Obstetrics and Gynaecology Cases - Reviews
Blasts on Blood Film after Treatment of Ovarian Cancer
Heong V1, Duong B2* and Wong S3
1Walter and Eliza Hall Institute, Australia
2The Royal Melbourne Hospital, Australia
3Western Hospital, Australia
*Corresponding author: Bich-Thu Duong, The Royal Melbourne Hospital, 300 Grattan Street, Parkville VIC 3050, Australia , Tel: +61 (3) 9342 7151, E-mail: bichdduong@gmail.com
Obstet Gynecol Cases Rev, OGCR-2-032, (Volume 2, Issue 2), Case Report; ISSN: 2377-9004
Received: September 29, 2014 | Accepted: April 11, 2015 | Published: April 13, 2015
Citation: Heong V, Duong B, Wong S (2015) Blasts on Blood Film after Treatment of Ovarian Cancer. Obstet Gynecol Cases Rev 2:032. 10.23937/2377-9004/1410032
Copyright: © 2015 Heong V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Secondary malignancies, Ovarian cancer, Alkylating agents
Case Presentation
A 40 year old female with a history of hypertension, depression and menorrhagia was admitted with a one week history of flu like symptoms, lethargy and neutropenia. Two years prior to admission, she was diagnosed with FIGO Stage IIC clear cell epithelial ovarian cancer and underwent a total abdominal hysterectomy and bilateral salphingo-oophorectomy (optimal debulking). She received adjuvant chemotherapy with carboplatin and paclitaxel (total dose 5190mg and 1833mg, respectively) and subsequent pelvic radiotherapy totalling 50.4Gy. She had a complete clinical, radiological and biochemical response.
On admission, her peripheral blood count was as follows: Hb 9.5, WCC 3.0, N 0.1 and Platelets 142. Circulating blasts accounted for 26% of the total white cell count.
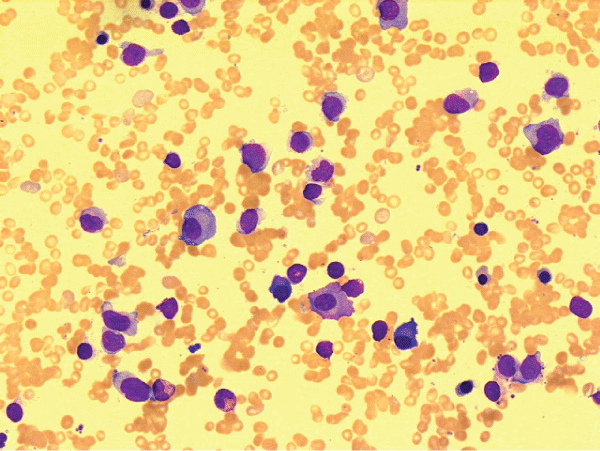
A bone marrow aspirate and trephine revealed hypocellularity with 48% blasts and incidentally 10–15% concomitant clonal (kappa) plasma cell infiltrate (Figure 1). Flow cytometry showed blasts expressing CD117 and MPO (weak) with clusters of plasma cells staining for CD138 with kappa light chain restriction. A diagnosis of therapy related Acute Myeloid Leukemia (AML) and Multiple Myeloma was made. The cytogenetic analysis demonstrated t(6;11) and involvement of the MLL gene which conferred a poor prognosis.
She went on to receive induction and consolidation chemotherapy with idarubicin and cytarabine. A repeat bone marrow biopsy confirmed complete morphological remission with a reduction in plasma cell burden and she subsequently underwent a successful sibling allogeneic stem cell transplant and has lived for more than 2 years from diagnosis of secondary leukemia.
Since 1999, the standard treatment for ovarian cancer has been cytoreductive surgery followed by platinum and paclitaxel combination chemotherapy [1,2] with a select subset of patients also going on to have radiation [3].
The incidence of secondary AML following treatment of epithelial ovarian cancer (EOC) is about 0.2%, with a median latency time between the diagnosis of EOC and development of leukemia being around 4-5 years [4]. More recently, Shimada et al conducted a retrospective review of 2482 patients with gynecologic malignancies at their institution between 1992 and 2012 [5]. 42.1% of patients were treated with chemotherapy and 14.7% (365) of patients had ovarian cancer. Four patients developed secondary leukemia during the 20-year period, following treatment of gynecologic malignancies (cumulative risk 0.38%). However, only one of these four patients had ovarian cancer. This patient was also diagnosed with breast cancer 2 years after the diagnosis of ovarian cancer and received 6 months of adjuvant cyclophosphamide for breast cancer, which may have increased her risk of developing secondary leukaemia. The incidence of secondary leukemia following a diagnosis of ovarian cancer, at this institution, was 0.27%.
Recently, it has been found that there is an 8-fold increased risk of developing secondary leukemia following chemotherapy for ovarian cancer, in comparison to the general population [6]. However, this rate of therapy-related leukemia post treatment for ovarian cancer appears to have declined since 1982, and this likely reflects the switch from mephalan to platinum-based regimens. Additionally the reduced use of combination chemotherapy and pelvic irradiation for ovarian cancer over the years, would also account for the decline in secondary leukemia rates. The combined use of these modalities was shown by Morton et al to have a higher incidence ratio for therapy related leukemia, compared to chemotherapy alone. However, this was not statistically significant due to small numbers [6].
Many of the reported cases of secondary leukemia have occurred in the recurrent setting after multiple exposures to cytotoxic chemotherapy, and largely due to the increasing cumulative doses of platinum compounds [7-9]. The relative risk rates of secondary leukemia after cumulative doses of less than 500mg, 500 to 750mg, 750 to 1000mg, and 1000mg or more are 1.9, 2.1, 4.1, and 7.6, respectively [10]. However, patients receiving adjuvant treatment are often unaware of this complication. This is a unique case where dual concomitant malignancies were diagnosed early, within two years, of adjuvant chemotherapy. Pelvic irradiation likely compounded the potential for side effects.
The most serious long term complication of any chemotherapy regimen is the development of a secondary malignancy with an estimated risk between 8 -12% by 20 years after initial cancer diagnosis and leukemia is the most common secondary malignancy [11]. This complication has been linked to the type of prior cytotoxic exposure such as anthracyclines, topoisomerase II inhibitors and paclitaxel [11,12]. The latency period between exposure to a cytotoxic agent and secondary AML is about 5-7 yrs with an alkylating agent and is usually preceded by myelodysplasia (MDS); it is 2-3 years with a topoisomerase II inhibitor [11]. Unfortunately, therapy related MDS or AML are usually resistant to standard induction chemotherapy and has a worse survival rate than their de novo counterparts [4,13]. Median survival is approximately 3 months from the time of secondary leukemia diagnosis compared to a median survival of 6 months in patients with primary leukemia [4].
In conclusion, our patient had several predisposing factors including ovarian cancer with exposure to an alkylating-like agent, paclitaxel and radiation. It is uncertain to what extent each of these elements contributed to her leukemia or Multiple Myeloma.
Despite the existing evidence, many patients are not routinely made aware of these complications when consenting to treatment of ovarian cancer. This is a very different scenario to breast cancer where patients are usually consented to long term treatment complications. Clinicians may feel that the poor prognosis of ovarian cancer may not necessitate a discussion regarding long term complications of chemotherapy. However, this case illustrates that secondary malignancies can occur within a short timeframe after treatment of ovarian cancer and as such, consent and monitoring for complications, particularly secondary MDS or AML is warranted.
Acknowledgements
Thank you to the Haematology Department at The Royal Melbourne Hospital for supplying the blood film.
Author contribution
Conception and design: Valerie Heong, Bich-Thu Duong, Shirley Wong
Collection of data and review of History: Valerie Heong, Bich-Thu Duong
Literature Review: Valerie Heong, Bich-Thu Duong
Manuscript Writing: Valerie Heong, Bich-Thu Duong
Final Approval of Manuscript: Valerie Heong, Bich-Thu Duong, Shirley Wong
References
-
McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, et al. (1996) Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med 334: 1-6.
-
Muggia FM, Braly PS, Brady MF, Sutton G, Niemann TH, et al. (2000) Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol 18: 106-115.
-
Hoskins PJ, Le N, Gilks B, Tinker A, Santos J, et al. (2012) Low-Stage Ovarian Clear Cell Carcinoma: Population-Based Outcomes in British Columbia, Canada, With Evidence for a Survival Benefit As a Result of Irradiation. J Clin Oncol 30: 1656-1662.
-
Vay A, Kumar S, Seward S, Semaan A, Schiffer CA, et al. (2011) Therapy-related myeloid leukemia after treatment for epithelial ovarian carcinoma: an epidemiological analysis. Gynecol Oncol 123: 456-460.
-
Shimada T, Saito T, Okadome M, Shimamoto K, Ariyoshi K, et al. (2014) Secondary leukemia after chemotherapy and/or radiotherapy for gynecologic neoplasia. Int J Gynecol Cancer 24: 178-183.
-
Morton LM, Dores GM, Tucker MA, Kim CJ, Onel K, et al. (2013) Evolving risk of therapy-related acute myeloid leukaemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood 121: 2996-3004.
-
Yeasmin S, Nakayama K, Ishibashi M, Oride A, Katagiri A, et al. (2008) Therapy-related myelodysplasia and acute myeloid leukemia following paclitaxel- and carboplatin-based chemotherapy in an ovarian cancer patient: a case report and literature review. Int J Gynecol Cancer 18: 1371-1376.
-
See HT, Thomas DA, Bueso-Ramos C, Kavanagh J (2006) Secondary leukemia after treatment with paclitaxel and carboplatin in a patient with recurrent ovarian cancer. Int J Gynecol Cancer 16: 236-240.
-
Ishikawa M, Nakayama K, Rahman MT, Rahman M, Katagiri H, et al. (2014) Therapy-related myelodysplastic syndrome and acute myeloid leukemia following chemotherapy (paclitaxel andcarboplatin) and radiation therapy in ovarian cancer: a case report. Eur J Gynaecol Oncol 35: 443-448.
-
Travis LB, Holowaty EJ, Bergfeldt K, Lynch CF, Kohler BA, et al. (1999) Risk of leukemia after platinum-based chemotherapy for ovarian cancer. N Engl J Med 340: 351-357.
-
Leone G, Mele L, Pulsoni A, Equitani F, Pagano L (1999) The incidence of secondary leukemias. Haematologica 84: 937-945.
-
Shah MA, Schwartz GK (2001) Cell cycle-mediated drug resistance: an emerging concept in cancer therapy. Clin Cancer Res 7: 2168-2181.
-
Thirman MJ, Larson RA (1996) Therapy-related myeloid leukemia. Hematol Oncol Clin North Am 10: 293-320.