Journal of Geriatric Medicine and Gerontology
Influence of Chemotherapy Schedule on the Efficacy of Radiotherapy in Stage III Non-Small-Cell Lung Cancer: Elders versus Youngsters
Regina Gironés1*, Jose Luis Monroy2, Alfredo Sánchez3, Francisco Aparisi4, Oscar Juan5 and GIDO: Grup of Investigation and Divulgation in Oncology
1Medical Oncolgy Unit, Hospital Lluís Alcanyís, Xativa, Spain
2Radiotherapy Unit, Hospital de La Ribera, Alzira, Valencia, Spain
3Medical Oncology Service, Hospital Provincial de Castellón, Valencia, Spain
4Medical Oncology Unit, Hospital Virgen de los Lirios, Alcoy, Valencia, Spain
5Medical Oncology Servive, Hospital Universitari, Politècnic, La FE, Valencia, Spain
*Corresponding author:
Regina Gironés, Oncologist, Physician, Medical Oncology Unit, Hospital Lluís Alcanyís, Crta Xàtiva a Silla km 2, Xàtiva 46800, Valencia, Spain, Tel: +34-96-228-95-79, Fax: +34-96-228-95-82, E-mail: girones_reg@gva.es
J Geriatr Med Gerontol, JGMG-2-019, (Volume 2, Issue 2), Research Article; ISSN: 2469-5858
Received: May 24, 2016 | Accepted: October 13, 2016 | Published: October 15, 2016
Citation: Gironés R, Monroy JL, Sánchez A, Aparisi F, Juan O, et al. (2016) Influence of Chemotherapy Schedule on the Efficacy of Radiotherapy in Stage III Non-Small-Cell Lung Cancer: Elders versus Youngsters. J Geriatr Med Gerontol 2:019. 10.23937/2469-5858/1510019
Copyright: © 2016 Gironés R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The optimal chemotherapy regimen to use with radiotherapy in stage III locally-advanced non-small-cell lung cancer (LA-NSCLC) is unknown. Considering the lack of clear data and guidelines for elderly patients, we designed this review to examine patterns of care for elderly patients with LA-NSCLC with a regional hospital database.
Purpose: The current study was conducted in order to evaluate the clinical outcome of concurrent chemoradiotherapy (CCR) related to chemotherapy schedule and comparing age groups. We tested the hypothesis that, elderly patients, treated with CCR in clinical practice, could benefit in terms of progression free survival (PFS) and overall survival (OS), in a similar magnitude that younger counterpart's do, independently of the chemotherapy schedule they receive.
Materials and methods: Between 2004 and 2014, 120 patients were treated with CCR. We reviewed the patients' chemotherapy schedules and prognostic factors. Survival rates by ages groups were compared using Cox proportional hazards regression models.
Results: 120 patients were analyzed; 73 young (60%), 47 elders (40%). Cisplatin-combination were mainly used in the young group (71% vs. 8%, p: 0.0001). Elders received more carboplatin-combination and monotherapy. Chemotherapy-schedule had not had an impact on survival in any age group. Median progression free survival (PFS) was similar for both groups: 13.0 (95% CI: 11.2-214.7) vs.11.0 (95% CI: 7.7-14.2) months (p: 0.519). Overall Survival (OS) did not differ by age groups: 18.0 (95% CI: 13.1-22.9) vs. 18.0 (95% CI: 12.8-23.1) months (p: 0.393). OS at 2-years was 20% and 10% at 5-years in both groups. The only therapeutic predictive factor for outcome (OS) was median radiotherapy dose (> 50 Gy).
Conclusions: Our date shown that in clinical practice, aged patients received less intensive treatment for LA-NSCLC. These less active schedules had not influenced the survival rate negatively. Elderly patients benefit from CRT, with schedules adjusted to age similar to a palliative intent. In the lack of a specific trial for elderly patients (needed), we advocate for the use of concurrent chemoradiotherapy in elderly patients. It doesn't matter the chemotherapy schedule is that you use; maintain radiotherapy dose higher than 50 Gy.
Keywords
Non-small-cell lung cancer, Locally advanced, Concurrent chemoradiation, Elderly, Platinum-combination, Monotherapy
Abbreviations
LA-NSCLC: Locally-Advanced Non-Small-Cell Lung Cancer; CCR: Concurrent Chemoradiotherapy; PFS: Progression Free Survival; OS: Overall Survival; NSCLC: Non-Small- Cell Lung Cancer; CC: Cisplatin-Combinations; CAC: Carboplatin-Combinations; M: Monotherapy
Introduction
Nearly half of patients with non-small-cell lung cancer (NSCLC) are elders. Almost one in four has stage III disease at presentation [1]. The outcome of patients (not elders) remains poor, with median survival times of only 15.3 to 21.7 months [1]. However, approximately 20% of patients achieve durable disease control, arguing for treatment with curative intent in those able to tolerate aggressive therapy [1]. The accepted standard treatment for inoperable stage III NSCLC today topically consists of concurrent chemoradiotherapy (CCRT) that is based on so-called platinum-doublet chemotherapy [2-6]. The optimal chemotherapy regimen to use with radiotherapy is unknown. In the absence of contraindications, the optimal chemotherapy to be combined with radiation in stage III NSCLC should be based on cisplatin. There are no firm conclusions supporting single-agent carboplatin as a radiation sensitizer [2].
Lung cancer mainly affects elderly patients worldwide. The care received by older lung cancer is often suboptimum. Poor functional status, coexisting comorbidities, limited life expectancy and physicians' concerns about toxicity and the effect of treatment on quality of life often limits the use of chemotherapy and radiotherapy in elderly patients [7]. This practice pattern is aggravated by the overwhelming lack of evidence from randomized controlled trials focused specifically on older patients. CCRT is considered standard treatment for patients with inoperable stage III NSCLC. However, elderly patients were not well represented in the trials.
Moreover, most elders are unsuitable for cisplatin-combinations, and there are few prospective data about how to treat stage III NSCLC on aged population. There is considerable concern that carboplatin-combinations, although better tolerated than cisplatin, may be inferior in terms of disease control [8].
To gain insight into the relative efficacy of these regimens on elder patients, we examined outcomes of patients with newly diagnosed inoperable stage III NSCLC using our date base. We hypothesize that elderly patients treated with CCRT in clinical practice could benefit from treatment in terms of PFS and OS in a similar magnitude that younger counterparts, independently the chemotherapy schedule they receive.
Methods
Patient selection
Using our data base registry, we identified patients diagnosed with stage III NSCLC from January 2004 to December 2014. We compared clinical characteristics and outcomes in two age groups; those under 70-years-old and those with 70 or more. Patients were required to have cytological or histologically confirmed NSCLC by biopsy. The eligible patients were those who meet the following criteria: Performance Status 0-1, inoperable AJCC stage IIIA or IIIB, treatment with concurrent chemotherapy and radiotherapy.
Chemotherapy
Treatment was decided by the same oncologist, Dr Gironés. We considered patients unsuitable for cisplatin if they have comorbidities (especially cardiac ones), poor renal function, diabetes or other neuropathies.
Radiotherapy
All of the patients underwent a Phillips scan with intravenous contrast. Treatment planification was made with a Pinnacle three-dimensional conformal radiotherapy (3D-CRT). The gross tumor volume (GTV) included the primary disease as well as the involved regional lymph nodes. The clinical tumor volume (CTV) included primary tumor plus a 0.7-1.0 cm margin. The range dose was 8-60 Gy. Patients treated with less than 40 Gy received palliative treatment; dose higher than 40 Gy had radical intention.
Statistical Analysis
The primary outcome of this analysis was Overall Survival (OS), comparing elder versus young patients and type of treatment. OS was calculated from the day of diagnosis to death or the last follow-up (15th December 2014). Progression-free survival (PFS) was calculated as the time to progression or death without progression from the date of diagnosis, defined by days from histological diagnosis until death. We tested differences in baseline characteristics between groups using the Pearson x2 or t test for categorical and continuous variables, respectively. Student's t-test, nonparametric Mann-Whitney u test or X2-test was used to evaluate the difference between patient clinical characteristics. Actuarial survival curves were generated using Kaplan-Meier method. Survivals were compared by using the log-rank test. A value of p < 0.05 was considered statistically significant.
Results
Patients characteristics
120 accomplished inclusion criteria for the analysis; 73 (60%) young and 47 (40%) elders. Table 1 shows characteristics of both groups. Almost all patients were male and had smoking habit. Most elders were ex-smokers versus active smokers in the young group (p: 0.0001). Squamous histology was the main subtype.
![]()
Table 1: Comparison of patient characteristics between groups.
View Table 1
Treatment date
Table 2 shows differences in patterns of treatment. Elderly patients received less active treatment, similar to a palliative approach. For radiotherapy treatment, the median dose was similar between groups (60 Gy and 59 Gy, p: 0.33). But less elderly patients received radical doses (> 50 Gy), and less received mediastinum treatment (85% vs. 68%, p: 0.026). For chemotherapy, aged patients received less cisplatin-combination (CC) (71% vs. 8%; p: 0.0001), more carboplatin-combination (CAC) (24% vs. 68%; p: 0.0001) and monotherapy (M) (5% vs. 24%; p: 0.0001). Mean number of cycles was similar.
![]()
Table 2: Treatment patterns between groups.
View Table 2
![]()
Table 3: Efficacy.
View Table 3
Efficacy and survival outcomes
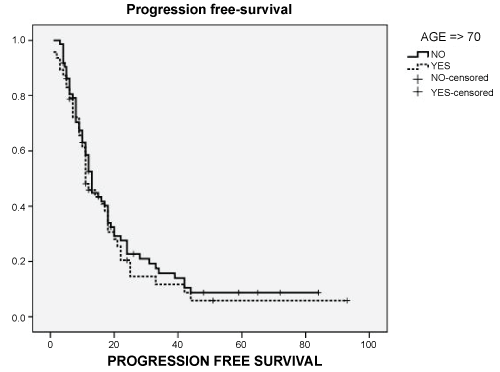
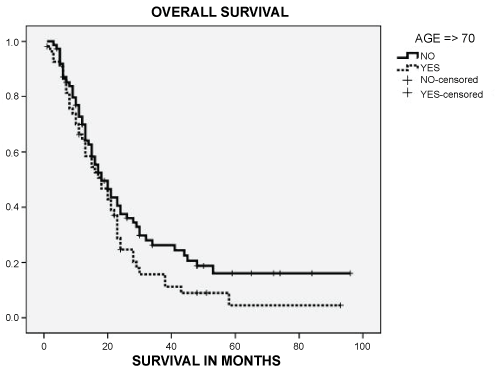
In terms of efficacy, response rate was similar between groups and progression after first response (Table 3). Second line use had not differences by age. Median progression free survival (PFS) was similar for both groups: 13.0 (95% CI: 11.2-214.7) vs. 11.0 (95% CI: 7.7-14.2) months (p: 0.519) (Figure 1). PFS at 2-years was nearly 30% for both groups. Overall Survival (OS) had not differences by age groups: 18.0 (95% CI: 13.1-22.9) vs. 18.0 (95% CI: 12.8-23.1) months (p: 0.393) (Figure 2). OS at 2-years was 20% and 10% at 5-years in both groups.
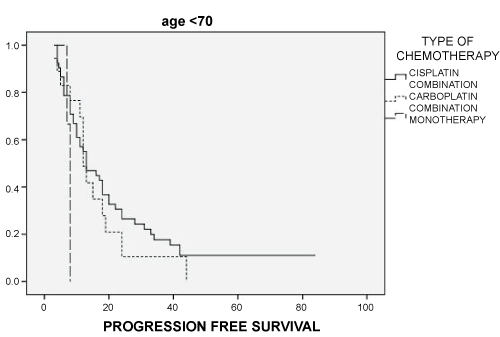
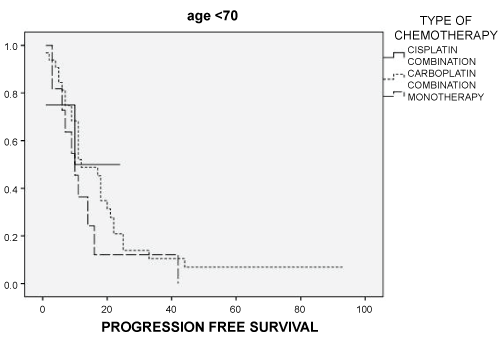
When we analyzed the impact of chemotherapy schedule combined with radiotherapy, no age group had differences related to the chemotherapy schedule chosen. In the young group, PFS was 13.0 (95% CI: 7.6-18.4) months for Cisplatin-Combinations (CC) vs. 16.0 (95% CI: 10-22.1) months for Carboplatin-Combinations (CAC) vs. 7.6 (95% CI: 6.9-8.4) months for Monotherapy (M), (p: 0.283) (Figure 3). For the aged group, PFS was 14.7 (95% CI: 5.1-24.3) months for CC versus 20.2 (95% CI: 12.3-28.1) for CAC versus 13.1 (95% CI: 5.5-20.8) months for M (p: 0.136) (Figure 4). In a multivariate analysis, the only treatment factor significantly related to overall survival for all series was median dose of Radiotherapy (50 Gy or more).
Discussion
The purpose of this study was to evaluate the clinical outcome of chemotherapy combined with radiotherapy, while analyzing the influence of chemotherapy schedule by age groups. Our date shown that, in a clinical practice scenario, elderly patients received less active chemotherapy combinations, concurrent with radiotherapy. In ananecdotic manner, they received cisplatin combination. Mainly were treated with carboplatin combinations or monotherapy. But this supposed under-treatment didn't seem impact on outcomes on this elder population. For young patients at our hospital, the chemotherapy schedule had impact on survival.
The accepted standard treatment for inoperable stage III non-small-cell lung cancer (NSCLC) today topically consists of concurrent chemoradiotherapy (CCRT) that is based on so-called platinum-doublet chemotherapy [3-6]. After the establishment of CCRT as the standard of care [4], little research has addressed the choice of chemotherapy [1]. If we sum up the published evidence from randomized trials, a meta-analysis that was based on individual patient data, and further published experience from several phase II trials, there is currently no consensus regarding which chemotherapy is the best to combine with radiotherapy in this curative setting [2]. The broadest evidence concerning this issue comes from trials that have included cisplatin-based doublets-particularly, cisplatin and etoposide or cisplatin and vincaalkaloid [2]. Despite this accepted evidence, because concerns about toxicity associated with cisplatin, there has been a strong trend to prefer outpatient administration of carboplatin-based schedules, on the basis of the assumption that this is more convenient than and possibly just as effective as cisplatin-based doublets [9-11].
But, what happens for elderly patients? Most of elderly patients attended at clinical practice, are unsuitable for cisplatin-combinations. Moreover, If we consider the 9 trials that compared sequential versus concomitant chemotherapy and radiotherapy, they included few patients older than 70 years of age (only 16%), whereas the median age for diagnosis of lung cancer is currently about 72 years of age [2].
The consensus of ESMO clinical guidelines is that cisplatin-based doublets should be preferred in stage III disease multimodality protocols when treatment has a curative intent. Clinical guidelines recommended that age itself should not be a criteria to treatment decisions in stage III NSCLC [2], because age itself has not been shown to influence outcome for definitive concurrent chemoradiotherapy [I, A]. However, data are limited for the elderly population and, in particular, in patients above 75 years of age. The number of elderly patients in all randomized chemoradiotherapy trials is still too small to allow for robust conclusions. Median age of the articles collected is only 61 and only 9% had ≥ 70 years old [6].
So, how must we treat elderly patients if they are not included in these clinical trials? Are we sure that this decision evidence based? In our article we report our investigation of data derived from patients attended in clinical-based practice. Over a 10-year period (2004-2014), we combined patient groups by age and analyzed if they received cisplatin-schedules or carboplatin-schedules, administered concurrently with curative or palliative doses of radiation. The aim of our study was to compare the two aged-based groups and the different chemotherapy schedules with respect to the survival outcome of the treated patients. From our findings, we conclude that carboplatin-schedules for elderly patients, when administered concurrently with radiotherapy, result in survival outcomes that were comparable to cisplatin-schedules for young patients in a comparable clinical setting. The same analysis was done by the Santana-Davila, et al. from the Veterans Health Administration (VHA) database [1]. They had a huge number of patients (1842). Of course, we know that we hadn't presented a randomized clinical trial, and dates are not excluded from bias. Furthermore, the outcome of elderly patients in our clinical practice was not inferior in the chemoradiotherapy trials by the Cancer and Leukemia Group B and the RTOG [4-11].
Scientific rules tell us that we should treat patients according to high level evidence. Level of evidence comes from randomized phase III trials, and those tell us that stage III NSCLC unresectable, should be treated with concurrent QT-RT and the most level of evidence comes from cisplatin-combinations. But, molts elders are unsuitable for cisplatin. So, should we not treat these patients because they cannot resist the standard regimen? But, if they are excluded from these trials, why we have to extrapolated their conclusions to clinical practice? If they are not included in clinical trials, why should we treat them in this way; as the clinical trials conclude? We believe that elderly patients benefit from CRT, despite the chemotherapy schedule is not cisplatin-combination. Of course, we know that this is not a “cientific evidence” or have high grade of evidence (expert opinion is level IV), and it's only a clinical approach. If elderly are underrepresented, why should be them treated according to the conclusions of phase III trials from they are excluded? The median age of patients in these trials is much lower than the median age of patients with NSCLC in general.
The significant underrepresentation of elderly patients that we have uncovered in these studies logically draws into question the generalizability of their results to elderly patients [12]. Elderly patients often harbor increased comorbidities and have poorer glomerular filtration rate [13], making them unsuitable for cisplatin treatment, resulting in altered pharmacokinetics and increased toxicity from treatment. As such, therapies demonstrated to be efficacious in a significantly younger group of trial patients may not necessarily be effective in an increasingly elderly population of patients with NSCLC [12]. Trials that have included elderly patients, and even more so, elderly patients with a poor prognosis and unresectable, stage III LA-NSCLC, have been lacking. The treatment recommendations for this group have often conflicted. Some investigators have questioned the indications for CCRT [14,15] and others have recommend CRT only for patients with a good performance status (PS). Some have simply considered patient aged 75 years a contraindication for CRT. An additional problem has been that few of the existing trials concerning this patient group have adhered to the standard treatment of unresectable stage III LA-NSCLC [16-18]. It has been tenaciously argued that clinical trials of treatment in older populations are necessary [19-22].
A high dilemma on treating elderly patients is to know which is preferable; minimize toxicity or preserve efficacy [23]. Furthermore, quality of life in an older patient population is potentially more complex, and the impact of palliative chemotherapy on this important clinical endpoint in the elderly population is poorly understood. Thus, the balance of toxicity against the antitumor effect of these treatments and their effect on survival and quality of life may be significantly altered in an elderly real-world population.
A most concerning dimension of the underrepresentation of the elderly in NSCLC clinical trials: that the most highly cited and practice-changing trials in the field are the ones that Sacher, et al. have shown to exclude elderly patients [12]. The implication that the current treatment of advanced NSCLC is based on trials that largely exclude the same elderly patients who account for a large proportion of the population with advanced NSCLC is sobering.Until this normalization of trial patient age is achieved, we believe it is appropriate to continue to conduct elderly-only trials to validate current practice in the treatment of elderly patients despite the expense of such trials. Greater representation of elderly patients in phase III trials is required to better define evidence-based treatment paradigms in the increasingly elderly NSCLC population. But, in absence of clinical trial, elderly patients should be treated.
This study contributes to a growing body of literature indicating that elderly patients could benefit from treatment. Here, we have clearly demonstrated that a significant proportion of elderly patients in clinical practice are treated outside of recommended guidelines. A significant proportion of highly cited phase III trials overtly exclude elderly patients. If elderly patients are excluded from practice-changing trials in advanced NSCLC, this is a sufficient reason to exclude them from treated in clinical practice scenario?
What might be the most important conclusion from our date report? If we see an elderly patient who might have considerable problems with the administration of a cisplatin-based doublet-preferably a man and a patient with a squamous cell carcinoma we might able to safely switch to carboplatin-combination fromCCRT. That would be, from now, a reasonable conclusion to draw from these data. There is increasing agreement that treatment decisions for elderly patients should be based on performance status, comorbid conditions and patients preferences. Treatment decisions based entirely on chronological age and not informed by the tremendous knowledge gained in optimal assessment of older patients in recent years do not serve our patients well [23]. Finally, it is imperative to include functional assessment as an integral component of clinical trials designed for older patients [23].
Limits and Strengths
To our knowledge, the current analysis is the largest Spanish study comparing the survival of elderly patients receiving CCR in this clinical setting. Its mains strengths are the richness and robustness of date of real treatment world. But we know our study has limits. We know that there are included a few number of patients, only 120 (63 and 47). But we are only aware of one published phase II randomized prospective comparison of cisplatin and carboplatin-combinations. This was a study of only 65 patients by Wang, et al. [24], which found that EP had superior OS but similar PFS compared with CP not specifically for elderly patients.
Other limit is that is not currently possible to establish why young patients are unsuitable for cisplatin. We applied geriatric approach since 2004, so many reasons for carboplatin and monotherapy in elder patients was comorbidity (mainly cardiovascular). We had not applied a registry from young, so probably those young that received monotherapy or carboplatin also had comorbidity. Despite these limitations, we believe this study shows that there is considerable equipoise regarding which regimen should be preferred for elderly patients. Given the prevalence of unresectable stage III lung cancer on elderly patients, we believe a phase III randomized control trial should be considered to definitively answer this question. Pending the availability of such data, our results may help guide clinicians and patients trying to decide which chemotherapy regimen to pair with radiotherapy.
Conclusion
Our date shown that in clinical practice, aged patients received less intensive treatment for LA-NSCLC. But these dose reductions and less active schedules had not influenced the survival rate negatively. Elderly patients benefit from CRT, with schedules adjusted to age similar to a palliative intent. Although CRT was routinely been reserved for younger fit patients, the results of the present analysis have indicated that CRT can result in both PFS and OS benefits to elderly patients, unsuitable for cisplatin-combinations. The significant median PFS and OS in our clinical practice indicates that CRT, with the doses adjusted to a palliative intent, might be a practical and relevant treatment alternative for elderly patients.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
Ethics
Informed consent was obtained from all individual participants included in the study. The institution's ethical review board approved the study. The data base was approved by the institutional ethic committee of our hospital in 2004.
Funding
The study has no sponsor; no funding for design, data collection, data analysis, data interpretation nor writing of the report.
References
-
Santana-Davila R, Devisetty K, Szabo A, Sparapani R, Arce-Lara C, et al. (2015) Cisplatin and etoposide versus carboplatin and paclitaxel with concurrent radiotherapy for stage III non-small-cell lung cancer: an analysis of Veterans Health Administration data. J Clin Oncol 33: 567-574.
-
Eberhardt WE, De Ruysscher D, Weder W, Le Péchoux C, De Leyn P, et al. (2015) 2nd ESMO Consensus Conference in Lung Cancer: locally-advanced stage III non-small-cell lung cancer. Ann Oncol 26: 1573-1588.
-
Wilfried Ernst Erich Eberhardt (2015) Concurrent chemoradiotherapy in stage III non-small-cell lung cancer: what is the best regimen? J Clin Oncol 33: 532-533.
-
Curran WJ Jr, Paulus R, Langer CJ, Komaki R, Lee JS, et al. (2011) Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst 103: 1452-1460.
-
Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, et al. (1999) Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J ClinOncol 17: 2692-2699.
-
Aupérin A, Le Péchoux C, Pignon JP, Koning C, Jeremic B, et al. (2006) Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol 17: 473-483.
-
Wisnivesky JP, Strauss GM (2012) Treating elderly patients with stage III NSCLC. Lancet Oncol 13: 650-651.
-
Price A (2012) Emerging developments of chemoradiotherapy in stage III NSCLC. Nat Rev Clin Oncol 9: 591-598.
-
Belani CP, Choy H, Bonomi P, Scott C, Travis P, et al. (2005) Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol 23: 5883-5891.
-
Akerley W, Herndon JE Jr, Lyss AP, Choy H, Turrisi A, et al. (2005) Induction paclitaxel/carboplatin followed by concurrent chemoradiation therapy for unresectable stage III non-small-cell lung cancer: a limited-access study--CALGB 9534. Clin Lung Cancer 7: 47-53.
-
Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, et al. (2015) Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 16: 187-199.
-
Sacher AG, Le LW, Leighl NB, Coate LE (2013) Elderly patients with advanced NSCLC in phase III clinical trials: are the elderly excluded from practice-changing trials in advanced NSCLC? J Thorac Oncol 8: 366-368.
-
Repetto L (2003) Greater risks of chemotherapy toxicity in elderly patients with cancer. J Support Oncol 1: 18-24
-
Lee JH, Wu H-G, Kim HJ, Kim DW, Lee SH, et al. (2012) Influence of comorbidities on the efficacy of radiotherapy with or without chemotherapy in elderly stage III non-small cell lung cancer patients. Cancer Res Treat 44: 242-250.
-
Gridelli C (2012) Lung cancer: locally advanced NSCLC in the elderly: which treatment? Nat Rev Clin Oncol 9: 434-435.
-
Blanco JAG, Toste IS, Alvarez RF, Cuadrado GR, Gonzalvez AM, et al. (2008) Age comorbidity treatment decision and prognosis in lung cancer. Age Ageing 37: 715-718.
-
de Rijke JM, Schouten LJ, Velde ten GPM, Wanders SL, Bollen EC, et al. (2004) Influence of age, comorbidity and performance status on the choice of treatment for patients with non-smallcell lung cancer; results of a population-based study. Lung Cancer 46: 233-245.
-
Davidoff AJ, Tang M, Seal B, Edelman MJ (2010) Chemotherapy and survival benefit in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol 28: 2191-2197.
-
Beckett P, Callister M, Tata LJ, Harrison R, Peake MD, et al. (2012) Clinical management of older people with non-small cell lung cancer in England. Thorax 67: 836-839.
-
Gridelli C, Rossi A, Maione P (2008) Challenges treating older non-small cell lung cancer patients. Ann Oncol 19: 109-113.
-
Firat S, Pleister A, Byhardt RW, Gore E (2006) Age is independent of comorbidity influencing patient selection for combined modality therapy for treatment of stage III non small cell lung cancer (NSCLC). Am J Clin Oncol 29: 252-257.
-
Siegel R, Naishadham D, Jemal A (2012) Cancer Statistics 2012. CA Cancer J Clin 62: 10-29.
-
Owonikoko TK, Ramalingam SS (2015) Minimize toxicity or preserve efficacy? A delicate trade-off in the management of older patients with lung cancer. J Clin Oncol 33: 534-536.
-
Wang L, Wu S, Ou G, Bi N, Li W, et al. (2012) Randomized phase II study of concurrent cisplatin/etoposide or paclitaxel/carboplatin and thoracic radiotherapy in patients with stage III non-small cell lung cancer. Lung Cancer 77: 89-96.