Obstetrics and Gynaecology Cases - Reviews
Neoadjuvant Chemotherapy for Vulvar Cancer: Case Series from a Single Institution
Ismail Mert1*, Jacqueline Guterman1, Ira S. Winer1, Rouba Ali-Fehmi2 and Gunter Deppe1
1Department of Obstetrics and Gynecology, Wayne State University, Detroit, USA
2Department of Pathology, Wayne State University, Detroit, USA
*Corresponding author: Ismail Mert, M.D, Department of Obstetrics and Gynecology, Wayne State University, Detroit, USA, Tel: +313-600-6282, E-mail: imert@med.wayne.edu
Obstet Gynecol Cases Rev, OGCR-1-011, (Volume 1 Issue 2), Case Report; ISSN: 2377-9004
Received: October 21, 2014 | Accepted: November 24, 2014 | Published: November 27, 2014
Citation: Mert I, Guterman J, Winer IS, Ali-Fehmi R, Deppe G (2014) Neoadjuvant Chemotherapy for Vulvar Cancer: Case Series from a Single Institution. Gynecol Cases Rev 1:011. 10.23937/2377-9004/1410011
Copyright: © 2014 Mert I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Vulvar carcinoma accounts for approximately 5% of gynecologic cancers in the United States with an estimated 4,490 new cases and 950 deaths in 2012 [1]. Survival depends on the extent of cancer spread upon initial diagnosis [2]. The staging of vulvar cancer depends on tumor size and spread to lymph nodes and other organs. Stage I and II comprise the early stages of vulvar carcinoma, where there is localized disease without lymph node involvement. Whereas late stage disease, stage III and IV, involves tumor that has invaded into adjacent structures such as the anus, urethra or vagina as well as metastasized to lymph nodes or other organs.
The majority of vulvar carcinoma are squamous cell in origin and are diagnosed at an early stage [3]. Approximately 32% of cases are still identified in later stages [4]. Treatment for early stage cancer has been well standardized to include wide radical excision of the primary tumor with inguinal-femoral lymphadenectomy or sentinel lymph node biopsy [5]. More advanced lesions involving the anus or rectum may require permanent colostomy, or if the urethra or bladder is involved, urinary diversion might be considered. Unfortunately, radical surgical treatment for advanced disease is not always possible and adjuvant therapy may be required.
The therapeutic options for patients with inoperable vulvar cancers include chemoradiation [6-13] and neoadjuvant chemotherapy [14-23]. The goal of these therapies being the reduction of tumor volume to allow effective surgical and/or radiation treatment. Multiple chemotherapy agents have been used in the treatment of advanced disease, without identification of an optimal chemotherapeutic regimen to induce disease regression [24]. In recent years, chemotherapy with platinum compounds, such as carboplatin, and paclitaxel has been shown to have some activity against vulvar cancer [21]. Both carboplatin and paclitaxel are used in conjunction for the treatment of ovarian and endometrial cancers, but have not been explored in the treatment of vulvar carcinoma. In the present report, we describe our experience with neoadjuvant carboplatin/paclitaxel chemotherapy in three patients with locally advanced squamous cell vulvar cancer.
Case Presentation
Patient #1
57 year old Caucasian female with a history of bladder cancer presented with perineal pain. Physical exam demonstrated a right vulvar mass, approximately 5 cm x 4 cm in size, with enlarged right-sided inguinal lymph nodes. Biopsy of the mass indicated moderately differentiated squamous cell vulvar carcinoma. Positron emission tomography (PET) CT scan with 18-fluoro-2-deoxyglucose (FDG) demonstrated an avid vulvar mass with a 3 cm right groin and 2-3 cm right pelvic lymph node, suspicious for metastases.
Patient #2
Fifty seven year old African American female with history of Stage IIB cervical carcinoma which was diagnosed and treated eight years ago presented with complaints of vaginal itching and bleeding. Physical exam demonstrated a large, foul-smelling erythematous lesion on the right vulva, which was diagnosed as a well-differentiated invasive squamous cell carcinoma on biopsy. CT scan indicated a 1.6 cm thickening of the right vulva with possible involvement of the adjacent urethra (Figure 1). Slightly enlarged right external iliac and bilateral inguinal lymph nodes were also noted.
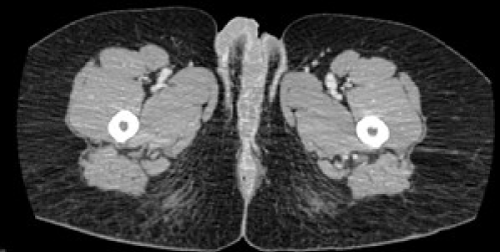
Figure 1: CAT scan of the abdomen and pelvis of patient #2, thickening of
right vulva.
View Figure 1
Patient #3
Forty one year old Caucasian female with a history of HPV and vulvar carcinoma in situ treated with multiple laser ablations and excisional biopsies presented with the re-occurrence of painful vulvar lesions. Physical exam demonstrated lesions at the 2, 6 and 8 o’clock positions on the right vulva as well as small lesions spread to clitoral hood. Excision of all three lesions indicated a poorly differentiated invasive squamous cell carcinoma, arising in condyloma acuminatum with positive margins.
Management and Outcome
All three patients presented above where diagnosed with locally advanced, inoperable squamous cell carcinoma of the vulva. Different treatment options were discussed with each patient and all three women decided to undergo neoadjuvant chemotherapy with paclitaxel 175mg/m2 and carboplatin (area under the curve, AUC, 5) every 3 weeks. Disease status was evaluated clinically and radiologically at baseline and following completion of 3 and 6 cycles of chemotherapy.
After three cycles of neoadjuvant chemotherapy, all of them presented with partial response with unchanged lymphadenopathy. All, except Patient 2 underwent additional cycles of chemotherapy, which yielded the following outcomes.
Three cycles of chemotherapy resulted in a 50% mass reduction as well as decreased lymph nodes size in Patient 1. After an additional three cycles, complete remission of the tumor with no evidence of malignancy on repeat PET scan was observed (Figure 2). Three months later, an approximate 1 cm small, firm, mobile right vulvar nodule was palpated by the patient and confirmed to be recurrence of disease on biopsy. The patient underwent three cycles of chemotherapy with paclitaxel and carboplatin followed by radical wide local excision. Pathology of the surgical specimen demonstrated invasive squamous cell carcinoma with free margins. Three years after completion of her therapy, the patient noted right sided groin swelling which was confirmed to be recurrence of her vulvar cancer and is currently being treated with carboplatin and paclitaxel chemotherapy regimen.
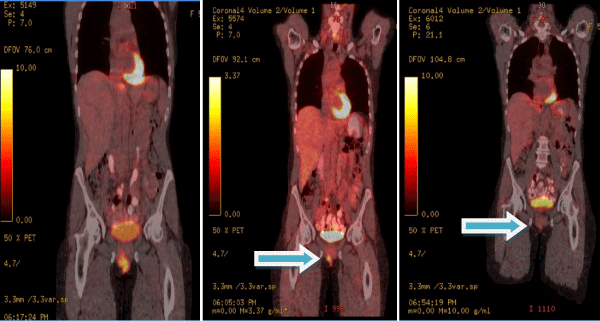
Figure 2: Regression of the vulvar lesion of patient # 1 after three cycles
and complete disappearance of the lesion after 6 cycles of chemotherapy.
View Figure 2
Patient 2 received four cycles of neoadjuvant chemotherapy. Clinical exam demonstrated more than 50% reduction in tumor size after three cycles of chemotherapy. Due to significant reduction of the size of her tumor after the fourth cycle, the patient decided to discontinue treatment and ultimately was lost to follow-up.
The tumor size in Patient 3 was negligible compared to the other four women, as all three of her exophytic lesions were excised. However, based on the pathology of the lesions and evidence of malignancy on CT scan, the patient received chemotherapy. After the completion of six chemotherapy cycles complete remission was achieved based upon clinical exam and negative PET scan and currently she is disease free after ten months of follow up.
Chemotherapy related side effects were minimal, the most common being myelotoxicity and nausea. No neutropenic fevers, grade 3 or 4 anemia, thrombocytopenia or peripheral neuropathy were reported or observed. Patient 1 did require granulocyte stimulating factor treatment after her fifth cycle of chemotherapy.
Discussion
Optimal management of locally advanced, inoperable vulvar cancer is a controversial and often debated topic. This has led to the establishment of different treatment modalities, some of which include primary chemoradiation [7,10] and neoadjuvant chemotherapy [24]. The intent of these therapies being reduction of disease to allow less radical surgery and decreased toxicity.
In 1990 Shimizu et al. [19] were the first to report that a neoadjuvant regimen of bleomycin, vincristine, mitomycin C, and cisplatin resulted in complete remission in a 57 year old woman with stage IV squamous cell vulvar cancer after three cycles of chemotherapy. Since then, other studies have demonstrated varying success rates in decreasing tumor burden with different chemotherapy regimes.
A phase II clinical trial by the European Organization for Research and Treatment of Cancer (EORTC) assessed disease response to treatment with a combination of bleomycin, lomustine and methotrexate in patients with primary (18 patients) or recurrent (10 patients) vulvar carcinoma not amenable to standard radical vulvectomy [14]. Of the 28 patients enrolled in the study, 67% showed an objective response with a decrease in tumor size. A follow up trial with modification of the chemotherapy regimen resulted in a decrease in tumor size in 56% of patients [23]. Among the 25 patients treated with neoadjuvant chemotherapy, 12 partial and 2 complete responses were observed.
After the initial promising results, Benedetti-Panici [16] evaluated similar combination chemotherapy with bleomycin, methotrexate and cisplatin substituted for lomustine The study demonstrated a 10% partial response with a 33% pathological down staging rate and a 3 year survival rate of 24%. However, 79% of cases still required radical surgery, suggesting that neoadjuvant chemotherapy did not offer any substantial benefit to the surgery alone.
On the other hand, Geisler et al. [15] treated 14 patients with advanced vulvar cancer involving the anal sphincter and/or urethra with neoadjuvant chemotherapy consisting of either cisplatin/5 FU or cisplatin alone. Of the patients treated with cisplatin/5 FU, all showed at least a partial response to therapy that allowed for conservation of the anal sphincter and/or urethra. In contrast, the patients treated with cisplatin alone had no measurable disease response.
Domingues et al. compared three chemotherapy regimens for neoadjuvant therapy [21]. 25 patients with locally advanced vulvar tumors were treated with one of three different chemotherpy regimens every three weeks; 10 patients with bleomycin, 5 with paclitaxel (100mg/m2 weekly) and 10 patients with 5-fluorouracil/cisplatin 60-80 mg/m2. Respective tumor size response rates to treatment were 60%, 40% and 20%.
Further evaluation of the role of cisplatin based neoadjuvant therapy in decreasing tumor burden to allow for less radical surgical resection was performed in a study done by Aragona et al. [22]. Their group described four different regimens. 5-fluorouracil/cisplatin, cisplatin/paclitaxel, cisplatin/paclitaxel/5-fluorouracil and cisplatin/vincristine/bleomycin. 33 patients completed the proposed treatment schemes and 30 patients were assessed for radical surgery. Partial response was seen in 90.9% of the patients (30 out of 33) and 27 patients had undergone radical surgery. After a median follow-up of 49 months, 24 of the 27 patients were without disease and they concluded that neoadjuvant chemotherapy increases the surgical feasibility in select cases and encourages adjacent organ preservation. Weekly administration of paclitaxel (60mg/ m2) and carboplatin (AUC of 2.7) showed limited clinical benefit in 6 patients with vulvar squamous cell cancer [17]. Raspagliesi et al. treated 10 patients with stage III and IV locally advanced squamous cell carcinoma of the vulva with 3 courses of paclitaxel-ifosfamide-cisplatin or paclitaxel-cisplatin and showed 80% clinical response rate (1 case with complete remission, 2 with persistent carcinoma in situ and 6 invasive cancer cases with tumor shrinkage of more than 50%). Nine of these patients subsequently underwent radical local excision or radical partial vulvectomy and bilateral inguino-femoral lymphadenectomy [25].
Other studies (Table 1) including the current case series have shown that cisplatin or carboplatin in combination with topotecan [20] or paclitaxel/taxotere [26] demonstrated activity without significant toxicity in the neoadjuvant setting. A phase II trial of targeted therapy with erlotinib (150mg by mouth daily for 28-42 days before definite treatment with surgery or chemoradiation) demonstrated a 27.5% response rate in 41 patients with a 13.2 weeks progression free survival [18].
![]()
Table 1: Platinum continuing neoadjuvant chemotherapy for advanced vulvar
cancer.
View Table 1
Our results showed objective response to neoadjuvant chemotherapy consisting of paclitaxel and carboplatin in patients with locally advanced vulvar cancer. However, there are significant limitations. First, there were only three patients presented here. Second, the role of other chemotherapy agents should also be evaluated. For firm conclusions to be made, randomized clinical trials are needed.
In conclusion; despite limited number of the patients included in this and previous studies, these encouraging results should provide the foundation for prospective international trials, perhaps comparing chemoradiation with neoadjuvant chemotherapy (platinum/paclitaxel) plus targeted therapy (erlotinib) in patients with locally advanced squamous cell carcinoma of the vulva not amenable to surgery or chemoradiation.
References
-
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62: 10-29.
-
Beller U, Quinn MA, Benedet JL, Creasman WT, Ngan HY, et al. (2006) Carcinoma of the vulva. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet 95.
-
Tabbaa ZM, Gonzalez J, Sznurkowski JJ, Weaver AL, Mariani A, et al. (2012) Impact of the new FIGO 2009 staging classification for vulvar cancer on prognosis and stage distribution. Gynecol Oncol 127: 147-152.
-
Ramanah R, Lesieur B, Ballester M, Darai E, Rouzier R (2012) Trends in of late-stage squamous cell vulvar carcinomas: analysis of the surveillance, epidemiology, and end results (SEER) database. Int J Gynecol Cancer 22: 854-859.
-
Levenback CF, Ali S, Coleman RL, Gold MA, Fowler JM, et al. (2012) Lymphatic mapping and sentinel lymph node biopsy in women with squamous cell carcinoma of the vulva: a gynecologic oncology group study. J Clin Oncol 30: 3786-3791.
-
Woelber L, Kock L, Gieseking F, Petersen C, Trillsch F, et al. (2011) Clinical management of primary vulvar cancer. Eur J Cancer 47: 2315-2321.
-
Tomao F, Di Tucci C, Marchetti C, Perniola G, Bellati F, et al. (2012) Role of chemotherapy in the management of vulvar carcinoma. Crit Rev Oncol Hematol 82: 25-39.
-
Mak RH, Halasz LM, Tanaka CK, Ancukiewicz M, Schultz DJ, et al. (2011) Outcomes after radiation therapy with concurrent weekly platinum-based chemotherapy or every-3-4-week 5-fluorouracil-containing regimens for squamous cell carcinoma of the vulva. Gynecol Oncol 120: 101-107.
-
Tans L, Ansink AC, van Rooij PH, Kleijnen C, Mens JW (2011) The role of chemo-radiotherapy in the management of locally advanced carcinoma of the vulva: single institutional experience and review of literature. Am J Clin Oncol 34: 22-26.
-
Moore DH (2009) Chemotherapy and radiation therapy in the treatment of squamous cell carcinoma of the vulva: Are two therapies better than one? Gynecol Oncol 113: 379-383.
-
Kumar PP, Good RR, Scott JC (1988) Techniques for management of vulvar cancer by irradiation alone. Radiat Med 6: 185-191.
-
Slevin NJ, Pointon RC (1989) Radical radiotherapy for carcinoma of the vulva. Br J Radiol 62: 145-147.
-
Moore DH, Ali S, Koh WJ, Michael H, Barnes MN, et al. (2012) A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: a gynecologic oncology group study. Gynecol Oncol 124: 529-533.
-
Durrant KR, Mangioni C, Lacave AJ, George M, van der Burg ME, et al. (1990) Bleomycin, methotrexate, and CCNU in advanced inoperable squamous cell carcinoma of the vulva: a phase II study of the EORTC Gynaecological Cancer Cooperative Group (GCCG). Gynecol Oncol 37: 359-362.
-
Geisler JP, Manahan KJ, Buller RE (2006) Neoadjuvant chemotherapy in vulvar cancer: avoiding primary exenteration. Gynecol Oncol 100: 53-57.
-
Benedetti-Panici P, Greggi S, Scambia G, Salerno G, Mancuso S (1993) Cisplatin (P), bleomycin (B), and methotrexate (M) preoperative chemotherapy in locally advanced vulvar carcinoma. Gynecol Oncol 50: 49-53.
-
Han SN, Vergote I, Amant F (2012) Weekly paclitaxel/carboplatin in the treatment of locally advanced, recurrent, or metastatic vulvar cancer. Int J Gynecol Cancer 22: 865-868.
-
Horowitz NS, Olawaiye AB, Borger DR, Growdon WB, Krasner CN, et al. (2012) Phase II trial of erlotinib in women with squamous cell carcinoma of the vulva. Gynecol Oncol 127: 141-146.
-
Shimizu Y, Hasumi K, Masubuchi K (1990) Effective chemotherapy consisting of bleomycin, vincristine, mitomycin C, and cisplatin (BOMP) for a patient with inoperable vulvar cancer. Gynecol Oncol 36: 423-427.
-
Achilli C, Palaia I, Perniola G, Donato VD, Marchetti C, et al. (2012) Complete remission after neoadjuvant chemotherapy of an advanced vulvar cancer patient: a case report. J Obstet Gynaecol Res 38: 1036-1039.
-
Domingues AP, Mota F, Durao M, Frutuoso C, Amaral N, et al. (2010) Neoadjuvant chemotherapy in advanced vulvar cancer. Int J Gynecol Cancer 20: 294-298.
-
Aragona AM, Cuneo N, Soderini AH, Alcoba E, Greco A, et al. (2012) Tailoring the treatment of locally advanced squamous cell carcinoma of the vulva: neoadjuvant chemotherapy followed by radical surgery: results from a multicenter study. Int J Gynecol Cancer 22: 1258-1263.
-
Wagenaar HC, Colombo N, Vergote I, Hoctin-Boes G, Zanetta G, et al. (2001) Bleomycin, methotrexate, and CCNU in locally advanced or recurrent, inoperable, squamous-cell carcinoma of the vulva: an EORTC Gynaecological Cancer Cooperative Group Study. European Organization for Research and Treatment of Cancer. Gynecol Oncol 81: 348-354.
-
Deppe G, Mert I, Winer IS (2014) Management of squamous cell vulvar cancer: a review. J Obstet Gynaecol Res 40: 1217-1225.
-
Raspagliesi F, Zanaboni F1, Martinelli F1, Scasso S2, Laufer J2, et al. (2014) Role of paclitaxel and cisplatin as the neoadjuvant treatment for locally advanced squamous cell carcinoma of the vulva. J Gynecol Oncol 25: 22-29.
-
Belotte J, Awonuga AO, Bolinjkar R, Alexis M, Tabassum F, et al. (2012) Platinum-based combination chemotherapy for the treatment of advanced-stage squamous cell carcinoma of the vulva. Obstet Gynecol 120: 458-460.





