Obstetrics and Gynaecology Cases - Reviews
Retroperitoneal Lymphoma Presenting as Gynecologic Malignancy: Case Report and Review of the Literature
Devin Miller, Sarah Andiman, Elena Ratner and Diana English*
Division of Gynecologic Oncology, Yale University School of Medicine, USA
*Corresponding author: Diana P. English, M.D., Division of Gynecologic Oncology, Yale University School of Medicine, 333 Cedar Street, PO Box 208063, New Haven, CT 06520-8063, USA, Tel: 203-785-7385, Fax: 203-737-4377, E-mail: Diana.english@yale.edu
Obstet Gynecol Cases Rev, OGCR-2-048, (Volume 2, Issue 4), Case Report; ISSN: 2377-9004
Received: May 11, 2015 | Accepted: July 01, 2015 | Published: July 04, 2015
Citation: Miller D, Andiman S, Ratner E, English D (2015) Retroperitoneal Lymphoma Presenting as Gynecologic Malignancy: Case Report and Review of the Literature. Obstet Gynecol Cases Rev 2:048. 10.23937/2377-9004/1410048
Copyright: © 2015 Miller D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Pelvic malignancy is commonly thought to represent a gynecologic primary on presentation. In reality, the differential diagnosis is wide and tumors which do not fit typical patterns on imaging or initial pathology can present a significant diagnostic dilemma as these patients may undergo radical gynecologic surgery unnecessarily.
We present the case of a 63 year old female presenting with abdominal pain, back pain, weight gain, and hydronephrosis with acute kidney injury. She was initially admitted to the gynecologic oncology service due to a finding of a large tumor within lower abdomen and pelvis with a heterogenous appearing uterus. She had an image guided biopsy of this mass and was ultimately found to have a primary retroperitoneal non- Hodgkin's lymphoma (diffuse large B cell). She then received medical management, as is appropriate for this diagnosis. Chemotherapy was initiated in a very timely manner and she showed significant improvement within weeks.
Introduction
While lymphoma is not typically on the forefront of the mind of the gynecologist, it is important to entertain pelvic lymphoma as a differential diagnosis when appropriate. In the past 30-40 years, the incidence of lymphoma has increased by an annual rate of approximately 4% in the 1970s and fortunately has tapered to a 2% annual increase since the 1990s [1].
In white women, NHL has an approximate incidence of 11.5 per 100,000 person years. In a study of 60,000 cases diagnosed over a 17 year period, the incidence in black women was about 40% lower [2]. The National Cancer Institute estimates new cases and deaths from NHL in the United States in 2015 to be approximately 17,850 and 19,790 respectively.
NHL can present in the female genital tract, as an extranodal tumor or as ovarian, uterine, cervical, vaginal, vulvar or even fallopian tube as the primary site. The prevalence of these female genital tract lymphomas tend to be much lower, with rates seen to be between 0.2% and 1.1% in separate studies of lymphoma cohorts [3,4].
Here we describe a case of an abdomino-pelvic retroperitoneal lymphoma in a female and review retroperitoneal lymphomas including epidemiology, typical presentation, diagnostic considerations and a brief explanation of staging, therapy and prognosis.
Case
A 63 year old woman presented to the emergency department at our institution due to severe abdominal pain. Prior to this presentation, she had been hospitalized at another institution for work-up and management of crippling abdominal, flank and back pain along with urinary frequency that had been present for six weeks. She also reported a change in bowel function noting that her stools had become "stringy," and she reported bloating and early satiety. She had an eighteen pound weight gain over the past eight months. The patient had a CT scan performed at the outlying institution and was told that the CT showed an "inoperable tumor" in her back.
Her past medical history was notable for a remote history of seizures and the patient was currently taking divalproex sodium 500mg daily. Her gynecologic history was significant for menarche at 11 years of age, menopause at 52 years and no hormone replacement use. She had a history of normal pap smears, no history of postmenopausal bleeding and no history of sexually transmitted infections. Her surgical history was significant for three cesarean section deliveries and the removal of an area of benign calcification in her left breast. Her family history was significant for both mother and maternal grandmother with breast cancer in their 50's.
On examination at the time of presentation, she had normal vital signs. She was thin appearing with no obvious surface lymphadenopathy. Her abdomen was distended, tympanic and minimally tender with a firm fixed mass measuring approximately 10 x 6cm palpable in the right lower quadrant and a soft mass in the left lower quadrant. There was no rebound tenderness or guarding. On pelvic exam the cervix was not visualized secondary to a tortuous irregular vaginal canal. There was a non-mobile palpable mass on the right that seemed to be fixed to the uterus.
Labs performed in the emergency department were significant for a normal white blood cell count, elevated creatinine at 1.3mg/dL, elevated LDH at 666U/L, mildly elevated AST at 39U/L and the remainder of her liver function tests were within normal limits. Urinalysis showed >30 RBCs/HPF. As the patient's imaging from the other institution was not available at the time of presentation, a computed tomography (CT) was performed in our emergency department. The imaging showed a diffuse soft tissue mass throughout the retroperitoneum encasing the aorta, inferior vena cava (IVC) and iliac vessels. The uterus was noted to be large, heterogeneous and fluid filled (Figure 1). Bilateral hydroureteronephrosis was noted (Figure 2). No bowel obstruction was visualized. Numerous liver hypodensities too small to characterize were noted. The gallbladder, pancreas, spleen, and adrenals were unremarkable. There were no osseous lesions.
.
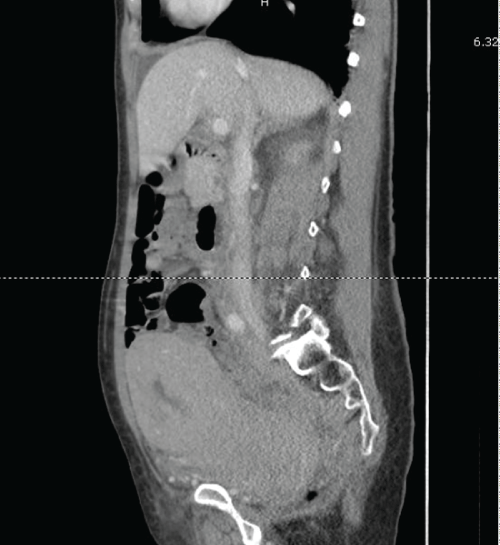
Figure 1: Coronal CT scan image showing enlarged heterogenous and fluid filled uterus along with retroperitoneal masses.
View Figure 1
.
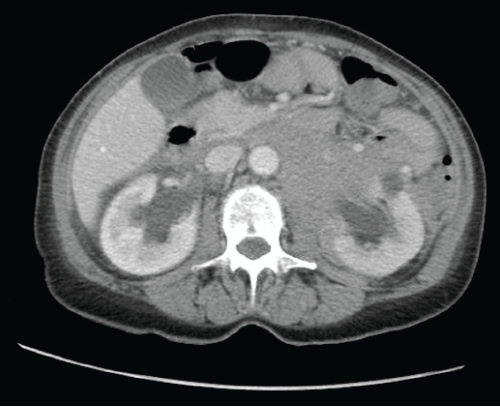
Figure 2: Axial CT scan image showing bilateral hydronephrosis and diffuse soft tissue mass throughout the retroperitoneum encasing the aorta and inferior vena cava.
View Figure 2
Transabdominal and transvaginal ultrasounds were performed for better evaluation of the uterus, ovaries and other pelvic structures. The uterus measured 16 x 7.5 x 15.5cm and was noted to have a markedly heterogeneous echotexture. The endometrial echo measured 4.2cm. An irregular contour to the left lower uterine segment was noted, and a poorly defined vascular mass was noted to be closely associated with the myometrium and of possibly cervical or uterine in origin. There was also a hypoechoic mass within the posterior lower uterine segment with appearance consistent with a myoma. The right ovary measured 3.8 x 1.6 x 1.5cm and the left ovary measured 1.8 x 1.1 x 1.7cm. Evaluation of the right and left kidney showed moderate bilateral hydronephrosis. A large amount of soft tissue was noted within the left retroperitoneum and appeared to be encasing the left ureter. There was a trace amount of free fluid within Morrison's pouch.
A magnetic resonance imaging scan (MRI) was then done for better characterization of the uterus, retroperitoneal mass and areas of metastatic disease. This showed a soft tissue mass in the left retroperitoneum and anterior left para-renal space extending into the right retroperitoneum and pelvis, encasing the left kidney, proximal abdominal aorta and IVC. Inferiorly the mass extended into the pelvis where it encased the iliac vasculature and was causing moderate bilateral hydroureteronephrosis. This mass was inseparable from the urinary bladder and displaced the bowel anteriorly (Figure 3). There were multiple subcentimeter hypodense intense T2 lesions throughout the liver, osseous pelvis and abnormal hypointense T2 signal throughout the spine, concerning for metastases.
.
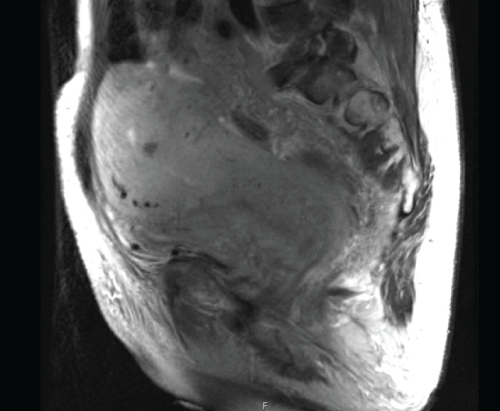
Figure 3: Coronal MRI image showing large heterogenous uterus and soft tissue mass in the retroperitoneum extended into the pelvis. This mass was inseparable from the urinary bladder and displaced the bowel anteriorly.
View Figure 3
The patient was admitted to the gynecology oncology service for further work up of the pelvic mass. She was started on intravenous hydromorphone for pain control and continued on her home divalproex sodium. A CA-125 was ordered and was elevated at 60.2U/mL, CA 19-9 and CEA were within normal limits. Interventional radiology was contacted to perform a biopsy of the pelvic mass and on hospital day number one; a biopsy of the left iliac chain lymph nodes and retroperitoneal mass was performed.
While the patient remained on the gynecologic oncology service, her creatinine continued to rise, reaching to 1.5mg/dL on hospital day 4. Her fractional excretion of sodium (FENa) was found to be 0.15%, consistent with prerenal azotemia, but likely with an obstructive component. Nephrology was contacted. The nephrology team felt the patient had acute kidney injury secondary to bilateral hydroureteronephrosis.
Her elevated urine protein/creatinine ratio was thought to be likely secondary to tubular damage, although the urine sodium at less than 20 was suggestive of a prerenal process. It was thought that this could be related to abnormal tubulo-glomerular reflex as well as involvement of the retroperitoneal mass vasculature, which could lead to decreased flow to the kidneys. The patient was also noted to be hypercalcemic (corrected calcium 11.7mg/dl) which itself could also lead to vasoconstriction and a prerenal state. The patient's hypercalcemia was evaluated with the usual studies although it was thought that in this situation the hypercalcemia was mostly likely secondary to an underlying malignant process with osseous involvement of the pelvis and/or spine. Calcitonin was administered to manage the hypercalcemia with a plan to continue if the patient responded. Both calcitonin and a bisphosphonate were started, and calcitonin was eventually discontinued after the bisphosphonate began to take effect.
Urology was also consulted for the placement of percutaneous nephrostomy tubes versus ureteral stents. The urology service recommended bilateral percutaneous nephrostomy tubes as it was felt that internal ureteral stents would be unsuccessful. The bilateral percutaneous nephrostomy tubes were successful placed and her creatinine decreased initially from 1.7 to 1.1mg/dl, and then stabilized at 0.8-0.9mg/dl.
She reported shortness breath during the hospitalization and had a CT scan of the chest with IV contrast performed which revealed bilateral pulmonary emboli in segmental and sub-segmental pulmonary arteries, involving predominantly the pulmonary arteries supplying the left lower lobe, but also the right middle and the right lower lobe. Further evaluation did not reveal evidence of right heart strain and she was subsequently started on therapeutic low molecular weight heparin.
Her pathology returned as a diffuse large B-cell lymphoma. The biopsied material consisted of a diffuse proliferation of large atypical lymphocytes with oval to irregular nuclei, dispersed chromatin, distinct nucleoli and small to moderate amounts of cytoplasm. Scattered mitotic figures and apoptotic bodies were also visualized.
Neoplastic B-cells were positive for CD20 and CD45, and negative for CD10. c-MYC rearrangement analysis, which is used as a diagnostic support for Burkitt's lymphoma, was assessed in 30 cells by interphase fluorescence in-situ hybridization (FISH).
c-MYC rearrangement as indicated by split green and red signals was not detected.
Additional studies showed that greater than 80% of the cells were positive for BCL6 and MUM1, which is a finding consistent with Diffuse Large B-Cell Lymphoma of post-germinal cell origin.
A bone marrow biopsy for staging was performed and revealed a moderately hypocellular marrow with trilineage hematopoiesis and no evidence of a lymphoproliferative process. Flow cytometry however showed very few B-cells, a restricted population of B cells that were CD19+ CD20+ CD10- CD5-. These findings were consistent with involvement of the bone marrow by B-cell lymphoma due to the corresponding profile in the prior biopsy of the retroperitoneal mass. Given the diagnosis of diffuse large B- cell lymphoma, the patient was treated with R-CHOP therapy by medical oncology. She received Doxorubicin 50mg/m2 IV push once on Day 1, Vincristine 2mg IV once on Day 1, Cytoxan 750mg/m2 once on Day 1 and Rituximab 375mg/m2 once on Day 2 and Prednisone 100mg PO daily days 1-5.
She was discharged after a 2 week hospital stay and received G-CSF support in the clinic. At her 3 month follow-up, she was doing well. Her nephrostomy tubes had been removed and her creatinine remained stable. She has since completed a regimen of 6 cycles of chemotherapy and has remained clinically free of disease. She will have a PET scan for disease surveillance in a few months.
Discussion
Primary pelvic NHL is a rare and only approximately 1.5% of extranodal NHL originate in the female genital tract and as such only a few cases have been reported [5]. Female genital tract NHL may be present in the retroperitoneum, ovary, uterus, cervix, vagina or vulva [3]. Lymphoma of the retroperitoneum and pelvis can therefore be due to a nodal primary, a female genital tract primary, or from other abdominal organs.
We presented a case of a primary non- Hodgkin's Lymphoma (NHL) presenting as a possible gynecologic malignancy. The diagnosis of retroperitoneal lymphoma can be difficult secondary to its ability to present as many other tumors, including pancreatic, hepatic, gynecologic, and urologic, as well as retroperitoneal fibrosis [6-10]. As in this case presentation, a large retroperitoneal tumor can easily restrict ureteral flow resulting in bilateral hydronephrosis and thus incurring renal injury. Prompt diagnosis and intervention aided this patient in regaining renal function as well as commencing chemotherapy without unwarranted surgery.
Background including classification and staging
The World Health Organization (WHO) has classified lymphomas first by differentiating Hodgkin's lymphomas from NHL. Historically, the Working Formulation and Rappaport classification was used, however currently the WHO classification is the updated classification system. Thus Hodgkin's lymphoma (HL) is categorized broadly as nodular lymphocyte-predominant Hodgkin's lymphoma versus classical Hodgkin's lymphoma which is further subdivided [11].
NHL is subdivided as B cell neoplasms and T-cell/natural killer (NK) cell neoplasms. These are both further subdivided into precursor and peripheral neoplasms. Precursor neoplasms are at the earlier stages of differentiation and peripheral neoplasms are more maturely differentiated [12]. As in this patient, diffuse large B cell lymphomas are the most common, comprising 30-58% of cases of lymphoma [13]. Lymphoma can present as nodal or extranodal. This patient presented with nodal lymphoma that is lymphoma arising from lymph nodes. There are many cases of extranodal lymphoma as well, arising from a non-lymph node source such as hepatic, renal, female genital tract, though these are much less common than nodal lymphoma, and typically represents 20-34% of all lymphomas [2].
In recent years, an increased rate of extranodal lymphomas has been noted in HIV positive patients. Although our patient was HIV negative, this is an important point to keep in mind and HIV testing should be performed in patients with a new diagnosis of lymphoma. Importantly, if managed appropriately, HIV status does not affect treatment outcomes for lymphoma if standard first line treatment for lymphoma is administered [14].
Immunohistochemistry is the most widely available and common way to diagnose types of lymphoma histologically. When lymphoma is determined to be the malignancy of a pathologic specimen, it must be determined whether it is a B cell neoplasm or T cell/NK cell neoplasm. Thus CD20, CD3, CD45, cytokeratin are initially performed, as CD20+ corresponds to B cell neoplasms, CD3 typically corresponds to T cell neoplasms, CD45+ corresponds to granulocytic sarcomas and cytokeratin to carcinomas [15]. Given that the majority of NHL is large B cell, most of the time immunohistochemistry is enough to make the diagnosis. However if there are differing characteristics of the tumor, such as inclusion of small cells, more studies must be undertaken. A significant amount of further stains can be obtained for further prognostic value in these cases and is detailed by the WHO in their monograph [16].
Staging is completed via the Ann Arbor system. This system was initially created for Hodgkin's lymphoma and many believe it more accurately predicts the course of that histology. NHL was adopted into the system in the 1970s. Due to the different patterns of spread, these diseases can be very different in their natural history and prognosis. The International Prognostic Index (IPI) was developed and takes into account Ann Arbor stage, age, number of nodal sites involved, serum LDH level and performance status and this system has proved more accurate for NHL [15].
Symptoms of lymphoma
Given the varying presentations of lymphoma, its heterogeneous anatomic locations, the presence of nodal versus extranodal disease and differing growth patterns, it is not surprising that standard presenting symptoms are rare, especially for patients with pelvic pathology. Traditionally regarded symptoms include weight loss, night sweats, abdominal pain or swelling, fevers, fatigue and swollen glands. Pelvic lymphomas can specifically present with abdominal pain, back pain as well as urinary manifestations including urinary urgency [17]. In our patient, the presence of hydronephrosis along with a large retroperitoneal mass contributed to her presentation with back pain and urinary symptoms. In general however, patients presenting with these or other concerning symptoms including vague pelvic pain, back pain or gynecologic symptoms should undergo proper workup.
Radiographic findings
The difficulty in diagnosis of pelvic masses by imaging is well known. Traditionally modalities of ultrasound (both transvaginal and transabdominal), MRI and CT of the pelvis have been employed. In the literature, several papers have examined the difficulty in diagnosing masses in the retroperitoneum, and it is well known that many other pathology and normal variants can mimic retroperitoneal masses and adenopathy [18]. Retroperitoneal lymphoma may be mistaken for idiopathic retroperitoneal fibrosis. Features more common to lymphoma include suprarenal location, peri-renal extension, anterior aortic displacement, heterogeneity and the presence of additional enlarged nodes [19]. The determination of involved lymph nodes is more accurately determined by PET/CT compared to CT scan and this may be helpful in differentiating lymphoma from other benign entities [20].
When renal involvement is present in lymphoma, then ultrasound will usually reveal a hypoechoic mass consistent with tissue heterogeneity. Ultrasound is used as a screening modality when renal pathology is suspected, or if disseminated lymphoma is suspected to involve the kidneys. However, CT is the imaging modality of choice and contrast is preferred as without it, entire masses can be missed in the renal parenchyma and perinephric area [21]. MRI can be helpful for delineating other abdominal or pelvic pathology or determining extent of the disease, but is not first line for renal involvement [22].
Female genital tract lymphomas are rare diseases and can often appear similar to other gynecologic malignancies on imaging. Typically, female genital tract lymphomas appear with more homogeneous organomegaly with diffuse infiltration and without necrosis with sometimes pelvic lymphadenopathy as an additional imaging finding. Ovarian lymphomas tend to present bilaterally and these malignancies present a significant diagnostic challenge [23].
Differential diagnosis of retroperitoneal disease
When masses are detected in the retroperitoneum, the differential diagnosis is wide secondary to both benign and malignant processes which can affect this area. Retroperitoneal lymphadenopathy can mimic many other processes on imaging including normal veins, aneurysms, hemorrhage, adhesions, and varices [18]. Retroperitoneal fibrosis, whether idiopathic or due to an autoimmune process or treatment of prior malignancy, can mimic lymphoma. Idiopathic retroperitoneal fibrosis remains a very rare disorder and one that would require biopsy for diagnosis [24]. These patients typically present in their 50s-60s with some degree of renal injury and require immunosuppressive therapy and often ureterolysis for treatment [25]. Castleman's disease (giant or angiofollicular lymphohyperplasia) can similarly present with non-malignant lymphadenopathy and while rare, should remain on the differential at the time of diagnosis [26]. Inflammatory and infectious processes are common in the pelvis and retroperitoneum, and thus lymphoma can mimic these processes. There have been reports of suspected abscesses diagnosed as lymphoma after attempted drainage [27]. Patients with retroperitoneal abscesses may present with fever, weight loss, and other symptoms typical of abscess or infection, but these symptoms are also possible in lymphoma making the diagnosis difficult.
Retroperitoneal lymphadenopathy is not uncommon in gynecologic malignancy such as cervical, vaginal, or uterine cancers. Cervical cancer is known to spread predictably through the pelvic and para-aortic lymph nodes and thus can cause hydronephrosis, which is identified in the FIGO staging system as a stage III B. Likewise, uterine cancer can spread by lymphatics to retroperitoneal lymph nodes in both the pelvic and para-aortic area. If a primary tumor is undiagnosed, as in the patient presented, then lymphoma can easily appear as a gynecologic malignancy.
Soft tissue sarcomas and liposarcomas are sometimes mistaken for pelvic or retroperitoneal lymphomas and vice versa. Several case reports exist of extra-nodal lymphoma being diagnosed after firstly being mistaken for a sarcoma [28].
Renal tumors and primary renal lymphoma are more common than NHL in the renal and peri-nephric regions, thus when NHL presents in the kidney it is difficult to make the diagnosis [29].
Female genital tract lymphoma
Extranodal lymphoma has been identified in all of the gynecologic organs and presents a significant diagnostic challenge due to the fact that it typically presents with symptoms typical of traditional gynecologic primary pathology. These tumors are rare; however multiple reviews have examined the data over the last several decades to determine some characteristics of the presentation and prognosis of these patients. A review of the literature performed by Trenhaile et al. noted some common characteristics of these patients [30]. Patients with NHL of the vagina, cervix, or uterus tended to present with pelvic pain, pelvic mass, and/or vaginal bleeding. Patients with ovarian NHL tended to present like those patients with epithelial ovarian cancer with bloating, abdominal pain, nausea, vomiting and pelvic mass [31]. Ovarian lymphoma can also be easily misdiagnosed initially as dysgerminomas, granulosa cell tumors or metastatic disease from gastrointestinal sites. Ovarian lymphoma is often found to be bilateral homogenous masses in 41-71% of cases. This knowledge may also be helpful in increasing the likelihood of diagnosis with concomitant acceleration of treatment initiation [30].
Patients with cervical lymphoma may be diagnosed on cervical cytology, and this diagnosis can also be made at the time of examination for abnormal bleeding [6]. Cervical lymphoma typically occurs in premenopausal women. Uterine lymphomas are more common in postmenopausal women and usually present with bleeding and the usual histology is large B cell lymphoma [32]. Vaginal lymphoma typically presents with an obstructing mass, bleeding, or dyspareunia [33]. These patients tend to do well with radiation or chemotherapy, however with advanced stage disease only one third were able to achieve long term remission in a previous study [33]. Patients with vulvar lymphoma tend to be the oldest and have the worst outcomes despite aggressive multimodal therapies [34].
Staging is complicated in these tumors because both the Ann Arbor (primary lymphoma staging system) and International Federation of Gynecology and Obstetrics (FIGO) systems have been used. Studies have attempted to evaluate whether one system is superior to the other, and the results have been inconclusive secondary to the very different criteria for staging within each system and the difficulty in comparing the prognostic value of the two systems as a result. Most authorities recommend using both systems to stage female genital tract lymphomas [15].
Treatment
Generally, extranodal lymphoma carries a worse prognosis than nodal lymphoma, however this also may be due to the fact that these patients are frequently treated with substandard treatment. As noted in the study by Trenhaile et al. according to the National cancer Database, only 46% of patients with extranodal NHL received chemotherapy whereas 70% nodal NHL patients received chemotherapy despite chemotherapy being recommended as first line treatment for these tumors [30]. A recent institutional 33 year review of 36 patients revealed a favorable prognosis for these patients if the diagnosis was made early and therapy initiated [6]. There is no consensus in the management of patients with extranodal female genital tract NHL but reports exist about different types of treatment depending on location of disease. Some patients undergo staging or debulking surgery because of suspected gynecologic pathology, and the diagnosis is only made intraoperative or on final pathology after surgery. Regardless of site of origin, NHL is frequently very chemosensitive depending on their pathology and thus expedited diagnosis can prevent at times unnecessary surgery and morbidity.
In NHL, early indolent tumors are generally treated with radiation therapy alone and are typically nodular or follicular in origin. For patients with diffuse B cell lymphoma such as in the case presented, the current first line therapy remains to be Rituximab in combination with Cyclophosphamide, Doxorubicin, Vincristine and Prednisone (R-CHOP) [11]. In patients with high risk for CNS involvement, prophylaxis is recommended with intrathecal methotrexate or cytarabine. Patients at high risk for CNS involvement include those with bone marrow, testicular, nasal/paranasal, orbital, bone, or peripheral blood involvement [35]. Multiple trials have evaluated treatment with or without radiation therapy, and thus far no difference was shown in overall survival [36].
A recent study showed that retroperitoneal fibrosis may actually result from the presence of B cell lymphoma, and that Rituximab was successful in treating this finding [37]. In these patients, the chemotherapy alone may treat symptoms of retroperitoneal fibrosis including hydronephrosis.
Prognosis
NHLs are divided into indolent and aggressive lymphomas. Indolent NHL types have a better prognosis and longer survival with median survival as long as 20 years but typically are not curable at advanced stages [38]. If disease remains low grade then this can remain a chronic process with need for re-treatment.
Indolent NHL may convert to more aggressive forms of NHL and these patients may still achieve long term survival [39]. Aggressive NHL tends to have a shorter natural history but overall survival at 5 years is over 60%. They are considered to be in complete remission (CR) if patients remain disease free for 24 months given the higher proportion of relapse in the first two years. In a large series of patients with diffuse aggressive lymphomas, late relapse, i.e. after 24 months of CR was 7% [40]. The cure rate is influenced by fast and appropriate treatment with chemotherapy without unnecessary delays with surgical treatment.
Extranodal lymphomas such as lymphomas of the female genital tract have differing prognoses based on the modalities of treatment. Many patients however do very well and achieve remission with targeted systemic therapy in addition to possible surgery and radiation where indicated with the exception of vulvar lymphoma which carries a poor prognosis overall.
Conclusion
The incidence of NHL is generally on the rise and as such this diagnosis may become a more prevalent finding for gynecologists. Bearing in mind the differing treatment approaches for more common gynecologic pathology compared to lymphoma, it is important to remember this entity as a differential diagnosis in order to maximize treatment outcomes and avoid unnecessary surgery. A multidisciplinary approach with the involvement of radiology, pathology, and gynecologic oncology may aid in a speedy diagnosis and prompt initiation of treatment.
References
-
Müller AM, Ihorst G, Mertelsmann R, Engelhardt M (2005) Epidemiology of non-Hodgkin's lymphoma (NHL): trends, geographic distribution, and etiology. Ann Hematol 84: 1-12.
-
Krol AD, le Cessie S, Snijder S, Kluin-Nelemans JC, Kluin PM, et al. (2003) Primary extranodal non-Hodgkin's lymphoma (NHL): the impact of alternative definitions tested in the Comprehensive Cancer Centre West population-based NHL registry. Ann Oncol 14: 131-139.
-
Freeman C, Berg JW, Cutler SJ (1972) Occurrence and prognosis of extranodal lymphomas. Cancer 29: 252-260.
-
Chorlton I, Norris HJ, King FM (1974) Malignant reticuloendothelial disease involving the ovary as a primary manifestation: a series of 19 lymphomas and 1 granulocytic sarcoma. Cancer 34: 397-407.
-
Glass AG, Karnell LH, Menck HR (1997) The National Cancer Data Base report on non-Hodgkin's lymphoma. Cancer 80: 2311-2320.
-
Ahmad AK, Hui P, Litkouhi B, Azodi M, Rutherford T, et al. (2014) Institutional review of primary non-hodgkin lymphoma of the female genital tract: a 33-year experience. Int J Gynecol Cancer 24: 1250-1255.
-
Kalil AN, Reck dos Santos PA, Azambuja DB, Beck PE (2004) A case of retroperitoneal lymphoma presenting as pancreatic tumor. Hepatogastroenterology 51: 259-261.
-
von Figura G, Hartmann D, Pauls S, Barth TF, Adler G, et al. (2010) Difficult diagnosis of a large cystic retroperitoneal tumor mimicking a hepatic origin. Z Gastroenterol 48: 1301-1304.
-
Celia A, De Stefani S, Bruschi M, Micali S, Sighinolfi MC, et al. (2004) Non Hodgkin lymphoma of the ureter: a rare disease. Archivio italiano di urologia, andrologia : organo ufficiale [di] Societa italiana di ecografia urologica e nefrologica / Associazione ricerche in urologia 76: 179-180.
-
Chen L, Richendollar B, Bunting S, Campbell S, Zhou M (2013) Lymphomas and lymphoproliferative disorders clinically presenting as renal carcinoma: a clinicopathological study of 14 cases. Pathology 45: 657-663.
-
(2015) Adult Non-Hodgkin Lymphoma Treatment. [http://www.cancer.gov/cancertopics/pdq/treatment/adult-non-hodgkins/HealthProfessional/page3 NCI].
-
Pileri SA, Milani M, Fraternali-Orcioni G, Sabattini E (1998) From the R.E.A.L. Classification to the upcoming WHO scheme: a step toward universal categorization of lymphoma entities? Ann Oncol 9: 607-612
-
Tilly H, Dreyling M (2010) Diffuse large B-cell non-Hodgkin's lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 21: v172-174.
-
Coutinho R, Pria AD, Gandhi S, Bailey K, Fields P, et al. (2014) HIV status does not impair the outcome of patients diagnosed with diffuse large B-cell lymphoma treated with R-CHOP in the cART era. AIDS 28: 689-697.
-
Lagoo AS, Robboy SJ (2006) Lymphoma of the female genital tract: current status. Int J Gynaecol Pathol 25: 1-21.
-
Jaffe ES (2009) The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology,Tthe Education Program: 523-531.
-
Takase Y, Kodama K, Motoi I, Terasaki Y, Saito K (2013) Urinary frequency as a presentation of bulky malignant lymphoma in the pelvis. Case Rep Urol 2013: 106820.
-
Koehler PR, Mancuso AA (1982) Pitfalls in the diagnosis of retroperitoneal adenopathy. J Can Assoc Radiol 33: 197-201.
-
Rosenkrantz AB, Spieler B, Seuss CR, Stifelman MD, Kim S (2012) Utility of MRI features for differentiation of retroperitoneal fibrosis and lymphoma. AJR Am J Roentgenol 199: 118-126.
-
Morimoto T, Tateishi U, Maeda T, Arai Y, Nakajima Y, et al. (2008) Nodal status of malignant lymphoma in pelvic and retroperitoneal lymphatic pathways: comparison of integrated PET/CT with or without contrast enhancement. Eur J Radiol 67: 508-513.
-
Cohan RH, Dunnick NR, Leder RA, Baker ME (1990) Computed tomography of renal lymphoma. Journal of Computer Assisted Tomography 14: 933-938.
-
Urban BA, Fishman EK (2000) Renal lymphoma: CT patterns with emphasis on helical CT. Radiographics 20: 197-212.
-
Alves Viera MA, Cunha TM (2014) Primary lymphomas of the female genital tract: imaging findings. Diagn Interv Radiol 20: 110-115.
-
van Bommel EF, de Mol M, Langerak AW, Westenend PJ (2011) Idiopathic retroperitoneal fibrosis mimicking malignant lymphoma. Pathol Int 61: 672-676.
-
Zhou HJ, Yan Y1, Zhou B, Lan TF, Wang XY, et al. (2015) Retroperitoneal fibrosis: a retrospective clinical data analysis of 30 patients in a 10-year period. Chin Med J (Engl) 128: 804-810.
-
Kim YS, Jung MH (2011) Retroperitoneal Castleman's disease presenting with hydronephrosis indistinguishable from lymphoma. J Obstet Gynaecol Res 37: 1145-1148.
-
McCammon J, Mascarenhas R, Monument MJ, Elyousfi A, Pilkey B (2014) Large B-cell lymphoma mimicking iliopsoas abscess following open revision of proximal femur infected non-union: a case report. BMC Res Notes 7: 470.
-
Katsura M, Nishina H, Shigemori Y, Nakanishi T (2015) Extranodal lymphoma originating in the gluteal muscle with adjacent bone involvement and mimicking a soft tissue sarcoma. Int J Surg Case Rep 7C: 161-164.
-
Boscolo-Berto R, Raduazzo DI, Vezzaro R, Marino D, Aversa SM, et al. (2011) Aggressive non-Hodgkin's lymphoma mimicking unilateral transitional cell carcinoma of renal pelvis. The risk of making a diagnostic mistake. Archivio italiano di urologia, andrologia : organo ufficiale [di] Societa italiana di ecografia urologica e nefrologica / Associazione ricerche in urologia 83: 163-165.
-
Trenhaile TR, Killackey MA (2001) Primary pelvic non-Hodgkin's lymphoma. Obstet Gynecol 97: 717-720.
-
Fox H, Langley FA, Govan AD, Hill AS, Bennett MH (1988) Malignant lymphoma presenting as an ovarian tumour: a clinicopathological analysis of 34 cases. Br J Obstet Gynaecol 95: 386-390.
-
Vang R, Medeiros LJ, Ha CS, Deavers M (2000) Non-Hodgkin's lymphomas involving the uterus: a clinicopathologic analysis of 26 cases. Mod Pathol 13: 19-28.
-
Vang R, Medeiros LJ, Silva EG, Gershenson DM, Deavers M (2000) Non-Hodgkin's lymphoma involving the vagina: a clinicopathologic analysis of 14 patients. Am J Surg Pathol 24: 719-725.
-
Vang R, Medeiros LJ, Malpica A, Levenback C, Deavers M (2000) Non-Hodgkin's lymphoma involving the vulva. Int J Gynecol Pathol 19: 236-242.
-
Abramson JS, Hellmann M, Barnes JA, Hammerman P, Toomey C, et al. (2010) Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer 116: 4283-4290.
-
Horning SJ, Weller E, Kim K, Earle JD, O'Connell MJ, et al. (2004) Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-Hodgkin's lymphoma: Eastern Cooperative Oncology Group study 1484. J Clin Oncol 22: 3032-3038.
-
Alvarez Argote J, Bauer FA, Posteraro AF 3rd, Dasanu CA (2014) Retroperitoneal fibrosis due to B-cell non-Hodgkin lymphoma: Responding to rituximab! JOncol Pharma Pract.
-
Tan D, Horning SJ, Hoppe RT, Levy R, Rosenberg SA, et al. (2013) Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: the Stanford University experience. Blood 122: 981-987.
-
Yuen AR, Kamel OW, Halpern J, Horning SJ (1995) Long-term survival after histologic transformation of low-grade follicular lymphoma. J Clin Oncol 13: 1726-1733.
-
Cabanillas F, Velasquez WS, Hagemeister FB, McLaughlin P, Redman JR (1992) Clinical, biologic, and histologic features of late relapses in diffuse large cell lymphoma. Blood 79: 1024-1028.





