Journal of Musculoskeletal Disorders and Treatment
Regenerative Therapy in Osteoarthritis of the Knee
Jonathan Theo1 and Edward Pang2*
1Touro University, California, USA
2Department of Physical Medicine and Rehabilitation, West Los Angeles Veterans Affairs Medical Center, California, USA
*Corresponding author:
Edward Pang, pain medicine fellow, Department of Physical Medicine and Rehabilitation, West Los Angeles Veterans Affairs Medical Center, Los Angeles, California, USA, E-mail: Edward.K.Pang@gmail.com
J Musculoskelet Disord Treat, JMDT-2-013, (Volume 2, Issue 2), Literature Review
Received: February 15, 2016: Accepted: May 18, 2016: Published: May 21, 2016
Citation: Theo J, Pang E (2016) Regenerative Therapy in Osteoarthritis of the Knee. J Musculoskelet Disord Treat 2:013.
Copyright: © 2016 Theo, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Osteoarthritis (OA) is the most common joint disorder in the world. It commonly affects the knee and current treatment options are limited, focusing mainly on symptom relief. It is now known that OA is the result of both mechanical and biological events that disrupt anabolic and catabolic processes in the joint. Recently, research in regenerative therapies has been gaining interest because of its potential to restore normal structure and function following tissue injury. The goal is to use the body’s own repair mechanisms in order to heal tissues that were previously irreparable. This article discusses the available research on such therapies, such as platelet rich plasma, mesenchymal stem cells, hyaluronic acid, and prolotherapy. There is a paucity of literature that examines these therapies in knee OA. More research is needed to establish the use of these therapies for the treatment of knee OA.
Keywords
Osteoarthritis, Knee, Regenerative medicine
Abbreviations
ACD: Acid citrate dextrose; ASCs: Adipose stem cells; bFGF: Basic fibroblast growth factor; BMP: Bone morphogenic protein; CTGF: Connective tissue growth factor; CJD:Cretuzfeldt-Jacob disease; EGF: Epidermal growth factor; G-CSF: Granulocyte colony stimulating factor; HA: Hyaluronic Acid; IGF-1 and IGF-2: Insulin-like growth factor-1 and 2; LIF: Leukemia Inhibitory Factor; L-PRF: Leukocyte and platelet-rich fibrin; L-PRP: Leukocyte and PRP; M-CSF: Macrophage Colony Stimulating Factor; MSCs: Mesenchymal stem cells; NSAIDs : Non-steroidal anti-inflammatory drugs; OA: Osteoarthritis; PGA: Patient Global Assessment; P2G: Phenol-glycerin-glucose; PDGF: Platelet derived growth factor; PRP: Platelet-Rich Plasma; P-PRP: Pure, Platelet-Rich Plasma; PPRF: Pure platelet rich fibrin; RBCs: Red blood cells; SCF: stem cell factor; TGF-β: Transforming growth factor beta; VEGF: Vascular endothelial growth factor; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; WBCs: White blood cells; WB: Whole blood
Introduction
Osteoarthritis (OA) is a chronic degenerative joint disease. As the most common joint disorder in the world, it is one of the leading causes of pain, loss of function, and disability in adults. It is estimated that nearly half the population at some point in their lives will be affected by OA [1]. Because of its weight-bearing function, OA in the knees is often debilitating, incurring significant health care costs. In the US alone, the number of total knee replacements was more than 600,000 in 2008 [2].
Current treatment options include non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroid injections, and oral supplements such as the combination of chondroitin sulfate and glucosamine, vitamin D and calcium, and invasive techniques such as surgery. However, these modalities have their limitations and none of them can help restore structural integrity. For instance, while NSAIDs provide pain relief and suppress inflammation, its therapeutic benefits are only palliative and do little to treat and prevent cartilage damage [3]. In the same manner, corticosteroids provide similar therapeutic benefits; however, they carry systemic and local adverse effects and the duration is short-lasting with one study showing pain relief lasting only 1 to 3 weeks [4]. Oral supplements such as chondroitin sulfate with glucosamine contain anti-inflammatory and anti-catabolic properties in-vitro [5]. Literature showed that this combination provided as much symptomatic relief as celecoxib, but no structural changes were observed [6,7]. Deficiencies in vitamin D and calcium have been shown to increase the risk of knee OA progression [8,9]. However, their use to improve pain and restore cartilage damage is controversial [10-12]. These are sold over the counter as dietary supplements and the actual content in these supplements can also vary from brand to brand. It is now known that OA is the result of mechanical and biological events that disrupt the balance between anabolic and catabolic processes in articular chondrocytes, extracellular matrix, and subchondral bone [13]. Because these therapies address only the symptoms of OA and not the root cause, interest in regenerative medicine has grown in popularity. Regenerative therapies aim to use tissue engineering and molecular biology in order to restore normal structure and function following tissue injury [14]. The goal is to use the body’s own repair mechanisms in order to heal tissues that were previously irreparable. From our literature search, there is not a comprehensive review of regenerative medicine for knee OA. Although the exact mechanisms of regenerative medicine are unknown, this paper will serve to review our current understanding of some of the most common treatment modalities for knee OA in regenerative medicine (i.e. platelet rich plasma, hyaluronic acid, stem cells, and prolotherapy) and describe the mode of action, therapeutic benefits, and adverse effects.
Platelet Rich Plasma Therapy
Definition: What is it?
Platelet Rich Plasma (PRP) is a concentration of autologous human platelets containing growth factors. There are four main families of PRP which are distinguished by their cell content and fibrin structure:
Pure platelet-rich plasma (P-PRP): Do not contain leukocytes and have a low-density fibrin network after activation.
Leukocyte and PRP (L-PRP): Contain leukocytes and have a low density fibrin network after activation.
Pure platelet rich fibrin (P-PRF): Do not contain leukocytes and have a high-density fibrin network.
Leukocyte and platelet-rich fibrin (L-PRF): Contain leukocytes and have high-density fibrin networks [15].
Preparation
Blood is drawn from a patient and subsequentially centrifugated, which separates the solution into three layers - the platelet poor plasma on the top, the platelet rich plasma in the middle, and the red blood cells on the bottom. There are many ways to prepare PRP such as using the PRP method or the Buffy coat method. The main difference between the two methods is that the Buffy coat method allows for the extraction of the Buffy Coat, which contains most of the white blood cells and platelets. Dhurat et al outlines the procedure as follows [15]:
PRP method
1. Obtain whole blood (WB) by venipuncture in acid citrate dextrose (ACD) tubes.
2. Do not chill the blood at any time before or during platelet separation.
3. Centrifuge the blood using a 'soft' spin.
4. Transfer the supernatant plasma containing platelets into another sterile tube (without anticoagulant).
5. Centrifuge tube at a higher speed (a hard spin) to obtain a platelet concentrate.
6. The lower 1/3rd is PRP and upper 2/3rd is platelet-poor plasma (PPP). At the bottom of the tube, platelet pellets are formed.
7. Remove PPP and suspend the platelet pellets in a minimum quantity of plasma (2-4 mL) by gently shaking the tube.
Buffy coat method
1. WB should be stored at 20°C to 24°C before centrifugation.
2. Centrifuge WB at a 'high' speed.
3. Three layers are formed because of its density: The
bottom layer consisting of RBCs, the middle layer consisting of platelets and white blood cells (WBCs) and the top PPP layer.
4. Remove supernatant plasma from the top of the container.
5. Transfer the Buffy-coat layer to another sterile tube.
6. Centrifuge at low speed to separate WBCs or use leukocyte filtration filter.
The platelets can then be activated using thrombin or calcium chloride, which causes the alpha granules to release growth and clotting factors from the platelets.
Significant variability in preparation procedures exist with no clear comparative evidence to date. Furthermore, some protocols include white blood cells, some involve activation with calcium or thrombin, and concentration of platelets can differ depending on preparation procedure [16].
In order to make future strides in PRP research, standardization of PRP preparation is paramount. Depending on the preparation technique, low quality PRP can confound research results. While there is still no uniform consensus, investigators have suggested that PRP have the following criteria [17]:
1.Platelet concentration of at least (3-8)x over baseline (i.e. a PRP platelet count of 1,000,000 per cubic mL may be therapeutic)
2.Centrifigutation process must be sterile and able to sequester high concentrations of platelets without damaging them. Platelet viability can be tested with pH, hypotonic stress, platelet aggregation levels, and P-selectin levels (low P-selecin levels indicate better platelet quality) [17].
Mode of action: how does it work?
PRP works through the degranulation of α granules in platelets, which in turn causes the release of growth factors. Some of these growth factors include transforming growth factor beta (TGF-β), basic fibroblast growth factor (bFGF), platelet derived growth factor (PDGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), insulin-like growth factor-1 and 2 (IGF-1 and IGF-2) and connective tissue growth factor (CTGF) [18]. Figure 1 illustrates the function of these growth factors in osteoarthritis treatment.
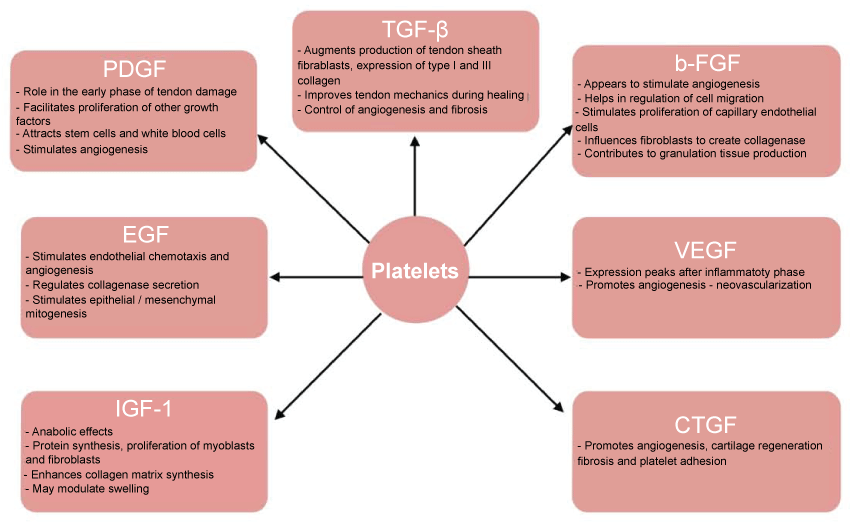
.
Figure 1: Illustrates the function of these growth factors in osteoarthritis treatment.
View Figure 1
Therapeutic effects
The growth factors released in PRP offer significant healing potential. For instance, PRP has been observed as a key potential mediator of angiogenesis and bone formation. In a review article by Eppley et al., it was observed that endothelial cells stimulated with PRP favored the proliferation and formation of new capillaries [19]. Similarly, an in vitro study by Hu et al. concluded that PRPs could start the process of angiogenesis by recruiting endothelial cells that line blood vessels [20]. The study also suggests PRPs role in the initiation of bone regeneration.
However, some have suggested that PRP is unlikely to directly enhance bone regeneration because platelets do not contain bone morphogenic protein (BMP) and is therefore not osteoinductive. However, adult mesenchymal stem cells, which are responsible for osteoblast formation, have been proven to respond to PRP resulting in accelerated bone formation [21].
In a prospective double-blinded randomized trial it was found that patients receiving PRP experienced all around improvements in WOMAC scores (pain, stiffness, physical function, and total score) compared to control (whose WOMAC scores deteriorated from baseline) [22]. In a more recent randomized controlled trial comparing PRP to hyaluronic acid, WOMAC pain score and bodily pain improved in both groups. However, PRP treatment was significantly more efficacious, showing an improvement in WOMAC score from 39.5 at baseline to 18.44 at week 52 (a change of 21.11). Hyaluronic acid went from a score of 28.69 at baseline to 27.46 (a change of 1.22) [23].
PRP seems to have long-term benefits as well. A recent meta-analysis by Campbell et al. found that the use of PRP led to significant improvements in patient outcomes at 6 months after injection and were maintained for up to 12 months. Improvements were seen starting at 2 months [24].
Overall, successful uses of PRP have been reported. However, many of the reports in the literature have been anecdotal or lack controls that could definitively determine the role of PRP [17]. In addition, there are also publications concluding PRP has little or no benefit in treating knee OA. The results from these studies could be traced to methodological weaknesses - the use of poor quality PRP or devices [19,25]. For instance, the use of leukocyte rich proteins could reduce the efficacy of PRP, as randomized trials that demonstrated the efficacy of PRP used leukocyte poor PRP [26].
Since not all PRP preparation protocols and devices are equal, it is important to create established standards in order to make the next step in this promising field.
Contraindications, side-effects, adverse effects
PRP is an autologous treatment and is therefore inherently safe. Transmittable diseases such as HIV, Cretuzfeldt-Jacob disease (CJD) and hepatitis should therefore not be a concern. In fact, it has been suggested that PRP actually inhibits bacterial growth [25].
Hyaluronic Acid/Viscosupplementation
Definition: what is it?
Hyaluronic Acid (HA) is a long polysaccharide glycosaminoglycan chain that makes up the main component of cartilage and synovial fluid. Its viscoelastic property is what allows cartilage to act as a shock absorber and synovial fluid to act as a joint lubricator [4].
However, OA presents with a qualitative and quantitative deficiency in HA. A normal glycosaminoglycan chain is 4-5 mD in a healthy joint but in OA it is 2-4 MD with only half the concentration [4].
Mode of action: how does it work?
Injection of HA is intended to have two therapeutic outcomes: mechanical and biological. HA restores mechanical function by supplementing the deficient HA, thus aiding shock absorption and cartilage protection. Biologically HA is taken up by specific joint receptors causing reduced cytokine-induced enzyme production, anti-oxidant actions, anabolizing efects on cartilage, and direct analgesia by masking joint nociceptors [4]. In a longitudinal study, Lurati et al. found that HA injection resulted in a significant decrease of CD4+ cells Th1, Th2, Th17 after 3 months when compared to control [27]. Akmel et al. found that articular chondrocytes cultured with HA demonstrated a greater rate of DNA proliferation and extracellular matrix production compared to cultures without HA [28]. While it is still not yet known why this happens, chondrocytes do express the glycoprotein CD44, which has the ability to act as a HA receptor. The effects of HA injections, therefore, may be mediated via CD 44 interactions [29]. The exact mechanism of action is unknown and more research is needed in this area.
Therapeutic effects
Currently, more than 100 clinical trials comparing HA derivitives with placebo, corticosteroids, NSAIDs, or a reference HA have been published. Overall, results were positive indicating an efficacy of approximately 20% compared to that of placebo (effect size -0.37) [4]. Furthermore, hyalruonic acid seems to be a reasonable treatment alternative for knee OA when compared current treatment options. Analgesics, the first-line treatment in OA for instance, have varying outcomes depending on the drug of choice [30]. For example, acetaminophen is well tolerated but has less efficacy (effect size -0.20). Opioids on the other hand possess greater analgesic efficacy (effect size -0.79) but patients exhibit poor tolerance, especially in the elderly [4]. In a meta-analysis by Bannuru et al., HA was shown to be just as efficacious as NSAIDs without the digestive, cardiovascular, and renal toxicity risks that one faces with long-term NSAID use [31]. Similiarly, corticosteroids have an efficacy comparable to that of HA (effect size -0.39) but differ kinetically [4]. Whereas corticosteroids have a quick onset and short duration (1 to 3 weeks), HA has a delayed onset and longer duration (several months) [32,33]. Corticosteroid efficacy was greater at 2 weeks, the same at 4 weeks, and lesser than that of HA at 8, 12, and 26 weeks [33].
Therefore, viscosupplementation seems to be a good alternative treatment for knee OA as it is well tolerated and has a better risk/benefit ratio than that of NSAIDs. Depending on the treatment plan and patient's goals, viscosuplementation may also be a suitable alternative for corticosteroids because of its longer duration of action. It has also been suggested that HA may have chondroprotective properties, making it an ideal candidate for OA prevention. These properties include the scavenging of reactive oxygen species, inhibition of immune complex adherence to polymorphonuclear cells, and inhibition of leukocyte and macrophage migration and aggregation [28,34,35]. However, these effects have yet to be proven in human clinical studies. HA is currently reserved for patients with symptomatic OA [4].
Currently, several in vitro studies and animal trials indicate a slower disease progression in knee OA patients treated with hyaluronic acid [4]. However, human studies have shown mixed results. In Wang et al., a randomized series of 78 patients with 4 cycles of 3 weekly hylan GF-20 (Synvisc®) injections every 6 months for 2 weeks was conducted [36]. A reduced annual condylar cartilage loss at 2 years was seen in MRI in the HA group compared to control. However, in Bard et al. arthroscopy score in the HA group did improve but radiologic impingement did not improve [4]. Further research is needed on the structural impact of HA in knee OA.
Saftey/contraindications, side-effects, adverse effects
Generally, HA injections are well tolerated with the occasional post-injection flare viewed as the only prominent toxic effect [37]. However, a meta-analysis by Rutjes et al. has raised concerns on the safety and efficacy of HA [38]. They pooled fourteen trials and showed that viscosupplementation is associated with an increased risk for serious adverse events (relative risk 1.41, CI 1.02 to 1.97). The most common disorders related to these events involve the cardiovascular system, gastrointestinal system, muscuoloskeletal system, and cancer. They, therefore, discourage the use of HA due to the increased risks of serious adverse and local events.
In response to the alarming findings by Rutjes, a recent literature review by McAlindon et al. reiterated the safety profile of viscosupplementation [37]. They argued that 10 of the 14 trials used to calculate the relative risk ratio had adverse events that were unrelated to the treatment. Of the remaining four, only one study reported HA related events - one severe skin reaction and one case of cutaneous vasculitis. The other three studies are unpublished and are difficult to evaluate because they are publically unavailable. Furthermore, the biological basis between viscosupplementation and severe adverse reactions is difficult to identify. Since the cancers reported in the studies were diagnosed soon after treatment, a biological causal relationship seems unlikely.
Recommendation
There are currently seven HA products that are FDA approved (Table 1). The active ingredient in these products is 20 mg sodium hyalruonate per dose [39]. The procedure for viscosupplementation is not yet universal. The current recommended number of injections per course ranges from one to five depending on the product. More research is needed in this area, as dose and scheduling influences treatment outcome.
![]()
Table 1: Characteristics of various hylans/hyaluronic acids [31].
View Table 1
Mesenchymal Stem Cells
Definition: what is it?
Stem cells are undifferentiated biological cells that have the ability to differentiate and self-renew [40]. These cells also have the ability to transdifferentiate, giving them a broad potential in regenerative medicine.
The Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy defines mesenchymal stem cells as having these minimum criteria [41,42]:
1. Plastic-adherent when maintained in standard culture conditions
2. Must express surface markers CD105, CD73, CD90 and lack CD45, CD34, CD14 (or CD11b), CD79a (or CD19), and HLA-DR
3. Capable of differentiating into: chondrocytes, osteoblast, and adipocytes in vitro
Stem cells for regenerative medicine applications should meet the following criteria [43]:
1. Can be found in abundant numbers (millions to billions of cells)
2. Can be harvested by a minimally invasive procedure with minimal morbidity
3. Can be differentiated along multiple cell lineage pathways in a controllable and reproducible manner
4. Can be safely and effectively transplanted to either an Autologous or allogeneic host
5. Can be produced in accordance with Good Manufacturing Practice guidelines
Preparation
Mesenchymal Stem cells may come a variety of sources such as bone marrow, adipose tissue, umbilical cord blood, synovial membrane, synovial fluid, periosteum, dermis, trabecular bone, infrapatellar fat pad, and muscle [44]. Each source has different differentiation abilities, which offer different advantages. For instance, bone marrow mesenchymal stem cells have good chondrogenic and osteogenic potential [43]. Synovial fluid on the other hand, has even greater chondrogenic but less osteogenic potential. Adipose tissue contains an abundant population of stem cells, making it an appealing source for MSCs. Unlike bone marrow aspiration, extracting ASCs (adipose stem cells) is less painful and can be retrieved in high number.
ASCs can be harvested from fat tissue wastes in surgery. There are numerous harvesting sites and harvesting techniques that are viable, including liposuction (syringe-based, pump-assisted, tumescent, ultrasound-assisted) and resection in the hip and abdomen [45]. However, their effects on cell yield and proliferation are still unknown. There are contradictory reports in the literature regarding the recovery of ASCs. For instance, Fraser et al concluded that the site of harvest and harvesting technique did not affect the number of ASCs harvested [46]. However, Oedayrajsingh-Varma et al. concluded that ultrasound-assisted liposuction yielded the lowest number of proliferative ASCs [47]. The differing reports make it difficult to conclude the most advantageous harvesting site, technique, and isolation procedure. There is currently no consensus for the minimum amount of cells needed in each harvest (Table 2).
![]()
Table 2: Amount of stem cells harvested.
View Table 2
Mode of action: how does it work?
The regenerative abilities of MSCs come from its 1) Structural contribution to tissue repair and 2) Its immunomodulatory action [48,49]. These abilities come from the following properties of MSCs:
Plasticity
• MSCs are able to contribute to tissue repair because of its capacity for self-renewal, maintenance of stemness, and cell potency. It is able to differentiate into multiple kinds of mesodermal tissues and migrate to injured tissue sites, where it displays tropic effects [50].
Tropic effects
• MSCs modulate the synthesis of proliferative, proangiogenic, and regenerative molecules [50,51]:
• G-CSF (granulocyte colony stimulating factor)
• SCF (stem cell factor)
• Leukemia inhibitory factor (LIF)
• Macrophage colony stimulating factor (M-CSF)
• IL-6, 11
• Decreased serum concentration of TNF-a
Immunosuppression
• MSCs inhibit B and T lymphocyte activation and proliferation by inhibiting the production of inflammatory cytokines released by CD4 TH and CD8 TC cells. Furthermore, they stimulate the production of IL-10, which promotes the generation of anti-inflammatory regulatory T cells [50,52].
• MSCs suppress NK activation and escape CTL mediated lysis because they are poorly recognized by T cells (due to their lack of MHC II or co-stimulatory molecules i.e. B7, CD40 or CD40L). MSCs are therefore immunopriveleged [53,54].
• They also modulate the secretion profiles of dendritic cells and macrophages, which allow for xenotransplantation [55].
Therapeutic effects
The use of MSCs in knee OA seems promising as it is able to differentiate into a wide variety of cells, such as myocytes, tendocytes and ligament cells [56-58]. The first reported use of MSCs for knee OA was in 2003 in a carbine menisectomy model. The treatment group demonstrated marked regeneration of the medial meniscus. Furthermore, articular cartilage degeneration, osteophytic remodeling, and subchondral sclerosis were all reduced. However, the injected MSCs were detected in soft tissue and not in articular cartilage, making it unlikely that MSCSs directly contribute to cartilage repair [59]. Therefore, rather than MSCs actively undergoing chondrogenic differentiation, it is more likely that MSCs tropic properties promote cartilage repair. Indeed, a study by Wu et al. demonstrated that MSCs induce chondrocytes proliferation and matrix deposition through the release of soluble factors [60].
A more recent study by Pak et al. showed positive results in two patients with knee OA treated with MSCs together with HA, dexamethasone, and PRP [61]. After 3 months both subjective outcomes (pain and function) and objective outcomes (MRI evidence of cartilage thickness) improved. However, due to the multi-modal treatment approach, it is difficult to ascertain which therapeutic intervention was most responsible for the outcomes.
In a 5-year follow up study by Davatchi et al., three patients were treated with MSCs exclusively. Pain and function (walking time, stair climbed, gelling pain, patella crepitus, flexion contracture, and visual analogue score on pain) all improved. Over time the therapeutic benefits gradually fade away but at 5 years these patients were still better than at baseline. Furthermore, Patient Global Assessment (PGA) improved from baseline to 5 years. The better knee at baseline, which did not receive MSC treatment, continued its deterioration and at 5 years it became the worst knee [62].
There is increasing evidence that inflammation in the synovium plays a large role in the progression of osteoarthritis. Because of its potential immunosuppressive properties in knee OA, MSCs also show promise in the prevention of occurrence of joint damage. The effects of MSCs in decreasing inflammation have only been shown in animal studies, and translation to patients is still unknown [63].
There is currently a lack of clinical data based on long-term randomized, double-blinded, controlled, multicenter studies with systemic follow up. Furthermore, the current literature shows a variety in the dosage and protocol of MSCs used making it difficult to compare clinical outcomes.
The optimal conditions for cartilage tissue regeneration is also unknown. More research is needed to determine the proper cell dosage of MSCs. Standardizing protocol will allow for a better understanding of MSCs in this field.
Saftey/contraindications, side-effects, adverse effects
While the use of stem-cells in the clincal setting is promising, caution is advised. Many biological pathways that ultimately determine the fate of transplanted MSCs in cartilage defects are still not understood [44]. Moreover, it is unknown how to control the chondrogenesis of MSCs in this setting.
Concerns over the use of MSCs have been raised regarding its:
Modification in vitro
during in vitro cultur
• MSCs can undergo modification during in vitro culture leading to serum-derived agents such as viruses and prions. Introduction of these agents into OA patients could be potentially harmful [64].
• Therefore, there is a great need for reliable culture protocols that reduce these risks.
Loss of immunogenicity
• During differentiation, MSCs may begin expressing MHC-II, resulting in the loss of its immunosuppressive property. This becomes a concern during allo- or xeno-transplantation. However, autologous transplantation is still widely used [51].
Formation of unwanted tissue at unwanted sites
• Wood et al. showed that MSC can show preferential migration towards the thymus and gastointestinal tract [65].
• Calcification at unwanted sites is a concern largely because osteogenesis is a main route of MSC differentiation. Breitbach et al. demonstrated the potential for bone formation in the myocardium of infarcted hearts [66].
Potential effect on tumor development
• Undifferentiated MSCs in mice have been seen migrating to tumor sites and support tumor growth and metastasis [67].
• However, this finding seems to be uncommon in human MSCs. A study by Prockop et al. concluded that malignant transformation of human MSC is rare when using standard culture techniques [68]. More research on the mechanism of tumor progression and MSCs possible role is needed.
There is currently a lack of clinical data based on long-term randomized, double-blinded, controlled, multicenter studies with systemic follow up. Furthermore, the literature shows a variety in dosage used making it difficult to compare clinical outcomes. In addition, the optimal conditions for cartilage tissue regeneration is unknown [44]. More research is needed to determine the proper cell dosage of MSCs.
According to the FDA, there is a potential safety risk when moving cells from one area of the body to another, as they are not performing the same biological function.
Prolotherapy
Definition: what is it?
Prolotherapy is an injection therapy for chronic musculotherapy. It involves repeated injections of an irritating or sclerosing solution at painful tendon, ligament insertions, and adjacent joint spaces [69].
Mode of action: how does it work?
The mechanism of action for prolotherapy is still unclear however several inflammatory and neural effects have been suggested. The three most common prolotherapy solutions and their hypothesized mechanism of action are as follows:
1 .Hypertonic dextrose via osmotic rupture of local cells
2. Phenol-glycerin-glucose (P2G) via local cellular irritation
3. Morrhuate Sodium via chemo tactic attraction of inflammatory mediators
The potential for prolotherapy to stimulate the release of growth factors for soft tissue healing is another proposed mechanism [69]. The prolotherapy solutions generate a temporary localized inflammatory response (or attract inflammatory cells in the case of Morrhuate sodium solution) inducing release of cytokines and growth factors synthesized by macrophages. Fibroblasts, recruited from growth factors, lay down new intercellular matrix including collagen. Because this inflammatory process is necessary to wound healing, patients are told to avoid taking anti-inflammatory medications [70].
Therapeutic Effects
A systemic review of prolotherapy in 2005 by Rabago et al. found 42 published reports of prolotherapy since 1937 [71]. Findings indicate that prolotherapy demonstrated positive findings for patients with chronic, painful, and refractory conditions. While promising, the studies in review do not have the same methodological rigor of today.
Modern research has been conducted and the results continue to show promise. An open label study using the Western Ontario McMaster University Osteoarthritis Index (WOMAC) found an overall improvement in WOMAC scores in intra-articular and extra-articular prolotherapy groups as early as 4 weeks progressing through 52 weeks (mean [standard deviation (SD)] point improvement, 15.9 [2.5]; P < 0.001) [72]. Similarly, a randomized controlled trial found a greater improvement in WOMAC scores among intra-articular and extra-articular prolotherapy recipients at 52 weeks than that of saline control and at home exercise participants (mean [SD] score change, 15.3 [3.5] vs. 7.6 [3.4] and 8.2 [3.3], respectively; P < 0.05). Both studies exceeded the minimum clinically important difference for the WOMAC of 12 points. Satisfaction for prolotherapy was high and there were no adverse reactions [70,73].
In a follow up study to determine the long-term effects of intra- and extra-articular prolotherapy, patients that had completed a 52-week prolotherapy study were observed at baseline, 12, 26, 52 weeks and 2.5 years. Again patients reached clinically important improvement benchmarks from 13.8 ± 17.4 points (23.6 %) at 12 weeks, to 20.9 ± 2.8 points, (p < 0.05; 35.8 % improvement) at 2.5 ± 0.6 years (range 1.6- 3.5 years) [72].
In a knee OA trial, Reeves et al. found that compared to control groups, patients receiving intra-articular prolotherapy reported significant improvements in pain, swelling, number of buckling episodes, and range of motion [74]. At 12 months follow up an increased patellofemoral cartilage thickness on knee radiography was observed, suggesting the possible disease modifying properties of prolotherapy. Furthermore, in a case series by Hauser et al., these patients were found to not only have pain reduction after prolotherapy, but also X-ray finding of joint space widening suggesting cartilage regeneration [75]. Similarly, Rabago et al. found that although prolotherapy and control groups demonstrated similar rate of cartilage loss over 52 weeks, those who had less cartilage loss in the prolotherapy group responded better clinically and improved dramatically in WOMAC pain scores [76].
Saftey/contraindications, side-effects, adverse effects
Contraindications include acute infection such as cellulites, local abscess or septic arthritis, and acute gouty arthritis and acute fracture. Side effects may include a post-injection flare during the first 72 hours that are self limited.
Conclusion
As one of the most common chronic health conditions, osteoarthritis of the knee poses a global economic burden costing tens of billions of dollars annually [77]. Current treatment options are primarily palliative, leading to an increased interest in regenerative treatments. PRP, viscosupplementation, MSCs, and prolotherapy all show promise as a potential regenerative therapy. While the direct mechanisms are different, each acts to ultimately promote wound healing through the modulation of growth factors and inflammation.
Current reports investigating the efficacy of PRP have generally been positive. A prospective, double-blinded randomized trial by Patel et al. showed that PRP was more effective in treating knee OA compared to placebo [22]. Although there are conflicting reports over PRPs efficacy compared with viscosupplementation [23,78], the differences in data could be traced to the type of PRP used. It is currently hypothesized that LP-PRP is more suitable than LR-PRP for the treatment of knee osteoarthritis [26]. Some attribute this to the injurious effects of proteases and reactive oxygen released from white blood cells [79].
Viscosupplementation has been shown to induce anabolic effects on cartilage, reduce inflammation, and mask analgesia in knee OA [4]. It has a longer duration of action and better safety profile than NSAIDs, making it a suitable alternative for corticosteroids. There is debate over the exact mechanism of viscosupplementation and whether it has chondroprotective properties. There are differing reports on whether viscosupplementation slows down disease progression in OA [36]. More research is needed on the structural impact of HA in knee OA.
Stem cells have a broad potential in regenerative medicine due to its ability to transdifferentiate [43]. In addition, there is evidence of MSCs therapeutic effects (i.e. tropic effects, immunosuppression, and cartilage repair) [50,59]. However, much of the evidence supporting MSCs have either been in animal trials or lacked controls that could clearly define the role of MSCs in knee OA. Furthermore, the exact biological pathways that determine the fate of transplanted MSCs in cartilage defects are still not understood [44]. There are also potential adverse effects, such as the formation of tissue in unwanted sites and potential tumor development [65-68]. More research is needed on the exact mechanisms of MSCs in knee OA and the optimal harvesting techniques and site. There is also a need for the standardization of dosages, as it can affect treatment outcome.
Similarly, phototherapy's exact mechanism of action is still unknown, with few pilot studies hypothesizing its mode of action [69]. However, the past few years have shown promising research. Compared to placebo, patients receiving prolotherapy demonstrated a significant improvement in knee OA. Furthermore, it is possible that prolotherapy may have disease-modifying properties. The safety-profile seems to be positive, with few contraindications and side-effects [74]. More research is needed on the mechanism of prolotherapy. Moreover, there is need for larger scale studies, as results may not be generalizable to all patients and the small sample size in the studies may have been too small to detect less common adverse effects.
Regenerative medicine is a relatively new field in managing osteoarthritis. Although more research is needed before it can be the standard of care, regenerative therapy presents as a very promising treatment option for knee OA.
References
-
Filardo G, Kon E, Buda R, Timoncini A, Di Martino A, et al. (2011) Platelet-rich plasma intra-articular knee injections for the treatment of degenerative cartilage lesions and osteoarthritis. Knee Surg Sports Traumatol Arthrosc 19: 528-535.
-
Losina E, Thorn hill TS, Rome BN, Wright J, Katz JN, et al. (2012) The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am 94: 201-207.
-
Hongsik Cho, Andrew Walker, Jeb Williams, Karen A Hasty (2015) Study of osteoarthritis treatment with anti-inflammatory drugs: cyclooxygenase-2 inhibitor and steroids. Biomed Res Int 2015: 595273.
-
Legre-Boyer V (2015) Viscosupplementation: techniques, indications, results. Orthop Traumatol Surg Res 101: S101-s108.
-
Yves Henrotin, Cécile Lambert (2013) Chondroitin and glucosamine in the management of osteoarthritis: an update. Current rheumatology reports 15: 361.
-
Castgillo J, MC Hochberg, J Martel-Pelletier, J Monfort, I Möller, et al. (2015) Combined chondroitin sulfate and Glucosamine versus celecoxib for painful Knee osteoarthritis: post-hoc analyses by Kellgren and Lawrence grade and C-reactive protein level from a randomized, double-blind, multicentre clinical trial. Clinical Therapeutics 37: e122.
-
Hochberg MC, Martel-Pelletier J, Monfort J, Möller, Castillo JR, et al. (2014) Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: a multicentre, randomized, double-blind, non-inferiority trial versus celecoxib. Ann Rheum Dis 75: 37-44.
-
Zhang FF, Driban JB, Lo GH, Price LL, Booth S, et al. (2014) Vitamin D deficiency is associated with progression of knee osteoarthritis. J Nutr 144: 2002-2008.
-
Demehri S, N Hafezi Nejad, TT Brown, F Roemer, A Guermazi, et al. (2016) Association of oral calcium intake with osteoarthritis progression and knee replacement: Data from the Osteoarthritis Initiative. Osteoarthritis and Cartilage 24: S256.
-
Jin X, Jones G, Cicuttini F, Wluka A, Zhu Z, et al. (2016) Effect of Vitamin D Supplementation on Tibial Cartilage Volume and Knee Pain Among Patients With Symptomatic Knee Osteoarthritis: A Randomized Clinical Trial. JAMA 315: 1005-1013.
-
Muraki S, Dennison E, Jameson K, Boucher BJ, Akune T, et al. (2011) Association of vitamin D status with knee pain and radiographic knee osteoarthritis. Osteoarthritis Cartilage 19: 1301-1306.
-
Jensen NH (2003) Reduced pain from osteoarthritis in hip joint or knee joint during treatment with calcium ascorbate. A randomized, placebo-controlled cross-over trial in general practice. Ugeskr Laeger 165: 2563-2566.
-
Klatt BA, Lopez HL, Segal NA, Chimes GP (2011) Treatment options in knee osteoarthritis: total knee arthroplasty versus platelet-rich plasma. PM R 3: 377-386.
-
Nelson TJ, A Behfar, A Terzic (2008) Strategies for therapeutic repair: The "R(3)" regenerative medicine paradigm. Clin Transl Sci 1: 168-171.
-
Dhurat R, Sukesh M (2014) Principles and Methods of Preparation of Platelet-Rich Plasma: A Review and Author's Perspective. J Cutan Aesthet Surg 7: 189-197.
-
Nguyen RT, Borg-Stein J, McInnis K (2011) Applications of platelet-rich plasma in musculoskeletal and sports medicine: an evidence-based approach. PM R 3: 226-250.
-
Choi, J, KW Minn, H Chang (2012) The efficacy and safety of platelet-rich plasma and adipose-derived stem cells: an update. Arch Plast Surg 39: 585-592.
-
Beny Charchian, Mauro Zappaterra, Mona Zall (2014) Regenerative Spinal Therapies for Low Back Pain. Current Physical Medicine and Rehabilitation Reports 2: 41-47.
-
Eppley BL, Pietrzak WS, Blanton M (2006) Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg 118: 147e-159e.
-
Zhen Ming Hu, Sean AF Peel, Stephen KC ,George KB Sándor, Cameron ML Clokie, et al. (2009) Platelet-rich plasma induces mRNA expression of VEGF and PDGF in rat bone marrow stromal cell differentiation. Oral and maxillofacial surgery 107: 43-48.
-
Slater M, Patava J, Kingham K, Mason RS (1995) Involvement of platelets in stimulating osteogenic activity. J Orthop Res 13: 655-663.
-
Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A, et al. (2013) Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med 41: 356-364.
-
Raeissadat SA, Rayegani SM, Hassanabadi H, Fathi M, Ghorbani E, et al. (2015) Knee Osteoarthritis Injection Choices: Platelet- Rich Plasma (PRP) Versus Hyaluronic Acid (A one-year randomized clinical trial). Clin Med Insights Arthritis Musculoskelet Disord 8: 1-8.
-
Campbell KA, Saltzman BM, Mascarenhas R, Khair MM, Verma NN, et al. (2015) Does Intra-articular Platelet-Rich Plasma Injection Provide Clinically Superior Outcomes Compared With Other Therapies in the Treatment of Knee Osteoarthritis? A Systematic Review of Overlapping Meta-analyses. Arthroscopy 31: 2213-2221.
-
Marx RE, (2004) Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 62: 489-496.
-
Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ, et al. (2015) Effect of Leukocyte Concentration on the Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis. Am J Sports Med 44: 792-800.
-
Lurati A, Mazzocchi D, Re KA, Marrazza M, Scarpellini M, et al. (2015) Effects of hyaluronic acid (HA) viscosupplementation on peripheral Th cells in knee and hip osteoarthritis. Osteoarthritis Cartilage 23: 88-93.
-
Akmal M, Singh A, Anand A, Kesani A, Aslam N, et al. (2005) The effects of hyaluronic acid on articular chondrocytes. J Bone Joint Surg Br 87: 1143-1149.
-
Chandra R, Mahajan S (2013) Role of viscosupplementation in osteo-arthritis of knee joint. J Indian Med Assoc 111: 337-340, 342.
-
Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, et al. (2003) EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 62:1145-1155.
-
Bannuru RR, Vaysbrot EE, Sullivan MC, McAlindon TE (2014) Relative efficacy of hyaluronic acid in comparison with NSAIDs for knee osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum 43: 593-599.
-
Bannuru RR, Natov NS, Dasi UR, Schmid CH, McAlindon TE, et al. (2011) Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis-meta-analysis. Osteoarthritis Cartilage 19: 611-619.
-
Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, et al. (2009) Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum 61: 1704-1711.
-
Balazs E J Denlinger(1984) The role of hyaluronic acid in arthritis and its therapeutic use. Osteoarthritis: Current Clinical and Fundamental Problems 165-174.
-
Goldberg RL, Toole BP (1987) Hyaluronate inhibition of cell proliferation. Arthritis Rheum 30:769-778.
-
Wang Y, Hall S, Hanna F, Wluka AE, Grant G, et al. (2011) Effects of Hylan G-F 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: a two-year single-blind clinical trial. BMC Musculoskelet Disord 12: 195.
-
McAlindon TE, Bannuru RR (2012) Osteoarthritis: Is viscosupplementation really so unsafe for knee OA. Nat Rev Rheumatol 8: 635-636.
-
Rutjes AW, Jüni P, da Costa BR, Trelle S, Nüesch E, et al. (2012) Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med 157:180-191.
-
Reid MC (2013) Viscosupplementation for osteoarthritis: a primer for primary care physicians. Adv Ther 30: 967-986.
-
Orkin SH (2000) Diversification of haematopoietic stem cells to specific lineages. Nat Rev Genet 1: 57-64.
-
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, et al. (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315-317.
-
Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, et al. (2005) Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 7: 393-395.
-
Lindroos B, R Suuronen, S Miettinen (2011) The potential of adipose stem cells in regenerative medicine. Stem Cell Rev 7: 269-291.
-
Filardo G, Madry H, Jelic M, Roffi A, Cucchiarini M, et al. (2013) Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc 21: 1717-1729.
-
Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, et al. (2009) Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut 58: 929-939.
-
Fraser, J, Wulur I, Alfonso Z, Zhu M, Wheeler E, et al. (2007) Differences in stem and progenitor cell yield in different subcutaneous adipose tissue depots. Cytotherapy 9: 459-467.
-
Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, Helder MN, Klein-Nulend J, et al. (2006) Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy 8:166-177.
-
Caplan AI (2005) Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng 11: 1198-1211.
-
Chen FH, Tuan RS (2008) Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther 10: 223.
-
Singer NG Caplan AL (2011) Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol 6: 457-478.
-
van der Kraan PM (2013) Stem cell therapy in osteoarthritis: a step too far. BioDrugs 27:175-180.
-
Riordan NH, Ichim TE, Min WP, Wang H, Solano F, et al. (2009) Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med 7: 29.
-
Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC, et al. (2003) Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation 75: 389-397.
-
Jorgensen C, Djouad F, Apparailly F, Noël (2003) Engineering mesenchymal stem cells for immunotherapy. Gene Ther 10: 928-931.
-
Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, et al. (2000) Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med 6: 1282-1286.
-
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143-147.
-
Caplan AI SP Bruder (2001) Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med 7: 259-264.
-
Brinchmann JE (2008) Expanding autologous multipotent mesenchymal bone marrow stromal cells. J Neurol Sci 265:127-130.
-
Murphy JM, Fink DJ, Hunziker EB, Barry FP (2003) Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 48: 3464-3474.
-
Wu L, Leijten JC, Georgi N, Post JN, van Blitterswijk CA, et al. (2011) Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A 17: 1425-1436.
-
Pak J (2011) Regeneration of human bones in hip osteonecrosis and human cartilage in knee osteoarthritis with autologous adipose-tissue-derived stem cells: a case series. J Med Case Rep 5: 296.
-
Davatchi F, Sadeghi Abdollahi B, Mohyeddin M , Nikbin B (2015) Mesenchymal stem cell therapy for knee osteoarthritis: 5 years follow-up of three patients. Int J Rheum Dis 19: 219-225.
-
Wolfstadt JI, Cole BJ, Ogilvie-Harris DJ, Viswanathan S, Chahal J, et al. (2015) Current concepts: the role of mesenchymal stem cells in the management of knee osteoarthritis. Sports Health 7: 38-44.
-
Simone A Glynn, Michael P Busch, Roger Y Dodd, Louis M Katz, Susan L Stramer, et al. (2013) Emerging infectious agents and the nation's blood supply: responding to potential threats in the 21st century. Transfusion 53: 438-454.
-
Wood JA, Chung DJ, Park SA, Zwingenberger AL, Reilly CM, Ly I, Walker NJ, et al. (2012) Periocular and intra-articular injection of canine adipose-derived mesenchymal stem cells: an in vivo imaging and migration study. J Ocul Pharmacol Ther 28: 307-317.
-
Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, et al. (2007) Potential risks of bone marrow cell transplantation into infracted hearts. Blood 110: 1362-1369.
-
Akay I, Oxmann D, Helfenstein A, Mentlein R, Schünke M, et al. (2010) Tumor risk by tissue engineering: cartilaginous differentiation of mesenchymal stem cells reduces tumor growth. Osteoarthritis Cartilage 18: 389-396.
-
Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, et al. (2010) Defining the risks of mesenchymal stromal cell therapy. Cytotherapy 12: 576-578.
-
Rabago D, Slattengren A, Zgierska A (2010) Prolotherapy in primary care practice. Prim Care 37: 65-80.
-
Banks AR (1991) A rationale for prolotherapy. Journal of Orthopaedic Medicine :13.
-
Rabago D, Best TM, Beamsley M, Patterson J (2005) A systematic review of prolotherapy for chronic musculoskeletal pain. Clin J Sport Med 15: 376-380.
-
Rabago D, Zgierska A, Fortney L, Kijowski R, Mundt M, et al. (2012) Hypertonic dextrose injections (prolotherapy) for knee osteoarthritis: results of a single-arm uncontrolled study with 1-year follow-up. J Altern Complement Med 18: 408-414.
-
Maynard JA, Pedrini VA, Pedrini-Mille A, Romanus B, Ohlerking F, et al. (1985) Morphological and biochemical effects of sodium morrhuate on tendons. J Orthop Res 3: 236-248.
-
Reeves KD, Hassanein K (2000) Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med 6: 68-80.
-
Hauser RA (2009) Cartilage Regeneration in Knees. Journal of Prolotherapy 1: 22-28.
-
Rabago D, Kijowski R, Woods M, Patterson JJ, Mundt M, et al. (2013) Association between disease-specific quality of life and magnetic resonance imaging outcomes in a clinical trial of prolotherapy for knee osteoarthritis. Arch Phys Med Rehabil 94: 2075-2082.
-
Chen A, C Gupte, K Akhtar, P Smith, J Cobb, et al. (2012) The Global Economic Cost of Osteoarthritis: How the UK Compares. Arthritis
-
Filardo G, Di Matteo B, Di Martino A, Merli ML, Cenacchi A, et al. (2015) Platelet-Rich Plasma Intra-articular Knee Injections Show No Superiority Versus Viscosupplementation A Randomized Controlled Trial. Am J Sports Med 43:1575-1582.
-
Elizaveta Kon, Giuseppe Filardo, Berardo Di Matteo, Maurilio Marcacci (2013) PRP for the treatment of cartilage pathology. Open Orthop J 7: 120-128.
-
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, et al. (2001) Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7: 211-228.
-
Aust L, Devlin B, Foster SJ, Halvorsen YD, Hicok K, et al. (2004) Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 6: 7-14.
-
Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, et al. (2006) Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 24: 376-385.
-
Lin K, Matsubara Y, Masuda Y, Togashi K, Ohno T, et al. (2008) Characterization of adipose tissue-derived cells isolated with the Celution system. Cytotherapy 10: 417-426.