Journal of Musculoskeletal Disorders and Treatment
Recovery of Functional Diaphragmatic Activity following Complicated Unilateral or Bilateral Phrenic Nerve Injuries using Multi-Modality Treatment
Matthew R Kaufman1,2,3*, Lisa Schneider1,2, Andrew I Elkwood1,2, Kameron S Rezzadeh3 and Reza Jarrahy3
1The Center for Treatment of Paralysis and Reconstructive Nerve Surgery, Jersey Shore University Medical Center, New Jersey, USA
2The Institute for Advanced Reconstruction, New Jersey, USA
3Division of Plastic & Reconstructive Surgery, David Geffen UCLA Medical Center, California, USA
*Corresponding author:
Matthew R Kaufman, MD, The Institute for Advanced Reconstruction, 535 Sycamore Avenue, Shrewsbury, NJ, USA, Tel: (732)-741-0970, E-mail: kaufmanmatthew@hotmail.com
J Musculoskelet Disord Treat, JMDT-2-026, (Volume 2, Issue 4), Original Article
Received: April 08, 2016; Accepted: October 20, 2016; Published: October 24, 2016
Citation: Kaufman MR, Schneider L, Elkwood AI, Rezzadeh KS, Jarrahy R (2016) Recovery of Functional Diaphragmatic Activity following Complicated Unilateral or Bilateral Phrenic Nerve Injuries using Multi-Modality Treatment. J Musculoskelet Disord Treat 2:026.
Copyright: © 2016 Kaufman MR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:Diaphragmatic paralysis may occur as a result of dysfunction in the central nervous system or phrenic nerves leading to inspiratory muscle weakness and a restrictive ventilatory deficit. Phrenic nerve reconstruction and diaphragm pacemakers have each been studied independently as effective therapeutic modalities.
Methods:We report three cases of diaphragmatic paralysis in patients with particularly complex pathological processes to investigate the use of multi-modality therapy consisting of phrenic nerve reconstruction and diaphragm pacemakers.
Results:The primary etiology of the diaphragmatic paralysis in all three patients was an iatrogenic injury. Two patients had underlying systemic disease complicating the phrenic nerve injury-central hypoventilation syndrome in one patient, and another patient with diabetic peripheral neuropathy. All patients reported significant improvement in respiratory activity and physical functioning after treatment.
Conclusion:The occurrence of diaphragmatic paralysis in patients with complex or recalcitrant neuromuscular pathology may be successfully reversed after phrenic nerve reconstruction and implantation of diaphragm pacemakers.
Introduction
Diaphragmatic paralysis may be caused by injuries or pathology in the central nervous system (CNS) or phrenic nerves. Symptoms range from mild dyspnea with exertion to severe respiratory disturbances even at rest that can lead to oxygen or ventilator dependency [1-3]. Chest fluoroscopy performed during nasal inspiration, or sniff testing, is performed to confirm the diagnosis, and in most cases of unilateral paralysis the normally functioning side is used as a reference [4]. Quantifying the neural injury or disorder is possible using electrodiagnostics, specifically nerve conduction studies (NCS) of the phrenic nerves and diaphragm electromyography (EMG) [5]. Although it is sometimes difficult to uncover the etiology of the paralysis, there are many patients who present with iatrogenic or traumatic occurrences as the cause of their phrenic nerve injury. Alternatively, there are established associations between certain CNS disorders, such as central hypoventilation syndrome or amyotrophic lateral sclerosis (ALS), and neuromuscular dysfunction in the respiratory system [6].
Treatment of diaphragmatic paralysis has traditionally been limited to plication of the diaphragm, a procedure that flattens the dome of the paralyzed diaphragm using sutures, in order to permit passive inflation of the lower lung during inspiration [7,8]. More recently, there have been evaluations of techniques intended to restore functional diaphragmatic activity, including the implantation of diaphragm pacemakers and reconstruction of the phrenic nerve [9,10]. Individually, these methods have shown great promise in reversing diaphragmatic paralysis, either through the application of functional neural stimulation (in the case of the pacemaker), or as a result of diaphragmatic reinnervation (with phrenic nerve reconstruction) [9]. Simultaneous application of both methods may provide a synergistic therapeutic effect most applicable to patients with complex, or recalcitrant neuromuscular pathology. Patients with remote injuries, multiple underlying etiologies, or extensive phrenic nerve pathology would generally be expected to have higher failure rates after therapeutic intervention using any single technique.
We present a case series of patients with diaphragmatic paralysis caused by complex or recalcitrant pathological processes treated using a combination of diaphragm pacemakers and phrenic nerve reconstruction.
Methods
We retrospectively reviewed three cases of symptomatic diaphragmatic paralysis refractory to conservative management. Treatment was offered after a thorough evaluation consisting of history & physical examination, sniff testing, pulmonary spirometry, relevant imaging (i.e. MRI cervical spine and/or CT neck/chest), and electrodiagnostics (NCS & EMG). Multi-modality therapy was recommended based upon recalcitrant neuromuscular pathology that was identified in each case, and included phrenic nerve injuries with concomitant underlying systemic neural disease, extensive phrenic nerve pathology, or remote injuries. Other indications for multi-modality therapy include aspects of the patient history that may suggest a "complex" presentation including bilateral diaphragmatic paralysis, prolonged unilateral paralysis, and co-morbidities such as obesity or advanced cardiac disease which would prevent the patient from participating in post-operative physical therapy. Of note, rates of nerve regeneration may be slower than expected in these patients; simultaneous pacemaker implantation represents an adjunct modality for clinical recovery and an opportunity to decrease morbidity associated with the patient's disease. This group was selected from a larger cohort of patients with diaphragmatic paralysis who otherwise did not reveal complex or refractory pathology. The Institutional Review Board at our hospital approved the study and informed consent was obtained in accordance with study approval.
All patients underwent phrenic nerve reconstruction, consisting of a meticulous neurolysis of the phrenic nerve and any involved cervical root contributions, and interposition sural nerve grafting to "bypass" the site of injury, typically in an end-to-side fashion when intraoperative nerve testing revealed at least partial axonal continuity. A diaphragm pacemaker was implanted either around the phrenic nerve in the neck (Avery Biomedical, Commack, NY), or through a laparoscopic approach into the nerve-muscle junction within the diaphragm (Synapse Biomedical, Oberlin, Ohio).
Patients were evaluated based upon subjective reporting of respiratory activity and physical functioning following treatment, and results of chest fluoroscopy and electrodiagnostic testing.
Results
Case 1
The patient was a 50-year-old woman with a long history of breathing difficulties related to central hypoventilation syndrome, exacerbated by right phrenic nerve injury following interscalene nerve block for shoulder surgery. Prior to the shoulder surgery she required only nocturnal BiPAP, however, complete oxygen dependency developed immediately following injury to the right phrenic nerve. Chest fluoroscopy confirmed paralysis of the right hemi-diaphragm. She was unable to perform any exertional activities, and could no longer participate as a tuba player in her local community marching band. The patient's medical history was significant for multiple pulmonary emboli and deep vein thrombosis (DVT) of the upper extremity related to Factor V Leiden deficiency.
On exam, the patient was in mild respiratory distress with obvious increased effort of breathing despite nasal cannula oxygen supplementation. Electrodiagnostic evaluation revealed phrenic nerve conduction latencies of 10.3 msec on the right and 6.2 msec on the left (ref. 8.0 ± 1.5 msec) and corresponding right and left diaphragm motor amplitudes of 0.3 mV and 0.5 mV (ref. > 0.33 mV), respectively. Radiographic imaging of the neck and chest, including vascular studies, failed to reveal evidence of organic pathology.
Based on these findings, a staged approach was offered to first correct the right phrenic nerve injury, and then address the underlying central hypoventilation syndrome. The patient underwent a right phrenic neurolysis and partial scalenectomy, including the release of vascular adhesions of engorged venous branches of the subclavian vein (possibly from prior upper extremity DVTs), that were clearly identified as compressing the phrenic nerve (Figure 1A and Figure 1B). Following the procedure, she reported modest improvement in her breathing. Post-operative electrodiagnostic evaluation revealed right and left phrenic nerve conduction latencies of 7.3 msec and 7.2 msec (ref. 8.0 ± 1.5msec), and diaphragm motor amplitudes of 0.4 mV and 0.9 mV (ref. > 0.33 mV), respectively. Chest fluoroscopy demonstrated increased movement of the right hemi-diaphragm. The patient continued to be dependent on daytime oxygen and nocturnal BiPAP. Approximately one year later, she underwent a second procedure to correct the disturbances caused by central hypoventilation syndrome, consisting of cervical implantation of bilateral diaphragm pacemakers (Avery Biomedical, Commack, NY) around both phrenic nerves. Six months following this procedure she reported near complete resolution of dyspnea, and indicated she was able to discontinue both daytime oxygen and nocturnal BiPAP. She is now participating in moderate exercise activities and has resumed playing the tuba in the marching band.
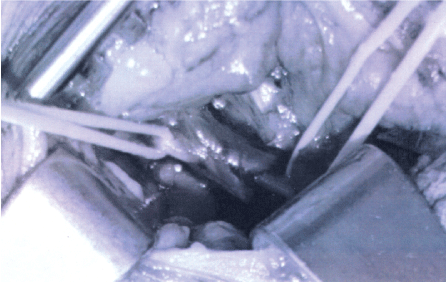
.
Figure 1A: Phrenic nerve (wrapped with loops) seen with adherent dilated, tortuous vein entwined circumferentially around it.
View Figure 1A
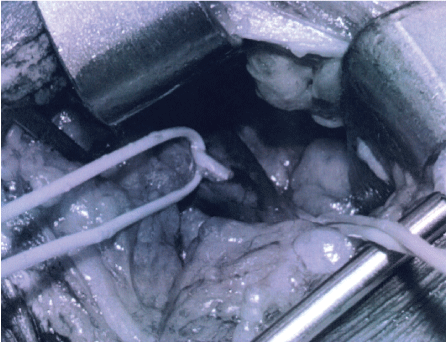
.
Figure 1B: Vein has been partially un-entangled from phrenic nerve (with loops) in preparation for vein ligation.
View Figure 1B
Case 2
The patient was a 59-year-old man with a symptomatic left diaphragmatic paralysis for approximately fifteen years. He reports sustaining a neck injury while playing football over twenty years ago which prompted him to visit a chiropractor regularly for neck manipulations over the next several years. The patient was diagnosed with left diaphragmatic paralysis on chest radiographs performed fifteen years ago after several emergency room visits for respiratory difficulties. He reported an exacerbation of symptoms beginning one year prior to presentation following removal of a benign left neck mass. Diagnosis of the left diaphragmatic paralysis was reconfirmed on chest fluoroscopy performed just after the neck surgery. Past medical history was also significant for coronary artery disease and mild emphysema. Despite the left phrenic nerve injury being quite remote, electrodiagnostic evaluation revealed axonal continuity and viable motor end-plates based upon detectable phrenic nerve conduction latencies and diaphragm motor amplitudes [Left = 13.7 msec; Right = 8.8 msec (ref. 8.0 ± 1.5 msec) & Left = 0.12 mV; Right = 0.6 mV (ref. > 0.33 mV), respectively]. Examination was significant only for diminished breath sounds at the left lung base.
Surgical treatment was offered based on the symptomatic nature of the patient's condition and clear evidence of residual neuromuscular activity. The patient underwent left C5 root and phrenic neurolysis, left partial scalene myomectomy, interposition sural nerve grafting (as an end-to-side graft from above-to-below the area of obvious abnormality), and implantation of a diaphragm pacemaker(Avery Biomedical, Commack, NY) around the left phrenic nerve distal to the site of grafting. Intraoperatively, there was evidence of both segmental phrenic nerve atrophy and post-inflammatory adhesions causing a compression neuropathy.
In the first three months following surgery, the patient began using the pacemaker and reported a mild reduction in his exertional dyspnea. At one year, the patient reported significant improvements in spontaneous respiratory activity without assistance from the pacemaker. Electromyography demonstrated a 50% improvement in left diaphragm motor amplitudes and chest fluoroscopy revealed increased motion of the left hemi-diaphragm. At this time, he had the receiver removed from his chest, under local anesthesia, given his overall improvement and with evidence of diaphragmatic reinnervation.
Case 3
The patient was a 58-year-old male with a one year history of a symptomatic right diaphragmatic paralysis that occurred during a life-saving, emergent right vertebral artery bypass. The patient presented to his local emergency room with symptoms consistent with a stroke, and was found, on MRI, to have posterior circulatory infarcts. Right vertebral artery stenting was attempted, however, the procedure was complicated by vertebral artery thrombosis post stent placement. The patient was taken emergently for right vertebral artery bypass and developed post-operative dyspnea. A right diaphragmatic paralysis was diagnosed on chest fluoroscopy. Electro-diagnostic testing revealed right and left phrenic nerve conduction latencies of 13.9 msec and 9.2 msec (ref. 8.0 ± 1.5 msec), and diaphragm motor amplitudes of 0.15 mV and 0.73 mV (ref. > 0.33 mV), respectively. Past medical history included diabetes and diabetic peripheral neuropathy. Physical examination revealed dense scar tissue in the right supraclavicualar area, diminished breath sounds at the base of the right lung, and mild paresthesias of the lower extremities.
Multi-modality surgical treatment was offered to maximize recovery in this patient with extensive scar tissue around the phrenic nerve from prior, emergent surgery, and poor neural regenerative capacity from underlying diabetic peripheral neuropathy. The patient underwent right phrenic neurolysis, partial scalene myomectomy, sural interposition grafting, and laparoscopic placement of a diaphragm pacemaker (Synapse Biomedical, Oberlin, Ohio). Intraoperatively, the right phrenic nerve was identified intact, but encased in dense adhesions throughout its course in the neck (Figure 2A and Figure 2B). The right hemi-diaphragm muscle exhibited moderate, diffuse atrophy compared to the normal left side.
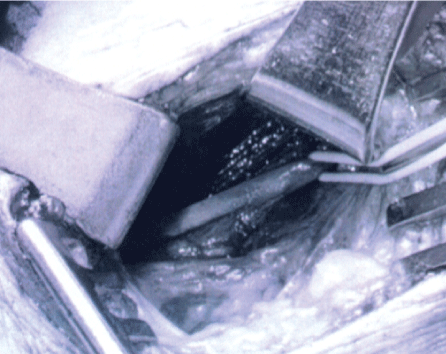
.
Figure 2B: After extensive microsurgical neurolysis the phrenic nerve (with loop) is seen clearly without visible abnormality.
View Figure 2B
Three months following treatment the patient reported modest improvement in respiratory activity while using the pacemaker. At six months, he reported that he was no longer using the pacemaker because his breathing was markedly better on its own. He indicated that he was able to return to a near-normal level of physical functioning. The patient lives in a remote location and has yet to have post-operative radiographs or electrodiagnostic studies.
Discussion
Diaphragmatic paralysis is a disorder that causes respiratory dysfunction yet the underlying causative process is neuromuscularly derived. Cervical tetraplegia, central hypoventilation syndrome, and ALS are CNS conditions resulting in diaphragmatic dysfunction that, if left untreated, will result in partial or complete dependence on respiratory support. Diaphragm pacemakers were first introduced in the mid 1970's and can provide an effective way of reducing or eliminating ventilator dependency in patients with these disorders. The mechanism by which the functional stimulators exert their effect is by transmitting an impulse to the phrenic nerve or nerve-muscle interface causing a contraction in the diaphragm muscle [11,12]. The result is a triggered inspiratory effort that generates negative pressure in the airway, unlike the positive pressure of mechanical ventilation. The inspiratory effort afforded by the pacemaker may generate enough of a tidal volume to reduce or eliminate oxygen therapy or positive pressure support, and the expiratory phase of breathing occurs passively. Absolutely critical to the successful use of a diaphragm pacemaker is phrenic nerve integrity, without which there will be no effective diaphragmatic contraction upon impulse transmission. Therefore, in patients with partial or complete phrenic nerve dysfunction, pacemakers will be limited in their effectiveness.
Phrenic nerve reconstruction has been investigated as an effective method for reinnervating the diaphragm in patients with iatrogenic or traumatic phrenic nerve injuries [9], and in patients with combined CNS disorders and loss of phrenic nerve integrity [10]. The basis for this work is the regenerative capacity of the peripheral nervous system supporting axonal regrowth after nerve decompression or through interposition nerve grafts. Microsurgical methods permit the meticulous reconstruction of peripheral nerves throughout the body, especially useful for functional restoration in the upper and lower limbs, and face following nerve injuries [13-15]. In applying these techniques to phrenic nerve injuries, the primary author (M.K.) has demonstrated diaphragmatic recovery in large numbers of patients, with regenerative rates comparable to those seen in the treatment of nerve injuries in other parts of the body (i.e. brachial plexus, peroneal nerve, etc.) [9]. Whereas nerve recovery and functional muscle activity is often successful following surgical treatment in patients with recent injuries, limited pathology, and an absence of contributing co-morbidities, the opposite is true when one or more of these factors are present [16].
Multi-modality therapy, consisting of diaphragm pacemakers and phrenic nerve reconstruction may improve the success of functional recovery under the most challenging of clinical circumstances. In Case 1, the patient had both a CNS disorder (central hypoventilation syndrome) and a peripheral phrenic nerve injury requiring dual treatment to effectively overcome respiratory symptomatology. Although the electrodiagnostic values following the first procedure demonstrated diaphragmatic reinnervation, she continued to require oxygen and positive pressure supplementation. It was only after implantation of the pacemaker did she overcome the respiratory dysfunction. In Case 2, the long duration of paralysis presented a formidable challenge, especially since it is difficult to predict the extent of irreversible diaphragm muscle atrophy over time. Multi-modality therapy was offered as a means to maximize re-innervation and muscular recovery. Case 3 was also complex in that the severe phrenic nerve entrapment occurred in a patient with diabetic peripheral neuropathy, a condition that has been demonstrated to impair nerve fiber regeneration after injury.
The success observed in these cases using multi-modality therapy may support the possibility of a synergistic effect. For example, there is evidence that electrical stimulation facilitates axonal regrowth and enhances activity in previously dormant neural circuitry [17]. Large scale comparative studies are necessary to further validate the findings in this study, yet it is increasingly evident that treatment options intended to restore functional activity to the respiratory musculature (i.e. pacemaker & phrenic nerve reconstruction) should be seriously considered in addition to diaphragm plication.
Acknowledgment
On behalf of the corresponding author and all co-authors, there is no conflict of interest.
References
-
Wang C, Yuan W, Zhou XH, Shi S, Wang X (2011) Neurotization of the phrenic nerve with accessory nerve: a new strategy for high cervical spinal cord injury with respiratory distress. Med Hypothes 76: 564-566.
-
Brown R, Dimarco AF, Hoit JD, Garshick E (2006) Respiratory dysfunction and management in spinal cord injury. Respir Care 51: 853-868.
-
Kalluri M, Huggins JT, Strange C (2008) A 56 year-old woman with arm pain, dyspnea, and an elevated diaphragm. Chest 133: 296-299.
-
McCool DF, Tzelepis GE (2012) Dysfunction of the diaphragm. N Engl J Med 366: 932-942.
-
Canbaz S, Turgut N, Halici U, Balci K, Ege T, et al. (2004) Electrophysiological evaluation of phrenic nerve injury during cardiac surgery- a prospective, controlled, clinical study. BMC Surg 4: 2.
-
Parhad IM, Clark AW, Barron KD, Staunton SB (1978) Diaphragmatic paralysis in motor neuron disease. Report of two cases and a review of the literature. Neurology 28: 18-22.
-
Mouroux J, Venissac N, Leo F, Alifano M, Guillot F (2005) Surgical treatment of diaphragmatic eventration using video assisted thoracic surgery: a prospective study. Ann Thor Surg 79: 308-312.
-
Groth SS, Rueth NM, Kast T, D'Cunha J, Kelly RF, et al. (2010) Laparoscopic plication for diaphragmatic paralysis and eventration: an objective evaluation of short-term and long-term results. J Thorac Cardiovasc Surg 139: 1452-1456.
-
Kaufman MR, Elkwood AI, Colicchio AR, CeCe J, Jarrahy R, et al. (2014) Functional restoration of diaphragmatic paralysis: an evaluation of phrenic nerve reconstruction. Ann Thorac Surg 97: 260-266.
-
Kaufman MR, Elkwood AI, Aboharb F, Cece J, Brown D, et al. (2015) Diaphragmatic re-innervation in ventilator dependent patients with cervical spinal cord injury and concomitant phrenic nerve lesions using simultaneous nerve transfers and implantable neurostimulators. J Reconstr Microsurg 31: 391-395.
-
Gordon T, Brushart TM, Amirjani N, Chan KM (2007) The potential of electrical stimulation to promote functional recovery after peripheral nerve injury- comparisons between rats and humans. In: Milessi H, Schmidhammer R, How to Improve the Results of Peripheral Nerve Surgery. Springer Vienna: 3-11.
-
Al-Majed AA, Neumann CM, Brushart TM, Gordon T (2000) Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci 20: 2602-2608.
-
Samii M, Matthies C (1994) Indication, technique and results of facial nerve reconstruction. Acta Neurochir (Wien) 130: 125-139.
-
Terzis J, Kalantarian B (2000) Microsurgical strategies in 74 patients for restoration of dynamic depressor muscle mechanism: a neglected target in facial animation. Plast Reconstr Surg 105: 1917-1931.
-
Hausamen JE, Schmelzeisen R (1996) Current principles in microsurgical nerve repair. Br J Oral Maxillofac Surgery 34: 143-157.
-
Lee SK, Wolfe SW (2000) Peripheral nerve injury and repair. J Am Acad Orthop Surg 8: 243-252.
-
Xu C, Kou Y, Zhang P, Han N, Yin X, et al. (2014) Electrical stimulation promotes regeneration of defective peripheral nerves after delayed repair intervals lasting under one month. PloS one 2: e105045.