Obstetrics and Gynaecology Cases - Reviews
Management of Early Pregnancy Failure in a Patient with Pancytopenia
Diana Cholakian and Jenell Coleman*
Johns Hopkins University School of Medicine, Department of Gynecology & Obstetrics, USA
*Corresponding author: Jenell Coleman, MD MPH, Johns Hopkins University School of Medicine, 600 N. Wolfe Street, Phipps Room 249, Baltimore, Maryland 21287, USA, Tel: 410-614-4496, Fax: 410-955-1003, E-mail: colemanj@jhmi.edu
Obstet Gynecol Cases Rev, OGCR-1-018, (Volume 1, Issue 3), Case Report; ISSN: 2377-9004
Received: October 28, 2014 | Accepted: December 04, 2014 | Published: December 08, 2014
Citation: Cholakian D, Coleman J (2014) Management of Early Pregnancy Failure in a Patient with Pancytopenia. Gynecol Cases Rev 1:018. 10.23937/2377-9004/1410018
Copyright: © 2014 Cholakian D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The safest and most effective therapy for an early pregnancy failure in women with pancytopenia is unknown. Here, we present a case report of a patient diagnosed with a 10-week embryonic demise who had a rare hematologic disorder called TnPolyagglutination syndrome that caused pancytopenia. The patient was offered expectant, medical, or surgical management, and she chose outpatient vaginal misoprostol. A week later, the patient presented to the emergency room with a septic abortion. She was given broad spectrum antibiotics and a suction dilation & curettage was performed. After 72 hours of antibiotic therapy, the patient was discharged home and made a full recovery to baseline. This case suggests that initial surgical management of early pregnancy failure in women with pancytopenia should be considered and strongly recommended over expectant or medical management. We review management options and surgical planning for patients with pancytopenia, regardless of the etiology.
Introduction
Tnpolyagglutination Syndrome (TnP) is a rare hematologic disorder characterized by pancytopenia with multi-lineage dysplasia [1]. In most cases of TnP, a defect in the synthesis of blood cell membrane glycoproteins (Gb3/CD77) allows exposure of the normally hidden Tn antigen to naturally occurring anti-Tn antibodies, thereby leading to polyagglutination [1-4]. The defect in the synthesis of this membrane is thought to be due a single point mutation in the Gb3/CD77 synthase and can also be found on the membranes of platelets, granulocytes, and lymphocytes [1,5]. Prevalence is unknown since it is estimated that many cases of Tnpolyagglutination are undiagnosed due to modern blood typing [2]. Previously, pooled human adult sera, which contains large amounts of naturally occurring anti-Tn, was used for ABO typing and TnP was suspected if there was diffuse polyagglutination of red blood cells, regardless of the ABO type [4]. Since monoclonal reagents are used for these tests today, cases of polyagglutination are not so obvious, and they usually are not noticed until human serum and red cells are mixed together in performance of an antibody screen or cross match. While TnP is not directly linked to malignancy, case reports of acute leukemia in patients with Tnpolyagglutination syndrome have been published [6]. Here we present the management of an embryonic demise in a woman with TnP.
Case Report
31-year-old woman para 2-0-1-2 ten weeks pregnant by last menstrual period presented emergently with vaginal bleeding. She denied pain, nausea/vomiting, and fever.
Medical history
The patient was born, full term, to a healthy young woman by vaginal delivery. At approximately three weeksof age she developed persistent petechiae. It is unknown which diagnostic tests were performed during her childhood, but during adulthood, a serum lectin panel confirmed the diagnosis of pancytopenia caused by Tnpolyagglutination syndrome (TnP). Previous hospitalizations included adenitis, otitis media, cellulitis, febrile neutropenia (absolute neutrophil count [ANC] < 1500 cells/microliter), and pyelonephritis. Bone marrow biopsies excluded malignancy. The patient had three pregnancies. The first pregnancy was terminated at 11 weeks after ultrasound findings of edema of the fetal head, neck, and body suggested fetal hydrops. She underwent chorionic villus sampling at 12 weeks, which did not reveal chromosomal abnormalities. Prophylactic washed-platelets, but no other blood products, were given for a platelet count of 41,000cells/microliter. Outpatient antibiotics were prescribed for severe neutropenia (ANC 486cells/microliter). She recovered uneventfully. The second pregnancy was complicated by re-admission for 48-hours of intravenous (IV) antibiotics for endometritis. No outpatient antibiotics were prescribed. She recovered uneventfully. The third pregnancy was uneventful. She denied prior sexually transmitted infections and was HIV negative. Menses were normal. She denied tobacco, excessive alcohol or illegal drugs.
Management of an embryonic demise
Pelvic sonogram demonstrated a crown rump length of 7-week/1-day without a heartbeat. Misoprostol (800micrograms/per vagina) was prescribed with 1-week follow-up. Three days later, she returned emergently with abdominal pain, nausea/vomiting, 38.4°C temperature, moderate vaginal blood, 1cm dilated cervical os, and a 13-week tender uterus. Sonogram demonstrated an intrauterine deformed gestational sac with blood flow. White blood cell count (WBC) 1.79K/microliter, ANC 1320cells/microliter, hemoglobin 8.7g/dL, platelets 67,000cells/microliter, normal coagulation profile, serum human chorionic gonadotropin 173.5mIU/mL, and Rh positive (Figure 1). A septic abortion was diagnosed, IV cefotetan was administered, and a suction dilation and curettage (D&C) was performed. Blood loss was 250 milliliters and unremarkable products of conception were obtained.
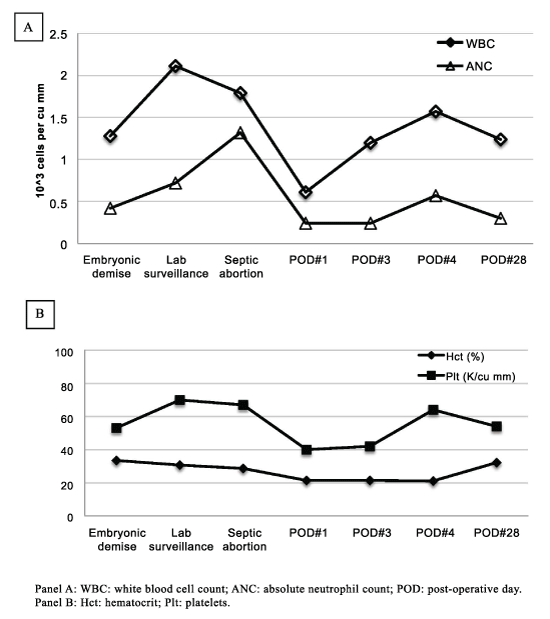
Figure 1: Figure 1: Trend in blood cell counts.
Panel A: WBC: white blood cell count; ANC: absolute neutrophil count; POD: post-operative day.
Panel B: Hct: hematocrit; Plt: platelets
View Figure 1
Twelve hours post-operatively, the patient spiked a temperature to 41°C and the antibiotic was changed to ertapenem. On post-operative day (POD)-1, pancytopenia worsened: WBC 0.61, ANC 240, hemoglobin 6.5, and platelets 40,000. Blood and urine cultures were negative as were gonorrhea/chlamydia tests. Hematology was consulted and a secondary consumptive process was ruled-out. Bone marrow biopsy was not performed because the severe neutropenia was attributed to acute infection. The patient declined packed red blood cell (PRBC) and platelet transfusions given minimal vaginal bleeding and lack of symptoms.
On POD-4, she was afebrile without complaints or examination findings. Laboratory values improved (Figure 1). Ten days of amoxicillin/clavulanate, iron supplements, and NuvaRing were prescribed. Four weeks after hospitalization, she was doing well and laboratory values were back tobaseline.
Discussion
To our knowledge, this is the first report of the management of early pregnancy failure in a patient with pancytopenia caused by TnP. Management of early pregnancy failure in patients with pancytopenia requires careful evaluation, as there may be increased risk of infection and bleeding.
Risk of infection increases as the ANC decreases. Some experts recommend hospitalization and parenteral antibiotics if the ANC drops below 500 [7]. Despite the seven hospitalizations for infections, this patient never required intensive care, and was aggressively managed with broad-spectrum antibiotics. At the time of the embryonic demise, ANC was greater than 1300, and there was moderate anemia and thrombocytopenia. Although expectant and medical management with misoprostol are not contra indicated, in retrospect, uterine aspiration might have been the best option. Initial surgical management might have avoided the additional ER visit, hospitalization, and IV antibiotics.
Second, none of her pregnancies resulted in hemorrhage or required transfusion of PRBCs despite anemia and thrombocytopenia. Post-operatively, the patient’s laboratory values dropped below transfusion thresholds for PRBCs (< 7) and platelets (< 50,000); however, she declined transfusion. If the patient had agreed, blood products need not be washed prior to transfusing because donor anti-Tn would be diluted with anti-coagulant and unlikely to cause hemolysis in vivo [8-10].
In sum, there is comparable risk of bleeding, infection, and curettage between medical and surgical management in healthy women, however these data are not available for patients with pancytopenia [11]. Other counseling issues include amount of pain or bleeding to expect, number of unscheduled clinic visits, time to completion of abortion, risk of hospitalization, and surgical complications. This case demonstrates that uterine aspiration should be strongly considered as first line; additionally, cross-matched blood products should be available, and prophylactic post-operative broad-spectrum antibiotics may be useful in women with severe neutropenia (ANC< 500). Given that surgical management allows for monitoring of a high risk patient in a controlled, hospital setting, consideration should be made to offer it as first line in patients with pancytopenia. Overall, the decision making process is complex in women with TnP given the lack of available data to guide management. This case demonstrates that obstetric or gynecologic complications are managed fairly well in patients with TnP, if promptly evaluated and empiric parenteral therapy is implemented.
Acknowledgement
The authors do not have any financial disclosures.
Written permission from the patient to use her information in the article was obtained.
References
-
Beck ML (2000) Red blood cell polyagglutination: clinical aspects. Semin Hematol 37: 186-196.
-
Hashmi G, Shariff T, Seul M, Vissavajjhala P, Hue-Roye K, et al. (2005) A flexible array format for large-scale, rapid blood group DNA typing. Transfusion 45: 680-688.
-
Nurden AT, Dupuis D, Pidard D, Kieffer N, Kunicki TJ, et al. (1982) Surface modifications in the platelets of a patient with alpha-N-acetyl-D-galactosamine residues, the Tn-syndrome. J Clin Invest 70: 1281-1291.
-
Bird GW, Shinton NK, Wingham J (1971) Persistent mixed-field polyagglutination. Br J Haematol 21: 443-453.
-
Suchanowska A, Kaczmarek R, Duk M, Lukasiewicz J, Smolarek D, et al. (2012) A single point mutation in the gene encoding Gb3/CD77 synthase causes a rare inherited polyagglutination syndrome. J Biol Chem 287: 38220-38230.
-
Ness PM, Garratty G, Morel PA, Perkins HA (1979) Tn polyagglutination preceding acute leukemia. Blood 54: 30-34.
-
Zuckermann J, Moreira LB, Stoll P, Moreira LM, Kuchenbecker RS, et al. (2008) Compliance with a critical pathway for the management of febrile neutropenia and impact on clinical outcomes. Ann Hematol 87: 139-145.
-
Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, et al. (2012) Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med 157: 49-58.
-
Slichter SJ (2007) Evidence-based platelet transfusion guidelines. Hematology Am Soc Hematol Educ Program .
-
Eversole M, Nonemaker B, Zurek K, South S, Simon T (1986) Uneventful administration of plasma products in a recipient with T-activated red cells. Transfusion 26: 182-185.
-
Sotiriadis A, Makrydimas G, Papatheodorou S, Ioannidis JP (2005) Expectant, medical, or surgical management of first-trimester miscarriage: a meta-analysis. Obstet Gynecol 105: 1104-1113.





