeffrey Krauss, MD, Department of Orthopaedic Surgery, Division of Physical Medicine and Rehabilitation, Stanford University, Stanford Medicine Outpatient Center, 450 Broadway St., Mailcode 6342, Redwood City, CA 94063, USA, Tel: 415-806-9278, E-mail: jkrauss@stanford.edu
Clin Med Rev Case Rep, CMRCR-3-084, (Volume 3, Issue 1), Original Research; ISSN: 2378-3656
Received: December 21, 2015 | Accepted: January 20, 2016 | Published: January 23, 2016
Citation: Krauss J, Nugent R, Bodor M, Fredericson M (2016) Therapeutic Efficacy of Platelet-Rich Plasma Injections in Treating Chronic High Hamstring Tendinopathy. Clin Med Rev Case Rep 3:084. 10.23937/2378-3656/1410084
Copyright: © 2016 Krauss J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: To determine whether ultrasound-guided platelet-rich plasma (PRP) injections reduce pain and improve function in patients with chronic high hamstring tendinopathy.
Design: Prospective case-control study in which patients who have failed treatment serve as their own controls.
Setting: Patients were recruited through two sports medicine clinics, one academically based and the other in a community medical center. Injections were performed by a single practitioner.
Patients: 14 adult patients with chronic high hamstring tendinopathy who previously failed physical therapy. All patients had both positive clinical findings and MRI evidence of high hamstring tendinopathy.
Methods: At baseline, patients received a single ultrasound-guided PRP injection, and completed a questionnaire assessing both average pain on a visual analog scale and functional levels, including the Lower Extremity Functional Scale (LEFS). Patients were then required to follow a physical therapy protocol and return for follow-up evaluation at 12 weeks post-treatment, at which time they completed the same questionnaire.
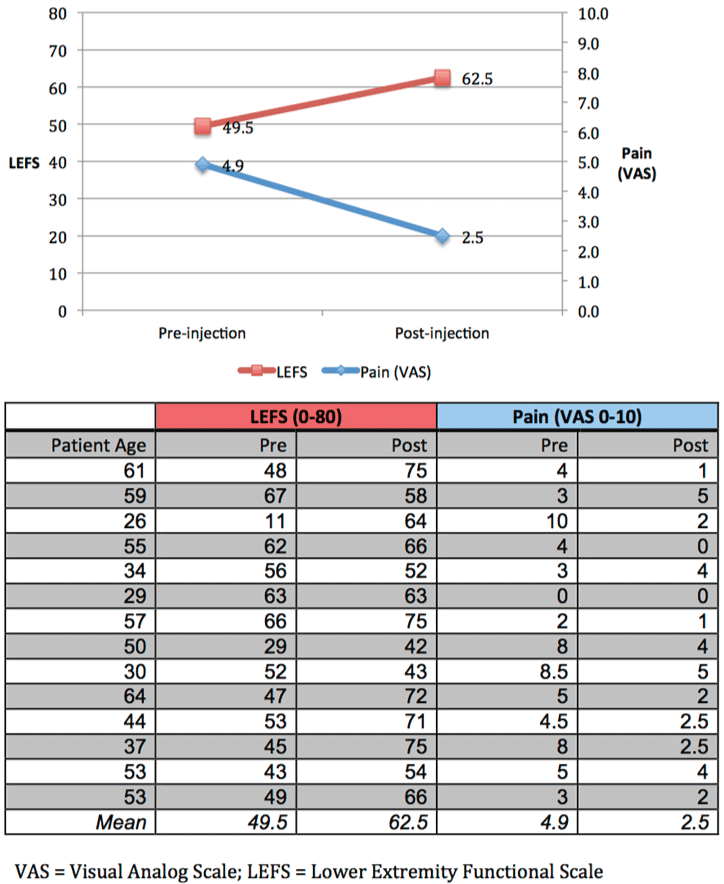
Results: Mean patient age was 46.6 years and 90% (13/14) were female. Mean duration of symptoms was 4.1 years. The mean LEFS score for patients pre-injection was 49.5 out of 80, and increased to 62.5 at 12 weeks post-injection (p = 0.02). The mean pain level prior to injection was 4.9 out of 10 and decreased to 2.5 at 12 weeks following injection (p = 0.01). Half of patients experienced at least a 50% reduction in average pain.
Conclusion: The use of PRP injections shows promise in treating chronic high hamstring tendinopathy, however it requires further evidence in order to become a widely accepted form of treatment. Future research in this area would benefit from larger patient populations, more objective measures of improvement and a randomized, controlled study design.
Keywords
Platelet-rich plasma injection, Hamstring tendinopathy, Hamstring syndrome
Introduction
High hamstring tendinopathy is an overuse injury mostly seen in middle to long distance runners [1]. It has been described in the literature since 1988, although it was originally termed “hamstring syndrome” [2]. However, its presence has been receiving more attention as an important cause for chronic pain in the lower extremities, and researchers have altered the nomenclature due to ambiguity of diagnosis in the previous terms [2-4]. Patients with high hamstring tendinopathy typically report deep buttock pain brought on by running, and in more severe cases the pain can be present during prolonged sitting [2]. Physical examination for tendinopathy is generally notable for local tenderness to palpation, pain with stretching, and reduced muscle activation. The pathophysiology is thought to be repeated heavy loading with an accumulation of micro-damage and failed healing attempts, and histologic evaluation reveals tendon thickening with collagen degeneration, rounding of tenocyte nuclei, increased ground substance, and increased vascular proliferation [3,5]. On MRI, increased tendon size, peri-tendinous T2 signal with a distal feathery appearance, and ischial tuberosity edema are significantly associated with symptomatic hamstring tendinopathy [6]. High-resolution ultrasound is also gaining acceptance in the diagnosis of tendinopathy, and chronically injured hamstring tendons appear hypoechoic with possible thickening and partial-thickness tears, along with possible irregularity of the ischium [7,8].
Conservative management is typically initiated with physical therapy modalities such as ice, electrical muscle stimulation, and pulsed ultrasound. The use of non-steroidal anti-inflammatory drugs (NSAIDs) is of limited benefit and should never be used chronically [9]. Correction of biomechanical factors, such as pelvic alignment and soft tissue mobilization, are also helpful, and the patient is encouraged to begin a progressive strengthening program as soon as possible. Hamstring strength training, especially eccentric exercises, is currently the best-supported treatment for hamstring muscle injuries [10]. Typically, conservatively managed hamstring injuries are fully resolved in 2-6 months, but in about 20% of patients symptoms may persist for more than 6 months and require more aggressive treatment methods [9]. Typically, corticosteroid injections are recommended when other pain management techniques have been unsuccessful, especially in cases of subcutaneous edema surrounding the hamstring attachment at the ischial tuberosity combined with a relatively normal appearing tendon. However, steroid injections are not a long-term solution to chronic tendinopathies, and they are generally prescribed in conjunction with physical therapy regimens to augment treatment [1]. Extracorporeal shockwave therapy (ESWT) has also shown effectiveness in treating chronic tendinopathies [11-14], including chronic proximal hamstring tendinopathy [15]. Persistent cases are approached surgically.
In recent years, the injection of endogenous substances to treat acute and chronic injuries has become a topic of great interest and research. The aim of this treatment is to inject growth factors or other substances with regenerative properties into the injury site in order to stimulate a more vigorous healing response and to promote growth and repair of normal cells and tissue. Platelet-rich plasma (PRP), in particular, has gained popularity in recent years. Although some studies have shown the potential efficacy of PRP injection in treating acute and chronic musculoskeletal injuries [16,17], such as lateral elbow tendinopathy [18,19] and patellar tendinopathy [20,21], there remain many questions as to the mode of action, preparation, timing and other factors involved in its clinical use [22-24].
The main rationale behind the use of PRP is that platelets are a readily available source of bioactive molecules essential to the inflammatory response and tissue repair. Platelets are non-nucleated bodies in the blood that contain over 1100 proteins, including growth factors, cytokines and other bioactive compounds [24,25]. Many of these compounds are contained within the platelet alpha-granules, which rapidly release their contents upon platelet activation [26]. The platelet concentrate seems to modify the natural healing pathway by providing increased concentrations of growth factors and bioactive proteins, which in turn stimulates regeneration of tissue with low baseline healing potential, resembling the initial stage of inflammation, and the attraction of leukocytes to the site of injury [22,24,26]. In vivo and animal studies have shown PRP addition resulted in cell proliferation, collagen deposition, improved gene expression, accelerated remodeling and angiogenic processes, increased anti-inflammatory response, increased fibrillogenesis and improved collagen matrix [27]. PRP also contains fibrin, fibronectin and vitronectin, proteins important for cell adhesion, osteoconduction and as a matrix for bone, connective tissue and epithelial migration [28]. In addition to the PRP adjuvant, studies suggest that the act of performing multiple injections into the diseased tissue is akin to tenotomy, which itself may play a role in the healing process due to induced local trauma, bleeding, and inflammation [20,29].
The preparation of PRP involves separating the cellular component from the plasma using centrifuges. After the blood is drawn from the patient, the blood is treated with an anti-coagulant and spun down resulting in a top layer of plasma, a middle layer of platelets and leukocytes, and a bottom layer of red blood cells. A second round of centrifugation is often performed to separate the platelet-poor plasma from the platelet-rich plasma [24]. However, at present, there are no standardized protocols for PRP injections for tendon and ligament injuries [22] with regards to concentrations of PRP, timing of injection relative to preparation, activation of platelets, and other factors [23,24].
While many studies have investigated the use of PRP for tendinopathy with mixed results [16,21,22,30-32], to our knowledge there are only three small studies, two retrospective and one prospective, reporting on the use of PRP in treating high hamstring tendinopathy [33-35]. Two of the studies have demonstrated significant improvement in pain and/or function [33,35], while the third showed at least 80% pain reduction in over half of patients, though statistical significance was not reported [34]. Given the lack of conclusive data, we sought to examine whether PRP injections reduced pain levels and improved function in patients with chronic high hamstring tendinopathy.
Materials and Methods
Patient population
Patients were recruited for this study through two sports medicine clinics, one academically based and the other in a community medical center. Athletes are a common patient population seen at these institutions, including elite collegiate and recreational athletes of various fitness levels. All patients had been referred by their physicians to receive PRP injections for the treatment of their tendinopathy and met predetermined inclusion/exclusion criteria. The inclusion criteria were: at least 18 years of age, failed physical therapy (including eccentric exercises), and positive MRI signs of high hamstring tendinopathy with or without adjacent bone marrow edema. In addition, patients had at least two of the following positive clinical findings: tenderness to palpation at the site of the ischial tuberosity, positive bent knee stretch test and positive supine plank test [1]. Exclusion criteria were: presence of other acute injuries to the lower limb, concurrent pregnancy, minors or the presence of malignant disease. Patients meeting the above criteria were approached and recruited. Additional information was gathered for complete baseline evaluation, including a Lower Extremity Functional Scale questionnaire [36]. Patients then received a single intratendinous PRP injection prepared and administered according to the study protocol.
PRP preparation
PRP was prepared using the Cascade Autologous Platelet System (ConMed Linvatec). Antecubital venipuncture was used to draw approximately 9 ml of blood, which was then centrifuged at 1100 g for 6 minutes. PRP was separated from the red blood cells and granulocytes by a layer of hydrophilic polysaccharide within the test tube and aspirated into a sterile syringe, yielding approximately 4 mL (range 2.5-5 mL) of PRP containing platelets, lymphocytes and monocytes. Calcium chloride was used to activate platelets. A hemocytometer (Horiba ABX Micros 60) was used to measure PRP counts in injections starting in 2014 (7 of 14 included injections). Mean # of platelets in these injections was 6.77 × 109 (range 3.55 × 109- 1.38 × 1010). Mean white blood cell concentration was 6.13 × 103 (range 3.6 × 103 - 11 × 103) prior to preparation and 6.35 × 103 (range 2.9 × 103 - 17.9 × 103) post preparation.
Injection procedure
The patient was placed on the examination table in prone position, and the gluteal region was examined under ultrasound. The proximal hamstring tendon origin was identified by transverse and longitudinal scanning using a high frequency transducer (Philips iU22). Local blood vessels and the sciatic nerve were identified. After identifying the target, a 21 gauge, 1.5 inch needle (longer needles were required in larger patients) was guided to the hamstring tendons using an in-plane approach with injection of 2-3 cc of 1% lidocaine for local anesthesia. The PRP was then injected slowly under ultrasound guidance, targeting areas of focal hypoechoic texture or areas of partial tearing. Injection was performed during gentle advance and retraction of the needle, in order to deposit the PRP deep, interstitial, and superficial to the tendons. Typically, 5 passes of the needle through different areas of the tendon were used for needle tenotomy. In addition, the spinal needle was used to probe the ischial tuberosity itself, with the aim of stimulating the periosteum or releasing marrow factors, though the bone was typically very firm and the marrow space was not entered directly. After PRP injection, mild pressure was applied to the site to distribute the injected materials further about the hamstring tendons and to minimize any local bleeding. Patients were kept prone for an additional 10 minutes following the procedure. All injections were performed by a single practitioner.
Post-treatment protocol
Following injection, all patients were asked to observe a 1 week rest period with non-weight bearing for the first 2 days, then progressive weight bearing using crutches advanced through the remainder of the week. After the first week, patients began physical therapy per protocol. All patients were then asked to revisit the clinic for a re-evaluation after the 12th week. Based on the clinical evaluation, a new MRI was recommended if the recovery level was not as expected. At this point, the patient was able to proceed with a second PRP injection or elect to undergo surgery consultation.
Statistical analysis
Calculations were performed using SPSS Statistics Version 22 (IBM Corp., Armonk, NY, USA). As the assumption of normality was violated in our data, pre- and post-injection data were compared using nonparametric Wilcoxon rank sum t tests. We defined patients with significant functional improvement as those in whom LEFS score increased by at least 9 points, according to the standards of the authors who defined the assessment [36].
Results
At the time of this paper, PRP injections had been performed on 18 patients meeting study criteria. Of these, 3 were excluded due to lack of completion of the initial LEFS questionnaire, and 1 was excluded due to lack of 12-week follow-up evaluation. As a result, 14 total patients were included in the analysis. The mean age of included patients was 46.6 years (range 26-64) and 90% (13/14) were female. Mean duration of symptoms was 4.1 years, with 1 patient (7%) reporting duration less than 6 months, 7 patients (50%) reporting 6 months to 1 year, and 6 patients (43%) reporting symptoms persisting for 1 year or more. All patients had previously undergone and failed physical therapy, including eccentric exercises, and 60% (8/14) had previously received corticosteroid injections. In addition, 60% of patients (8/14) had taken NSAIDs, and 29% of patients (4/14) had tried other complementary treatments, such as acupuncture or massage. The patient characteristics are summarized in table 1.
![]()
Table 1: Patient Characteristics and Prior Treatments.
View Table 1
Mean average pain level prior to injection on 0-10 visual analog scale (VAS) was 4.9 (range 0-10, SE 0.75) and decreased to 2.5 at 12 weeks following injection (range 0-5, SE 0.45; z = -2.63, p = 0.01) (Figure 1). 79% of patients (11/14) reported an improvement in average pain, 7% (1/14) reported no change, and 14% (2/14) reported worsened pain. Of note, the patient who reported no change in pain score had 0/10 average pain both pre- and post-injection. 50% of patients (7/14) reported at least 50% improvement in average pain.
With regards to functional change, the mean LEFS score for patients pre-treatment was 49.5 (range 11-67, SE 4.03) and increased to 62.5 at 12 weeks following injection (range 42-75, SE 3.03; z = -2.35, p = 0.02) (Figure 1). The mean percentage of maximal function prior to injection was 61.8% (range 13.8-83.8%, SE 5.03) and increased to 78.1% at 12 weeks post injection (range 52.5-93.8%, SE 3.79; z = -2.35, p = 0.02). 57% of patients (8/14) had a significant improvement in LEFS (defined as at least 9 points), 14% of patients (2/14) had non-significant improvement, and 29% of patients (4/14) had worsened LEFS score.
Discussion
In this prospective study, significant improvements in both pain and functional scores were demonstrated at 3 months following PRP injection in 14 patients. All patients had failed physical therapy with eccentric hamstring strengthening, the gold standard for chronic tendinopathy, suggesting that symptoms were unlikely to improve without intervention. An increase in LEFS score greater than 9 is suggestive of significant functional improvement [36], and we saw a mean LEFS improvement of 13.0 in the current study (p = 0.02). 57% patients in the study reported significant improvements in their symptoms and overall athletic capabilities, while a smaller percentage reported insignificant benefit from the treatment or a lower LEFS score. This study also demonstrated a significant decrease in average pain levels of 2.4 points on a 10-point scale at 12 weeks (p = 0.01). 50% (7/14) of patients experienced at least a 50% reduction in average pain, and 35% (5/14) of patients experienced both a significant improvement in LEFS (≥ 9) and at least a 50% reduction in pain.
Numerous studies have evaluated the effect of PRP on chronic tendinopathies with mixed results. A 2013 Cochrane analysis found no significant difference in functional improvement in PRP vs. control groups, and suggested only a possible short-term reduction in pain in favor of PRP [21]. Similarly, a recent meta-analysis of five randomized control trials showed that PRP did not provide significant benefit over placebo or dry needling at 6 months, though benefit was seen over 2-3 months [30]. However, another recent review of 9 RCTs showed benefit only in patellar and lateral epicondylar tendinopathy, but not for Achilles and rotator cuff tendinopathy [31]. Lastly, a retrospective cross-sectional study of 180 patients found a significant short- and long-term decrease in pain following PRP injection in patients with a variety of chronic tendinopathies [16].
Currently, to the best of our knowledge, only three small studies report the effects of injecting PRP into patients with chronic high hamstring tendinopathy [33-35]. Wetzel et al. showed a significant reduction in pain and function in 10 patients treated with PRP, but no significant improvement in a control group treated with conservative measures alone [33]. Fader et al. reported that 10 of 18 patients with chronic high hamstring tendinopathy had 80% or greater improvement in pain scores following PRP injection; however statistical significance was not reported. Lastly, in a randomized control trial comparing PRP and whole blood injections, Davenport et al. recently demonstrated statistically significant improvements in ADL and quality of life scores in 11 patients receiving PRP injections, though outcomes were not statistically significant between PRP and whole blood groups. The current prospective study further suggests the efficacy of PRP for treating chronic high hamstring tendinopathy. Compared with prior studies, patients in the current study had longer duration of symptoms (mean 4.1 years).
It is unclear why studies of the efficacy of PRP in chronic high hamstring tendinopathy have demonstrated consistently positive results, while reviews of its use in a variety of chronic tendinopathies are mixed. Of note, prior reviews have included very few patients with high hamstring tendinopathy. The cross-sectional survey by Mautner et al. included only 17 hamstring patients out of 180, and it is not clear if any suffered from high hamstring tendinopathy [16]. The analyses by Tsikopoulos et al, Moraes et al, and Balasubramaniam et al. did not include any patients with hamstring tendinopathy [21,30,31]. While the pathophysiology of high hamstring tendinopathy is likely similar to that of other tendinopathies, and morphologic changes in hamstring tendinosis are largely identical to those in Achilles and patellar tendinosis [3], prior reviews show a wide variation in results between different tendinopathies. For example, as noted previously, Balasubramaniam et al. reported significant effects of PRP in patellar and lateral epicondylar tendinopathy, while no benefit was seen for Achilles and rotator cuff tendinopathy [31]. Another explanation for the mixed results may be the lack of standardization regarding PRP indications, number of platelets injected, presence of white blood cells in the injectate, activation of platelets, and method of delivery [21-23,26]. However, we were unable to identify common preparation techniques among studies of PRP in high hamstring tendinopathy to explain the consistently positive findings. Lastly, it should be noted that all studiesof PRP in high hamstring tendinopathy have been small (less than or equal to 18 patients) and none have been randomized control trials comparing PRP injection to placebo. Furthermore, these studies may have significant selection bias as high hamstring PRP injections are typically performed in athletes who have failed conservative treatments.
There were multiple limitations that potentially affected the outcomes of our research. First, the small sample size limited the statistical power of the study and our ability to perform subgroup analysis. Second, questionnaires can lead to subjective results, can be influenced by recall, and it is difficult to compare the results of one patient with another. Objective data regarding the effects of PRP on hamstring tendinopathy can be obtained by comparing pre- and post-treatment imaging studies, such as MRI and ultrasound, however this would not necessarily correlate to patient symptoms or functional status. Third, the standard follow-up time was set at 12 weeks, but it is possible that the healing timeline is highly variable and the full healing potential of PRP may take significantly longer for some patients. Currently, there are no clear guidelines of duration of treatment effect and follow-up timeline. Including follow-ups for times longer than 12 weeks may better demonstrate the long-term outcome of the PRP treatment. Lastly, given the study design, neither the patients nor the clinicians were blinded. There were no controls beside historical control, which cannot rule out the influence of other confounding factors.
The results of this study show promise for the use of PRP in treating high hamstring tendinopathy. Future studies with larger patient sample sizes can help validate and further characterize the clinical effect seen in this study. Subgroup analysis with sufficient follow-up may better delineate the onset and the duration of treatment benefit, the degree of symptoms and functional improvement, and the demographic or biomechanical factors influencing them. Pre- and post-treatment imaging studies using MRI or ultrasound could help demonstrate the macroscopic tissue changes, which could then be correlated with symptoms and function. Lastly, randomized, double-blinded controlled studies are needed to remove the effects of confounding factors on study outcomes.
Conclusion
PRP injections show promise in treating chronic high hamstring tendinopathy. This study demonstrated significant improvements in both pain and functional scores in patients who previously failed physical therapy. Future research in this area should include a larger patient population, more objective measures of improvement, and a randomized, controlled study design.
Acknowledgements
No funding was received for this study. The authors wish to acknowledge Yvette Uribe and Dr. Julia Arroyo Martin for their significant assistance in coordinating the study.
References
-
Fredericson M, Moore W, Guillet M, Beaulieu C (2005) High hamstring tendinopathy in runners: meeting the challenges of diagnosis, treatment, and rehabilitation. Phys Sportsmed 33: 32-43.
-
Puranen J, Orava S (1988) The hamstring syndrome. A new diagnosis of gluteal sciatic pain. Am J Sports Med 16: 517-521.
-
Lempainen L, Sarimo J, Mattila K, Vaittinen S, Orava S (2009) Proximal hamstring tendinopathy: results of surgical management and histopathologic findings. Am J Sports Med 37: 727-734.
-
Zissen M.H, Wallace G, Stevens KJ, Fredericson M, Beaulieu CF (2010) High hamstring tendinopathy: MRI and ultrasound imaging and therapeutic efficacy of percutaneous corticosteroid injection. AJR Am J Roentgenol 195: 993-998.
-
Warden SJ (2007) Animal models for the study of tendinopathy. Br J Sports Med 41: 232-240.
-
De Smet AA, Blankenbaker DG, Alsheik NH, Lindstrom MJ (2012) MRI appearance of the proximal hamstring tendons in patients with and without symptomatic proximal hamstring tendinopathy. AJR Am J Roentgenol 198: 418-422.
-
Koulouris G, D Connell (2005) Hamstring muscle complex: an imaging review. Radiographics 25: 571-586.
-
Jacobson JA (2013) Fundamentals of musculoskeletal ultrasound. (2nd edn), Saunders/Elsevier, Philadelphia, PA, 400.
-
Brukner P, K Khan (2012) Brukner & Khan's Clinical Sports Medicine. (4th edn), Sports medicine series, Sydney, McGraw-Hill, New York, 1296.
-
Sherry MA, TM Best (2004) A comparison of 2 rehabilitation programs in the treatment of acute hamstring strains. J Orthop Sports Phys Ther 34: 116-225.
-
Al-Abbad H, JV Simon (2013) The effectiveness of extracorporeal shock wave therapy on chronic achilles tendinopathy: a systematic review. Foot Ankle Int 34: 33-41.
-
Galasso O, Amelio E, Riccelli DA, Gasparini G (2012) Short-term outcomes of extracorporeal shock wave therapy for the treatment of chronic non-calcific tendinopathy of the supraspinatus: a double-blind, randomized, placebo-controlled trial. BMC Musculoskelet Disord 13: 86.
-
van der Worp H, van den Akker-Scheek I, van Schie H, Zwerver J (2013) ESWT for tendinopathy: technology and clinical implications. Knee Surg Sports Traumatol Arthrosc 21: 1451-1458.
-
Wang CJ (2012) Extracorporeal shockwave therapy in musculoskeletal disorders. J Orthop Surg Res 7: 11.
-
Cacchio A, Rompe JD, Furia JP, Susi P, Santilli V, et al. (2011) Shockwave therapy for the treatment of chronic proximal hamstring tendinopathy in professional athletes. Am J Sports Med 39: 146-153.
-
Mautner K, Colberg RE, Malanga G, Borg-Stein JP, Harmon KG, et al. (2013) Outcomes after ultrasound-guided platelet-rich plasma injections for chronic tendinopathy: a multicenter, retrospective review. PM R 5: 169-175.
-
A Hamid MS, Mohamed Ali MR, Yusof A, George J, Lee LP (2014) Platelet-Rich Plasma Injections for the Treatment of Hamstring Injuries: A Randomized Controlled Trial. Am J Sports Med.
-
Mishra A, Pavelko T (2006) Treatment of chronic elbow tendinosis with buffered platelet-rich plasma. Am J Sports Med 34: 1774-1778.
-
Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T (2010) Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med 38: 255-262.
-
Dragoo JL, Wasterlain AS, Braun HJ, Nead KT (2014) Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. Am J Sports Med 42: 610-618.
-
Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC (2014) Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev 4.
-
Taylor DW, Petrera M, Hendry M, Theodoropoulos JS (2011) A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med 21: 344-352.
-
Dohan Ehrenfest DM, Andia I, Zumstein MA, Zhang CQ, Pinto NR, et al. (2014) Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J 4: 3-9.
-
Arnoczky SP, Sheibani-Rad S (2013) The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc 21: 180-185.
-
Leslie M (2010) Cell biology. Beyond clotting: the powers of platelets. Science 328: 562-564.
-
Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA (2009) Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 37: 2259-2272.
-
de Vos RJ, van Veldhoven PL, Moen MH, Weir A, Tol JL, et al. (2010) Autologous growth factor injections in chronic tendinopathy: a systematic review. Br Med Bull 95: 63-77.
-
Marx RE (2004) Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 62: 489-496.
-
Yelland MJ, Glasziou PP, Bogduk N, Schluter PJ, McKernon M (2004) Prolotherapy injections, saline injections, and exercises for chronic low-back pain: a randomized trial. Spine (Phila Pa 1976) 29: 9-16.
-
Tsikopoulos K, Tsikopoulos I, Simeonidis E, Papathanasiou E, Haidich AB, et al. (2016) The clinical impact of platelet-rich plasma on tendinopathy compared to placebo or dry needling injections: A meta-analysis. Phys Ther Sport 17: 87-94.
-
Balasubramaniam U, Dissanayake R, Annabell L (2015) Efficacy of platelet-rich plasma injections in pain associated with chronic tendinopathy: A systematic review. Phys Sportsmed 43: 253-261.
-
Kon E, Filardo G, Di Martino A, Marcacci M (2011) Platelet-rich plasma (PRP) to treat sports injuries: evidence to support its use. Knee Surg Sports Traumatol Arthrosc 19: 516-527.
-
Wetzel RJ, Patel RM, Terry MA (2013) Platelet-rich plasma as an effective treatment for proximal hamstring injuries. Orthopedics 36: e64-70.
-
Fader RR, Mitchell JJ, Traub S, Nichols R, Roper M, et al. (2014) Platelet-rich plasma treatment improves outcomes for chronic proximal hamstring injuries in an athletic population. Muscles Ligaments Tendons J 4: 461-466.
-
Davenport KL, Campos JS, Nguyen J, Saboeiro G, Adler RS, et al. (2015) Ultrasound-Guided Intratendinous Injections With Platelet-Rich Plasma or Autologous Whole Blood for Treatment of Proximal Hamstring Tendinopathy: A Double-Blind Randomized Controlled Trial. J Ultrasound Med 34: 1455-1463.
-
Binkley JM, Stratford PW, Lott SA, Riddle DL (1999) The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther 79: 371-383.