Journal of Geriatric Medicine and Gerontology
Short Term Quality of Life Following Transcatheter Aortic Valve Replacement
Yaar Aga1*, Ximena Marincic2, Christian Marin y Kall2, Peter de Jaegere1 and Eduardo De Marchena2
1Department of Cardiology, Rotterdam, Erasmus MC, Thorax center, The Netherlands
2Department of Medicine, University of Miami, Miller School of Medicine, USA
*Corresponding author:
Yaar Aga, Erasmus Medical Center, Thoraxcenter, Department of Cardiology, 's-Gravendijkwal 230, 3015 CE Rotterdam, The Netherlands, E-mail: yaar.aga@gmail.com
J Geriatr Med Gerontol, JGMG-2-012, (Volume 2, Issue 1), Research Article; ISSN: 2469-5858
Received: January 30, 2016 | Accepted: March 29, 2016 | Published: April 01, 2016
Citation: Aga Y, Marincic X, Kall CMY, de Jaegere P, Marchena ED (2016) Short Term Quality of Life Following Transcatheter Aortic Valve Replacement. J Geriatr Med Gerontol 2:012. 10.23937/2469-5858/1510012
Copyright: © 2016 Aga Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Transcatheter aortic valve replacement (TAVR) is the recommended therapy for patients with severe calcific aortic stenosis (AS) who are not suitable candidates for surgery. The aim of this study is to evaluate the quality of life (QOL) for patients with severe AS with extreme and high surgical risk following TAVR with the Core Valve Medtronic prosthesis.
Methods: An analysis of patients who underwent TAVR with the Medtronic self- expanding Core Valve system (Medtronic, Inc.) at the University of Miami hospital was conducted between April 2011, and March 2014. Demographic and clinical data were obtained from inpatient and outpatient clinical records. QOL assessment was realized with the SF-12 Health-Survey. The questionnaire was administered at baseline, 6 months and 1 year after TAVR. Missing values were treated with median based imputation.
Results: A total of 71 patients underwent TAVR therapy. Of these, 63 patients successfully completed or partially completed the SF-12 questionnaire at 6 months follow up. Mean age was 85.0 ± 8.2 years and 65% were male. Eight patients (11.3%) died during follow up. After 6 months, a significant improvement in both scores (PCS 37.0; p < 0.001 and MCS 52.5 p < 0.001) was observed.
Conclusions: The main finding of this study is that TAVR in patients with severe AS is followed by early improvements in quality of life. The results show a marked improvement in functional status and a significant improvement in quality of life in both physical and mental components.
Introduction
Transcatheter Aortic Valve Replacement (TAVR) has emerged as an alternative treatment modality for patients with severe aortic stenosis (AS) who are at high or extreme risk for surgical aortic valve replacement (SAVR) [1]. Previous studies have shown that TAVR results in substantial reductions in symptoms and mortality [2-5]. Despite these health benefits, the assessment of quality of life (QOL) remains critical in these patients who often express quality of life over quantity of life [6]. The aim of this study is to evaluate the changes in quality of life in elderly patients with severe AS who underwent TAVR.
Methods and Patient selection
From April 2011 until March 2014, 71 consecutive patients were referred to the University of Miami Hospital for evaluation of TAVR. All patients eligible for the procedure had to be considered at high or extreme risk for SAVR if 2 cardiac surgeons and 1 interventional cardiologist at the clinical site estimated high (≥ 15% and ≤ 50%) or extreme (≥ 50%) risk for mortality or irreversible morbidity at 30 days with SAVR. Inclusion criteria were as follows: severe symptomatic aortic stenosis defined as an aortic valve area 0.8 cm2 or aortic valve index 0.5 cm2/m2 and either a mean aortic valve gradient > 40 mmHg or a peak aortic valve velocity > 4.0 m/s at rest or with a dobutamine stress if the left ventricular ejection fraction was < 50%.
Principal exclusion criteria were an active gastrointestinal hemorrhage within the prior 3 months, a major stroke within the prior 6 months, or a life expectancy < 1 year. All patients underwent TAVR via an iliofemoral, transaortic or subclavian approach, using the Medtronic self- expanding Core Valve system (Medtronic, Inc.). All patients gave their informed written consent after receiving complete information about risk and benefits of the study and procedure.
Quality of Life assessment
QOL was prospectively assessed using the SF-12 Health-Survey at baseline, 6 months and 1 year follow-up [7,8]. The SF-12 questionnaire consists of 12 questions, which measure functioning in 8 multi-item domains of daily life, such as: physical functioning, role limitation to physical health problems, bodily pain, general health, vitality as a measure of energy and fatigue, social functioning, role limitations due to emotional problems, and mental health related to psychological distress and psychological well-being. The Mental Component Summary (MCS) and the Physical Component Summary (PCS) are the 2 meta-scores of the SF-12, which combines the 8 domains and represent overall physical and mental functioning [8]. Higher SF-12 scores indicate better QOL, positive changes in SF-12 scores indicate improvement in QOL, and a negative change indicates deterioration. The scoring system and the interpretation of results of SF-12 has been explained elsewhere [8]. The questionnaires were administered by dedicated research staff to the participating patients during their screening for the TAVR procedure at the outpatient clinic and at their scheduled 6-months and 1-year clinical visit. After explaining the method and the purpose, the patients were then asked to complete the questionnaire.
Statistical Analysis
Continuous variables were presented as mean + standard deviations and compared with the use of the paired t-test or the Wilcoxon signed-rank test, as appropriate. Categorical variables were presented as counts and percentages and compared with the use of McNemar's test. A two-sided P-value of less than 0.05 was considered of statistical significance. Missing values were handled using multiple imputation. All data were processed using the Statistical Package for Social Sciences, version 15 (SPSS, Chicago, IL, USA).
Results
In this cohort 71 (100%) of the patients successfully underwent TAVR. In total 63 out of the 71 (89%) patients (67% male, median age 85 years) fully or partially completed SF-12 both at baseline and 6 months follow-up. The 30-day mortality was 2% and 8 (11.3%) patients had died at 6 months follow-up. In the all cohort, 1 patient died for cardiovascular causes, 2 for respiratory failure, 1 for aspiration pneumonia, 2 for sepsis and 2 for cancer. The remaining 6 patients were not able or willing to return to follow-up. For these 6 patients, multiple imputation was used for the missing data. Patients with missing SF-12 data at 6 months tended to be sicker than those is the analytic cohort, with more lung disease and higher STS mortality scores and euroscore (Figure 1) shows the postoperative follow-up of the entire cohort. Of the total of 63 analyzed, 51 patients underwent TAVR via iliofemoral approach, 7 via transaortic approach and 4 via subclavian approach. Baseline characteristics of the patients are summarized in (Table 1).
![]()
Table 1: Baseline patient characteristics.
View Table 1
Clinical Outcomes
There was a significant difference in mean gradient (49.1 ± 11.1 to 10.0 ± 4.5 mmHg; p < 0.001) one day after TAVR compared to baseline. Echocardiographic parameters 6 months after TAVR showed a significant difference in mean gradient (49.1 ± 11.1 to 8.4 ± 6.4 mmHg; p < 0.001) and aortic valve area (0.60 ± 0.17 to 1.65 ± 0.45 cm2; p < 0.001) compared to baseline. The left ventricular ejection fraction increased after 6 months, but this change did not achieve statistical significance (61 ± 11 to 63 ± 8 p = 0.4741) Seventeen (27%) of the patients required a new PPM within 30 days after TAVR. The reason for PPM was a new onset complete heart block in 7 (11%) of the patients. Ten patients (16%) developed a new left bundle branch block within one month after the procedure. Two patients (3%) suffered from a non-fatal stroke and remained event-free during the 6 month follow up. NYHA class improved from 2.5 ± 0.6 to 1.3 ± 0.5 after 1 month and from 2.5 ± 0.6 to 1.2 ± 0.5 (P < 0.001) after 6 months when compared to baseline. The average post-procedural length of stay was 6.4 ± 2.3 days. Other clinical outcomes of the studied patients are reported in (Table 2).
![]()
Table 2: Clinical outcomes within 30 days.
View Table 2
Quality of Life
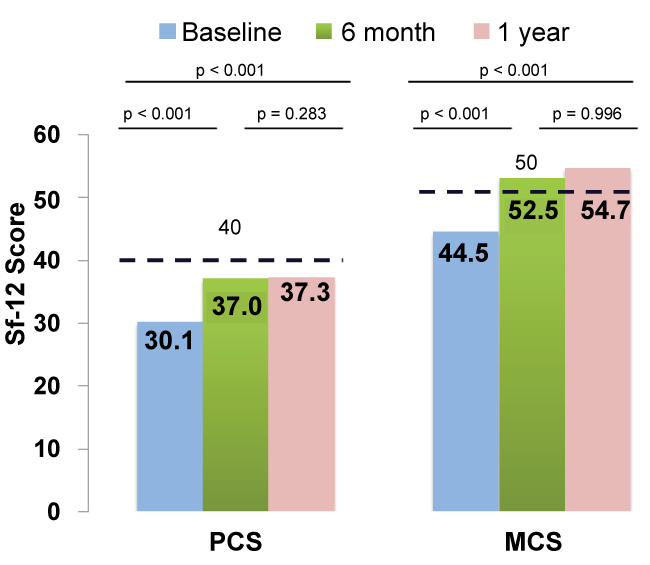
Mean pre-procedural SF-12 scores showed an impairment of perceived quality of life compared with general U.S. population > 75 years, both for physical (baseline-PCS 30.1 vs 40.0) and mental scores (baseline-MCS 44.5 vs. 50.0) [9]. Summary scores of quality of life at both baseline and at follow-up are schematized in (Figure 2). Mean pre-procedural PCS and MSC of SF-12 showed a significant improvement after 6 months of follow-up (30.1 vs 37.0, p = < 0.001; 44.5 vs 52.5, p = < 0.001).
.
Figure 2: SF-12 summary component scores at baseline, 6 months (n = 63) and preliminary 1-year (n = 46) results after TAVR. Means of a USA standard population are indicated (dashed line).
View Figure 2
Forty-six (65%) of the patients completed 1 year follow up by the time of inclusion. Preliminary results are schematized in (Figure 2).
Discussion
The main finding of this single center observational study is that the quality of life in both physical and mental component significantly improved 6 months after TAVR in patients with severe aortic stenosis with excessive surgical risk. Pre-procedural SF-12 scores of this cohort showed impairment in quality of life compared with the general USA population over the age of 75 years, both for mental and physical scores. Higher MCS score at 6-month follow up might be related to the relative absence of cognitive disorders in our population.
The present study adds additional evidence regarding QOL following TAVR and the results are consistent with previous published studies on this topic [10-17]. Correspondingly to an improved in QOL, an improvement in functional class and echocardiographic parameters was achieved. Eight (11.3%) patients did not reach 6 months survival threshold, this is comparable with previous published literature on QOL and TAVR [10-12,18].
QOL changes after TAVR have to be assessed to guide clinical decision-making and in particular in this patient population. In this population, clinicians are often challenged with a heterogeneous group of individuals in terms of life expectancy, presence of comorbidities, and degree of disability. Also, these patients may have different expectation of treatment than younger or less sick patients; quality of life may outweigh quantity of life after treatment. The current population showed a high frequency of comorbidities in patients selected for TAVR (Table 1). The presence of multiple comorbidities could limit QOL improvements despite an effective restoration of AS by valve replacement. Arnold et al. found that patients with poorer baseline health status assessed by the KCCQ tended to have more comorbidities, higher estimated mortality risk, and worse mobility [19]. Thus, confirming the importance of using QOL changes in clinical practice to guide decision making. To further emphasize this, a larger study is needed to identify predictors of either worsening or improvement of QOL that may help stratify patients for treatment when QOL is the main treatment goal.
The SF-36 has been used widely in cardiac patients, and has been used in studies investigating changes in QOL in patients older than 80 years after surgical aortic valve replacement (SAVR) [20]. Although it does not contain disease specific questions, it is a validated measurement of overall physical and mental health status. However, the SF-36 contains 36 items and therefore places a considerable burden on both patients and investigators [21]. In this study the widely accepted QOL assessment instrument, the SF-12, was used because of it is easy to understand, short and high feasible [8]. All these factors reduce the respondent burden and improve the response rate. Previous studies assessing the QOL following TAVR using the SF-12 showed similar results [11,13-15], thus confirming the results of the present study.
Study limitations
The small sample size of study population and the single-site data collection are limitations of the present study. Because of the small cohort we were not able to identify predictors for patient selection regarding the worsening or improvement of QOL. The single-site data collection potentially limits the generalizability of the present study.
Another limitation of the present study is missing data due to drop-out or incomplete questionnaires. These missing patients tended to be sicker and had a higher risk for morbidity and mortality. Therefore, we may have overestimated the results. We attempted to address this limitation by performing a multiple imputation of the missing data. The reported results encompass a follow-up period of only 6 months. The 6 months period might have been too short to establish whether the QOL keeps improving, worsens or reaches a plateau. The extrapolations from our own data suggest that after one year, there might be a greater improvement in the mental score and physical score, however these changes are not significant. Hence, further inclusion of patients and longer follow up is needed to observe the longer-term dynamics of QOL after TAVR.
Conclusion
The main finding of this study is that TAVR in patients with severe AS is followed by early improvements in quality of life. The results show a marked improvement in functional status and a significant improvement in quality of life in both physical and mental components. The question whether this effect will last in the longer term remains to be determined.
References
-
Joint Task Force on the Management of Valvular Heart Disease of the European Society of C, European Association for Cardio-Thoracic S, Vahanian A, Alfieri O, Andreotti F, et al. (2012) Guidelines on the management of valvular heart disease (version 2012). European heart journal 33: 2451-2196.
-
Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, et al. (2012) Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. The New England journal of medicine 366: 1696-1704.
-
Leon MB, Smith CR, Mack M, Miller DC, Moses JW, et al. (2010) Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. The New England journal of medicine 363: 1597-1607.
-
Rodes-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, et al. (2010) Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. Journal of the American College of Cardiology 55: 1080-1090.
-
Reynolds MR, Magnuson EA, Wang K, Thourani VH, Williams M, et al. (2012) Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial (Cohort A). Journal of the American College of Cardiology 60: 548-558.
-
Gurvitch R, Webb JG (2010) Life after transcatheter aortic valve implantation: quality still matters. Heart. 96: 1083-1084.
-
Newnham EA, Harwood KE, Page AC (2007) Evaluating the clinical significance of responses by psychiatric inpatients to the mental health subscales of the SF-36. Journal of affective disorders 98: 91-97.
-
Ware J Jr, Kosinski M, Keller SD (1996) A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical care 34: 220-233.
-
U.S. Population Norms (1998).
-
Bekeredjian R, Krumsdorf U, Chorianopoulos E, Kallenbach K, Karck M, et al. (2010) Usefulness of percutaneous aortic valve implantation to improve quality of life in patients > 80 years of age. The American journal of cardiology 106: 1777-1781.
-
Georgiadou P, Kontodima P, Sbarouni E, Karavolias GK, Smirli A, et al. (2011) Long-term quality of life improvement after transcatheter aortic valve implantation. American heart journal 162: 232-237.
-
Krane M, Deutsch MA, Piazza N, Muhtarova T, Elhmidi Y, et al. (2012) One-year results of health-related quality of life among patients undergoing transcatheter aortic valve implantation. The American journal of cardiology 109: 1774-1781.
-
Osnabrugge RL, Arnold SV, Reynolds MR, Magnuson EA, Wang K, Gaudiani VA, et al. (2015) Health Status After Transcatheter Aortic Valve Replacement in Patients at Extreme Surgical Risk: Results From the CoreValve U.S. Trial. JACC Cardiovascular interventions 8: 315-323.
-
Ussia GP, Barbanti M, Cammalleri V, Scarabelli M, Mule M, et al. (2011) Quality-of-life in elderly patients one year after transcatheter aortic valve implantation for severe aortic stenosis. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 7: 573-579.
-
Ussia GP, Mule M, Barbanti M, Cammalleri V, Scarabelli M, et al. (2009) Quality of life assessment after percutaneous aortic valve implantation. European heart journal 30: 1790-1796.
-
Reynolds MR, Magnuson EA, Lei Y, Leon MB, Smith CR, et al. (2011) Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation 124: 1964-1972.
-
Tully PJ, Roshan P, Rice GD, Sinhal A, Bennetts JS, et al. (2015) Change in quality of life after transcatheter aortic valve implantation and aortic valve replacement surgery in Australian patients aged >/= 75 years: the effects of EuroSCORE and patient operability. Journal of geriatric cardiology : JGC 12: 30-36.
-
Goncalves A, Marcos-Alberca P, Almeria C, Feltes G, Hernandez-Antolin RA, et al. (2013) Quality of life improvement at midterm follow-up after transcatheter aortic valve implantation. International journal of cardiology 162: 117-122.
-
Arnold SV, Spertus JA, Vemulapalli S, Dai D, O'Brien SM, et al. (2015) Association of Patient-Reported Health Status With Long-Term Mortality After Transcatheter Aortic Valve Replacement: Report From the STS/ACC TVT Registry. Circulation Cardiovascular interventions 8.
-
Lam BK, Hendry PJ (2004) Patients over 80 years: quality of life after aortic valve replacement. Age and ageing 33: 307-309.
-
Iglesias C, Torgerson D (2000) Does length of questionnaire matter? A randomised trial of response rates to a mailed questionnaire. Journal of health services research & policy 5: 219-221.