Journal of Sleep Disorders and Management
Sleep Characteristics in Blind Subjects
Fructuoso Ayala-Guerrero* and Graciela Mexicano
Laboratorio de Neurociencias, Universidad Nacional Autónoma de México, México
*Corresponding author:
Fructuoso Ayala-Guerrero, Facultad de Psicología, Departamento de Posgrado, Universidad Nacional Autónoma de México, Ciudad Universitaria, 04510, México, Tel: 52-5622-2222, E-mail: fayala@unam.mx
J Sleep Disord Manag,
JSDM-1-003, (Volume 1, Issue 1),
Research Article
Received: July 28, 2015: Accepted: September 12, 2015: Published: September 14, 2015
Citation: Ayala-Guerrero F, Mexicano G (2015) Sleep Characteristics in Blind Subjects. J Sleep Disord Manag 1:003
Copyright: © 2015 Ayala-Guerrero F. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are
credited.
Abstract
The sleep patterns of ten blind adults and their matched controls were studied during three consecutive nights. The first night was allowed for adaptation. Significant electroencephalographic and quantitative findings were obtained from nights 2 and 3. Although alpha-like rhythm was registered in only one blind subject during wakefulness, it was displayed by 8 of the 10 blind participants of this study during REM sleep. This rhythm was also present during the N2 sleep stage. The delta phase of sleep was markedly reduced as compared to the healthy subjects. Mean duration of REM sleep episodes was significantly longer in blind subjects; in contrast, their frequency was lower than in the corresponding control subjects. Total sleep time and sleep efficiency was noticeably shorter in blind subjects.
Atypical brain activity and quantitative sleep abnormalities found in the blind subjects may be explained on the basis of degeneration of cerebral organization and circadian rhythm alterations induced by absence of visual stimulation.
Keywords
Sleep in blind adults, Delta sleep, Alpha-like rhythm, REM sleep
Introduction
Two different sleep states, Non- REM sleep (NREM) and rapid eye movement (REM) sleep, have been described in humans based on electrophysiological parameters [1,2]. NREM sleep is conventionally subdivided into 3 stages [3], which are characterized by different EEG patterns. The third stage is commonly described as delta or N3 sleep [4]. In addition, muscular activity is hypotonic and ocular movements are scarce or absent. REM sleep, in contrast, is defined by EEG activation, presence of sawtooth waves (trains of frontocentral waves at 2.5 hz and 20 to 100 μv of amplitude), muscle atonia, and episodic bursts of rapid eye movements [4].
The normal human adult enters sleep through NREM sleep. REM sleep does not occur until 80 min or longer thereafter and both sleep states alternate cyclically through the night with a period of about 90 min [1,5].
REM sleep tends to be more abundant in the last third of the night. The preferential distribution of REM sleep towards the latter portion of the night in normal adults is thought to be linked to a circadian oscillator [6].
Sleep length depends on genetic determinants and on a large number of factors where volitional control and processes associated with circadian rhythms are among the most significant in humans [7-11].
According to previous information, blind people with no perception of light, exhibit continual circadian desynchrony resulting in sleep disturbances and daytime dysfunction [12-14]. The most common sleep-related problem among the blind subjects is related to longer sleep latency, fragmented sleep, short sleep duration, and daytime naps.
On the other hand, the magnitude of EEG waves recorded from the scalp is a function of both, the size of the potentials generated by each neuron and the number of neurons discharging synchronously [15-17]. There is direct evidence that visual deprivation can alter the neuronal organization of the cerebral cortex leading to its irreversible deterioration [18].
Accordingly, the decrease in the network size due to visual pathway degeneration could lead to a perturbation in electroencephalographic and sleeping processes. Additionally, since the biological rhythms are regulated by visual inputs; the absence of these inputs may affect the circadian and ultradian organization of sleeping processes inducing an alteration in the sleep architecture.
Methods
This study was carried out according to human criteria and institutional approval. Likewise, verbal consent from the subjects was obtained.
Subjects
Ten blind adults, 7 men and 3 women, chosen from a sample of 110 subjects entered the nocturnal sleep study. Their age average was 30.50 ± 12.50 years; all of them reported no light perception at all, which was corroborated through photic stimulation delivered by an Alvar "Soneclat" photostimulator. Selection of the subjects, who had similar education background, was based on their responses to a questionnaire in which we inquired on their ophthalmic and systemic health, including sleep quality and lifestyle (e.g., those with alcohol and tobacco consumption were excluded). In order to be included in the present study, the subjects were required to remain awake during the day and not to take diurnal naps, which could result in circadian desynchrony of sleep distribution.
Control subjects
Ten Control healthy subjects, matched in sex and age with blind individuals, were recruited from the community. They did not present any sleep problems or medical disorders requiring medical treatment that could influence sleep or alertness.
Procedure
The selected subjects, who had been free of stimulant, sedative-hypnotic, or other psychotropic medications, were polygraphically monitored during the night. Silver disk electrodes were attached with collodion to obtain monopolar EEG from C3/A2 and C4/A1 derivations according to the 10-20 International System [19]. Electrodes for monitoring the electro-oculogram (EOG) were fixed at the external canthus of each eye. Electromyogram (EMG) was obtained from electrodes placed on the chin. The electrocardiogram (EKG) was recorded from electrodes placed on the wrist of the left hand and on the ipsilateral leg. Respiratory rate was obtained by means of a thermistor placed in the nostrils.
Collection and analysis of data
Subjects laid on a bed placed inside a shielded, sound-attenuated chamber. Recordings were obtained by a 10-channel Alvar Reega X electroencephalograph at a paper speed of 15 mm/s. Lights were turned off at 23:00 hours and subjects were urged to stay in bed until 06:00 hours the following morning. This interval was established according to the customary sleep schedule of the studied individuals. Subjects were monitored for 3 successive nights. Night 1 was allowed for adaptation and data presented here were obtained during nights 2 and 3.
Polygraphic recordings were visually analyzed and scored in 20 s epochs according to the system by the American Academy of Sleep Medicine and Rechtschaffen and Kales [3,20]. REM sleep latency in minutes was defined as the time elapsed from sleep onset to the 0first epoch scored as REM sleep. The total time spent in each sleep stage during the recording period was assessed and the percentage within the period calculated. Mean duration of each sleep state as well as of each sleep cycle were obtained. Scoring reliability was 89%, when comparing the independent judgment of two scorers. Data from night 2 and 3 were compared using paired t-tests.
Heart and respiratory rate values obtained throughout the vigilance states were compared by a 2 way ANOVA and subsequent contrast using Tukey's test.
Results
Control subjects displayed typical polysomnographic patterns across the sleep-wake cycle. Blind subjects, in general, presented both NREM sleep and REM sleep as described for normal individuals. However, important qualitative and quantitative differences were detected throughout the nocturnal recordings.
NREM sleep
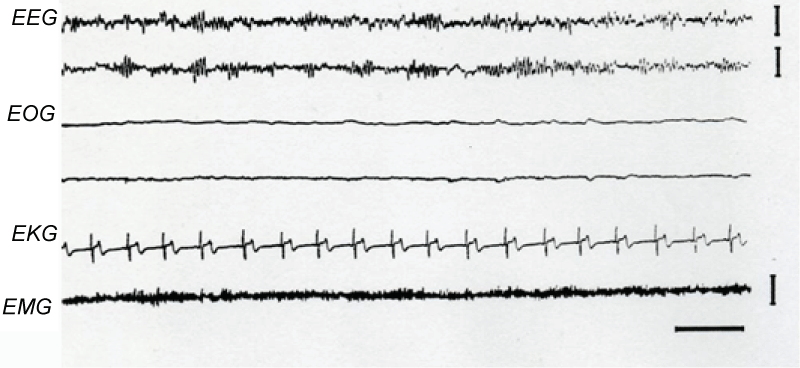
At the beginning of the recordings, subjects were awake. During this period, brain activity consisted of a low voltage mixed frequency wave pattern. Alpha-like rhythm was absent in all, excepting one of the ten blind recorded subjects. In the exceptional case, alpha-like waves displayed a spindle-like distribution in a waxing-waning amplitude sequence (Figure 1).
.
Figure 1: Alpha Rhythm during Wakefulness.
Note the spindle-like distribution of the alpha rhythm on the C3/A2 and C4/A1 (EEG). This activity was displayed by only one of the ten blind recorded subjects. EOG, right and left electro-oculogram; EKG, electrocardiogram; EMG, Electromyogram. Calibration: 2 sec, 50 μv.
View Figure 1
After a variable period of relaxed wakefulness, blind subjects entered stage N1 of sleep, a transitional phase between full wakefulness and clear sleep. During this stage, the EEG patterns were similar to those in normal individuals.
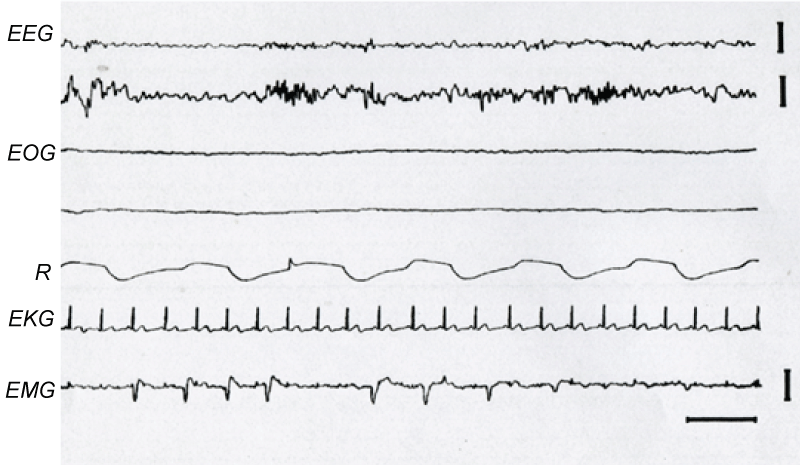
In addition to the typical EEG patterns of stage N2 of sleep, there were bursts of alpha-like rhythm in two of the studied blind subjects (Figure 2). These alpha-like bursts frequently preceded or followed sleep spindles or K complexes. Moreover, faintly perceptible low amplitude sleep spindles appeared near the transition between stages N1 and N2, early during sleep. These electrographic signs became greater as stage N2 progressed.
.
Figures 2: Presence of Alpha-Like Rhythm during N2 sleep stage.
A burst of alpha-like rhythm followed by a sleep spindle is observed in the middle of the second EEG tracing. Calibration: 2 sec, 50 μv.
View Figures 2
As stage N2 of sleep developed, sporadic high voltage slow waves appeared gradually. However, this activity hardly met the criteria for N3 sleep stage. When present, this stage had a very brief duration reaching 2.5 and 10.50 minutes in the second and third night respectively in all the studied blind subjects (Table 1).
![]()
Table 1: Quantitative data.
TST: Total Sleep Time; SE: Sleep Efficiency; B: Blind subjects; C: Control subjects.
View Table 1
REM sleep
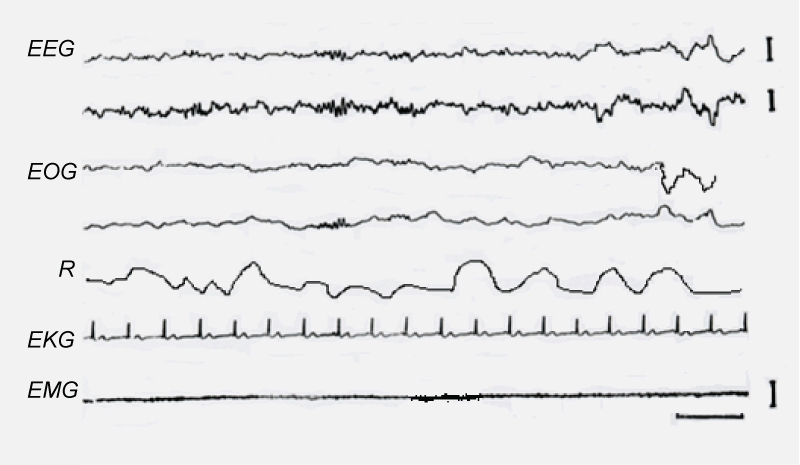
The EEG pattern of this state of sleep was similar to that described in normal subjects. Sawtooth waves preceded or concurred with eye movements in subjects that had eye balls, showing a mean of 2.3 sawtooth waves bursts per REM sleep stage. In addition, bursts of alpha-like wave activity were displayed during REM sleep by 8 of the 10 recorded blind subjects (Figure 3).
.
Figure 3: Presence of alpha-like rhythm during REM sleep.
Rhythmic alpha activity is observed on the second EEG tracing and Respiratory (R).
View Figure 3
EMG
Low amplitude EMG activity was present throughout all NREM sleep stages. No discrete change in EMG amplitude in the waking to sleep transition was usually observed, although a gradual decrease in EMG signal amplitude sometimes occurred. The EMG during N2 sleep stage was tonically active, generally at lower amplitude than that observed in wakefulness. During delta sleep, this signal was tonically active, although its tracing occasionally fell to very low levels similar to those of REM sleep, where muscular tone was extremely low or absent. However, frequent twitches were present during this period.
Heart rate
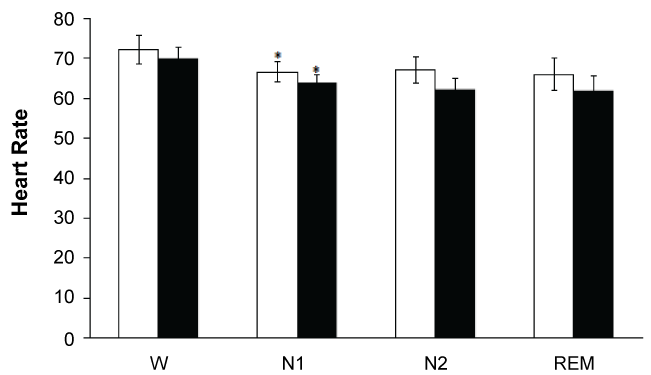
Heart rate decreased significantly (p < 0.05) when passing from wakefulness (72.75 beats/min) to stage N1 sleep (66.67 beats/min). This value did not show additional significant variations throughout the sleep cycle as judged by the mean frequency observed during stage N2 sleep (67.02 beats/min) and REM sleep (66.02 beats/min). Similar heart rate tendency was observed in control subjects. (W, 70.02; N1, 63.89; N2, 62.26; REM sleep 62.00) (Figure 4). Data differences between blinds and control subjects were not statistically significant.
.
Figures 4: Both in blinds (white bars) and control (black bars) subjects was a significant HR decrement* when passing from waking state to N1. No significant differences were observed between both groups.
View Figures 4
Respiratory activity
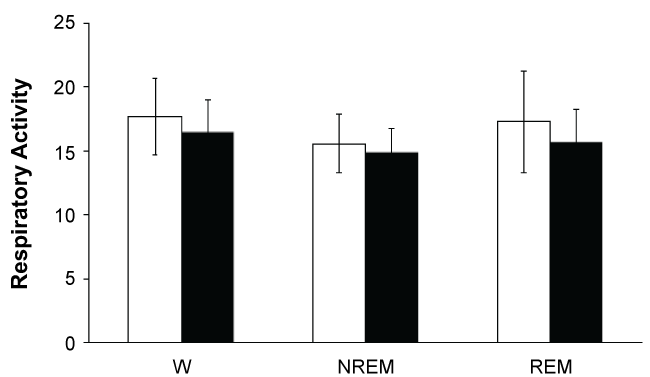
During steady NREM sleep, breathing was remarkably regular in both amplitude and frequency showing the lowest index of variability of all vigilance states. Breathing frequency averaged 17.70 breaths/min in blind subjects during wakefulness. This frequency decreased to 15.58 breaths/min when passing to NREM sleep, and it increased slightly (17. 30) in REM sleep (Figure 5).
.
Figure 5: No significant differences were observed in respiration rate across the vigilance states in both blind (white bars) and control (black bars) subjects.
View Figure 5
Breathing during REM sleep was irregular displaying a mean frequency similar to that in wakefulness. The irregularity consisted of sudden changes in both respiratory amplitude and frequency, at times interrupted by central apneas lasting 10 to 30 sec. The breathing irregularities did not occur at random but were linked to bursts of REMs. Control subjects displayed similar respiratory data.
NREM/REM cycle sequence
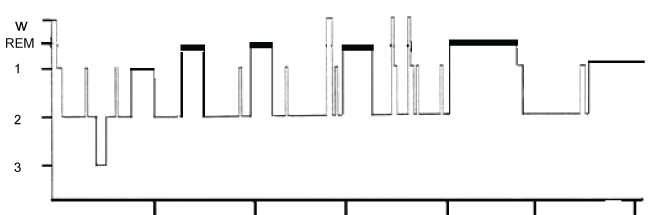
Blind subjects entered sleep by stage 1 NREM sleep, followed by stage N2 and then by REM sleep. As mentioned above, stage N3 sleep was almost absent. REM sleep was followed either by stage N1 or stage N2 and then by REM sleep again, so NREM sleep stages N1 or N2 alternated with REM sleep cyclically through the night (Figure 6). Duration of REM sleep stage in the first cycle of the night was usually short and it generally became longer throughout the night.
.
Figure 6: Hypnogram displayed by a blind subject.
Note the almost absence of the N3 sleep stage
View Figure 6
In contrast to blind subjects, stage N3 sleep was present in control subjects, particularly in the first part of the night. In this case, sleep entered by stage N1 followed by stages N2 and then N3. Before the start of the REM sleep, subjects returned to stage N2 sleep.
Data description
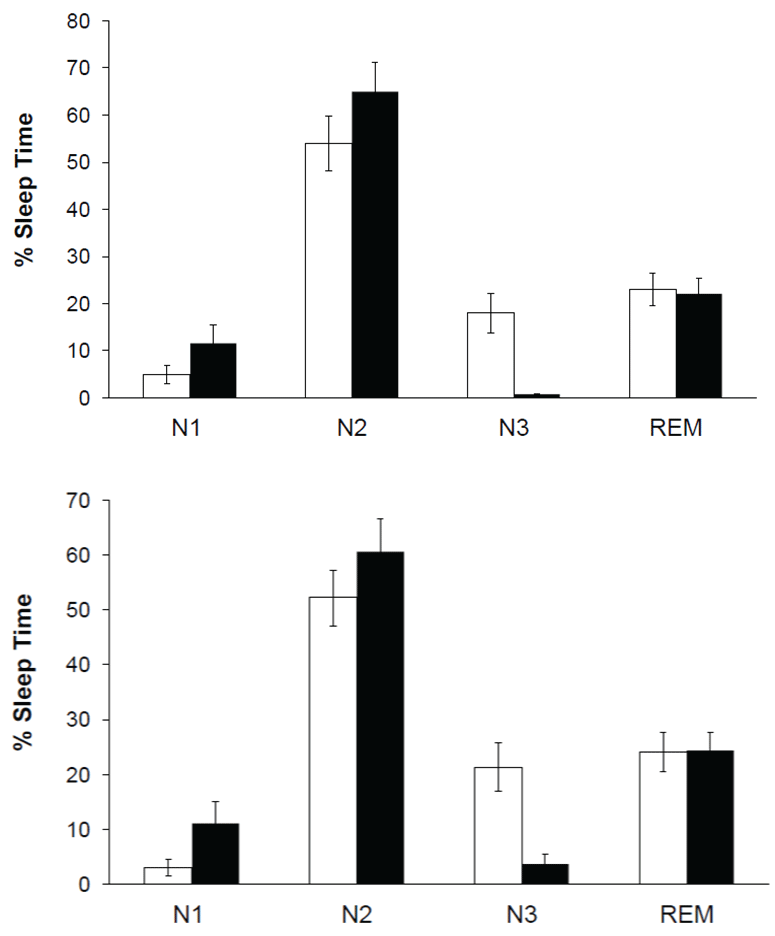
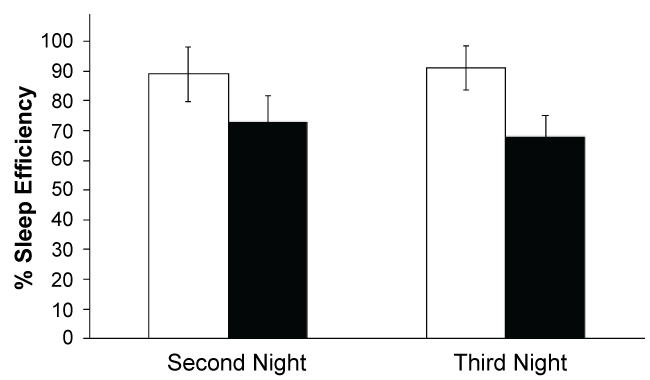
In spite of the fact that blind subjects were urged to stay in bed from 23:00 to 06:00 hours, they hardly completed 6 hours of sleeping time. Their sleep efficiency was 73% and 68% for the second and third recording nights, respectively. In contrast, the sleep efficiency in control subjects was 89% and 91% respectively (Figure 7, Table 1).
.
Figure 7: Sleep efficiency was significantly lower in blind subjects (*) White bars, control subjects; black bars, blind subjects.
View Figure 7
Both blind and control subjects spent most of the total sleeping time in light sleep (N1 + N2) during the two evaluated nights (Figure 8, Table 1). REM sleep percentages displayed during the second and third recording nights were similar to those in control subjects (Table 1). Delta sleep (N3) was practically absent since it was present only in 6 subjects, with a mean of 2.50 min (0.82% of total sleep time) for the second recording night and 10.50 min (3.66% of total sleep time) for the third one. Mean latency of REM sleep stage for the second recording night was 84.75 ± 41.10 min and 105.68 ± 36.07 min for the third night. Similar REM sleep latencies were observed in control subjects (88.43 ± 32.13 and 97 ± 33. 19 min for the second and the third night respectively). The number of sleep cycles was very variable from one subject to the next. An average of 3.35 ± 0.87 cycles for the second and 2.62 ± 0.99 cycles for the third recording night were observed. Control subjects exhibited 3.70 ± 1.09 and 6 ± 1.10 cycles for the second and the third night respectively. Mean duration of REM sleep episodes in blind subjects was 20.05 ± 6.70 min (Mean ± SD) for the second night and 26.78 ± 8.32 min for the third night. In contrast, values showed by control subjects were significantly shorter (13.40 ± 5.10 and 17. 67 ± 4.90 respectively). Mean of total sleeping time showed by blind subjects was 303.57 ± 89 min and 287.01 ± 92 min for the second and third recording nights, respectively. These values were significantly lower (p < 0.05) than those in control subjects (374 ± 72 min and 382 ± 76 min respectively).
Discussion
EEG patterns
Brain electrical activity shown by the blind subjects throughout the sleep-waking cycle was similar to controls and to that previously reported in normal individuals [1,5]. However, some important differences must be mentioned. Among these differences, the presence of alpha-like rhythm during N2 sleep stage in blind subjects is worthy of note. This sleep pattern was first described in patients with psychiatric disorders [21]. A similar pattern has been observed in patients with muscle-skeletal pain or fibrositis who complain of non-restorative sleep and excessive daytime sleepiness [22-24]. These sleep disorders might be originated by frequent brief arousals caused by the disease during nocturnal sleep. This would explain the intrusion of alpha-like rhythm into N2 sleep stage in blindness people as shown in other studies [25]. Such brief arousals are a common feature in elderly subjects [26]. In our experimental subjects, perhaps the intrusion of alpha-like rhythm during sleep could reflect imperceptible brief arousals; even though, these individuals seemed to be behaviorally sleeping deeply.
On the other hand, it is quite probable that this alpha-like rhythm really corresponds to mu waves since existing evidence indicates that mu and other alpha-like rhythms are independent phenomena because they generate from different sources [27]. In a study using closely-spaced Laplacian derivations, it was possible to rule out volume-conduction effects, providing evidence that both rhythms are, in fact, independently generated by the underlying neocortical circuitry [28]. Whole-head MEG recordings assessing the distribution of the estimated sources of alpha and mu rhythms led to similar conclusions. The results indicate that alpha and mu rhythms have distinct spatial distributions [29].
Further information is necessary to understand the origin and functional meaning of these waves in blind individuals.
As described in the results section, delta sleep was almost absent in the blind subjects. These observations are similar to those reported in another sleep study featuring a blind man where no explanation was given for this finding [8]. Experimental section of the two optical nerves in the baboon induced a significant decrease in delta sleep [30]. In this case, the delta sleep decrease was attributed to retino-hypothalamic fiber degeneration.
On the other hand, EEG waves recorded from the scalp are produced by slow voltage changes (inhibitory and excitatory post-synaptic potentials) occurring synchronously in large groups of neurons [17,31,32]. The magnitude of these voltage changes is a function of both the size of the potentials generated by each neuron and the number of neurons varying synchronously. A decrease in the size of neuronal networks could therefore account for the reduction in delta wave amplitude in blind subjects, as it has been suggested for schizophrenic patients and elderly individuals [33].
The aforementioned results are supported by information obtained from studies related to sleep pattern ontogeny in normal subjects, where high voltage waves of delta sleep follow a parallel development to that of cortical synaptic density [33-36].
The other physiological variables recorded in this study, EKG, respiratory rate and EMG did not vary significantly with respect to those of normal subjects.
Analysis of data
Although all sleep phases exhibited by control subjects were also present in the blind subjects, some significant quantitative differences were detected in total sleeping time and phase duration between the two groups of subjects. Total sleeping time shown by the blind subjects of this study was markedly lower than that observed in the controls and that reported by other authors in normal individuals [1] (Table 1). Similar low sleep values were reported in the study of a blind man [8].
Slow wave sleep was the most affected phase showing a lightening in depth, since duration of N1 sleep stage was significantly longer than that reported by other authors for normal subjects [1]. This increment occurred at the expense of a highly significant reduction in delta sleep, considered the deepest stage of NREM sleep, which was almost absent. The N2 sleep stage showed normal duration.
A similar reduction in delta sleep and increase in light sleep was observed in baboons with optic nerve section, where sleep disturbances were attributed to retino-hypothalamic fiber degeneration [30]. Similar findings were obtained by other researchers [7].
Although mean duration of the REM sleep episodes displayed by blind subjects was significantly longer than that in normal individuals, but the percentage of the total recording time spent in this sleep phase was similar to that in control subjects. These data are explained on the basis of their recurrence which was lower in the blind subjects.
In mammals, circadian rhythms, including that of sleeping-wakefulness are controlled by cells located in the suprachiasmatic nucleus of the hypothalamus [11,37,38]. Light is one of the most powerful environmental factors that influences this internal biological clock through fibers originated in the retina [9,39-41]. Blind individuals with no light perception are deprived of the influence of the light-dark cycle. This may account for the alterations observed in the sleep organization of blind subjects [42,43]. However, alternative or complementary explanations may exist.
In conclusion, sleep disturbances previously described such as longer sleep latency, fragmented sleep, and short sleep duration, were confirmed in this study. Additionally, abnormal sleep architecture was observed since delta sleep stage is almost absent.
Conflict of interest
The authors declare that there is no conflict of interest in this study
References
-
Carskadon AM, Dement WC (1989) Normal human sleep: An overview. In: Kryger MH, Dement WC, Principles and practice of sleep medicine. WB Saunders Company, Philadelphia 3-13.
-
Dement W, Kleitman N (1957) Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalogr Clin Neurophysiol 9: 673-690.
-
Iber C, Ancoli-Israel S, Chesson A, Stuart F (2007) The AASM Manual for the scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. (1st edn) American Academy of Sleep Medicine.
-
"Glossary (2008) A resource from the Division of Sleep Medicine at Harvard Medical School, Produced in partnership with WGBH Educational Foundation" Harvard University.
-
Ayala-Guerrero F, Mexicano G, Huicochea-Arredondo S (2014) Sleep characteristics in patients with autism spectrum disorders. J Neurol Disord Stroke 2: 1070.
-
Zulley J (1980) Distribution of REM sleep in entrained 24 hour and free-running sleep--wake cycles. Sleep 2: 377-389.
-
Karacan Y, Moore CA (1979) Genetics and human sleep. Psychiatr Ann 9: 11-23.
-
Klein T, Martens H, Dijk DJ, Kronauer RE, Seely EW, et al. (1993) Circadian sleep regulation in the absence of light perception: chronic non-24-hour circadian rhythm sleep disorder in a blind man with a regular 24-hour sleep-wake schedule. Sleep 16: 333-343.
-
Morin LP (1994) The circadian visual system. Brain Res Brain Res Rev 19: 102-127.
-
Sharma B, Feinsilver S (2009) Circadian rhythm sleep disorders: An update. Sleep Biol Rhythms 7: 113-124.
-
Emens JS, Laurie AL, Songer JB, Lewy AJ (2013) Non-24-Hour Disorder in Blind Individuals Revisited: Variability and the Influence of Environmental Time Cues. Sleep 36: 1091-1100.
-
Leger D, Guilleminault C, Defrance R, Domont A, Paillard M (1999) Prevalence of sleep/wake disorders in persons with blindness. Clin Sci (Lond) 97: 193-199.
-
Lockley SW, Skene DJ, Butler LJ, Arendt J (1999) Sleep and activity rhythms are related to circadian phase in the blind. Sleep 22: 616-623.
-
Leger D, Guilleminault C, Santos C, Paillard M (2002) Sleep/wake cycles in the dark: sleep recorded by polysomnography in 26 totally blind subjects compared to controls. Clin Neurophysiol 113: 1607-1614.
-
Csercsa R, Dombovári B, Fabó D, Wittner L, Eross L, et al. (2010) Laminar analysis of slow wave activity in humans. Brain 133: 2814-2829.
-
Dang-Vu TT, Desseilles M, Laureys S, Degueldre C, Perrin F, et al. (2005) Cerebral correlates of delta waves during non-REM sleep revisited. Neuroimage 28: 14-21.
-
Elul R (1972) The genesis of the EEG. In: Pfeiffer CC, Smythies JR, eds. International review of neurobiology. Academic Press, New York, NY, 15: 227-272.
-
Hubel DH, Wiesel TN, LeVay S (1977) Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci 278: 377-409.
-
Jasper HH (1958) The ten twenty electrode system of the international federation. Electroencephalogr Clin Neurophysiol 10:371-375.
-
Crofts HS, Wilson S, Muggleton NG, Nutt DJ, Scott EA, et al. (2001) Investigation of the sleep electrocorticogram of the common marmoset (Callithrix jacchus) using radiotelemetry. Clin Neurophysiol 112: 2265-2273.
-
Hauri P, Hawkins DR (1973) Alpha-delta sleep. Electroencephalogr Clin Neurophysiol 34: 233-237.
-
Moldofsky H, Scarisbrick P, England R, Smythe H (1975) Musculosketal symptoms and non-REM sleep disturbance in patients with "fibrositis syndrome" and healthy subjects. Psychosom Med 37: 341-351.
-
Roizenblatt S, Moldofsky H, Benedito-Silva AA, Tufik S (2001) Alpha sleep characteristics in fibromyalgia. Arthritis Rheum 44: 222-230.
-
Hernández BMA, Ramos PJ (2005) Estudio polisomnográfico en pacientes con fibromialgia primaria. Archivo de Neurociencias 10:9-14.
-
Stepanski E, Salava W, Lamphere J (1984) Experimental sleep fragmentation and sleepiness in normal subjects: A preliminary report. Sleep Res 13:193.
-
Carskadon MA, Brown ED, Dement WC (1982) Sleep fragmentation in the elderly: relationship to daytime sleep tendency. Neurobiol Aging 3: 321-327.
-
Pineda JA (2005) The functional significance of mu rhythms: translating "seeing" and "hearing" into "doing". Brain Res Brain Res Rev 50: 57-68.
-
Andrew C, Pfurtscheller G (1997) On the existence of different alpha band rhythms in the hand area of man. Neurosci Lett 222: 103-106.
-
Manshanden I, De Munck JC, Simon NR, Lopes da Silva FH (2002) Source localization of MEG sleep spindles and the relation to sources of alpha band rhythms. Clin Neurophysiol 113: 1937-1947.
-
Vuillon-Cacciuttolo G, Seri B (1978) [Effects of optic nerve section in baboons on the EEG activity and sleep-waking cycles]. Electroencephalogr Clin Neurophysiol 44: 769-777.
-
Crunelli V, Hughes SW (2010) The slow (< 1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat Neurosci 13: 9-17.
-
Nir Y, Staba RJ, Andrillon T, Vyazovskiy VV, Cirelli C, et al. (2011) Regional slow waves and spindles in human sleep. Neuron 70: 153-169.
-
Feinberg I, Hibi S, Carlson VR (1977) Changes in the EEG amplitude during sleep with age. In: Nandy K, Sherwin I, Aging brain and senile dementia. Plenum Press, New York, 85-98.
-
Dobbing J, Sands J (1973) Quantitative growth and development of human brain. Arch Dis Child 48: 757-767.
-
Feinberg I (1974) Changes in sleep cycle patterns with age. J Psychiatr Res 10: 283-306.
-
Feinberg I (1976) Functional implications of changes in sleep physiology with age. In: Gershon S, Terry RD, Neurobiology of aging. Raven Press, New York.
-
Lydic R, Albers HE, Tepper B, Moore-Ede MC (1982) Three-dimensional structure of the mammalian suprachiasmatic nuclei: a comparative study of five species. J Comp Neurol 204: 225-237.
-
Moore RY (1983) Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed Proc 42: 2783-2789.
-
Johnson RF, Moore RY, Morin LP (1988) Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res 460: 297-313.
-
Moore RY, Card P (1993) Visual afferents and suprachiasmatic nucleus pacemaker function. In: Nakagawa H, Oomura Y, Nagai K, New functional aspects of the suprachiasmatic nucleus of the hypothalamus. John Library and Company LTD, New York 1-13.
-
Moore RY, Lenn NJ (1972) A retinohypothalamic projection in the rat. J Comp Neurol 146: 1-14.
-
Leger D, Guilleminault C, Defrance R, Domont A, Paillard M (1996) Blindness and sleep patterns. Lancet 348: 830-831.
-
Sack RL, Lewy AJ, Blood ML, Keith LD, Nakagawa H (1992) Circadian rhythm abnormalities in totally blind people: incidence and clinical significance. J Clin Endocrinol Metab 75: 127-134.