Journal of Genetics and Genome Research
Analysis of the Methylation Pattern of SOX2 and OCT4 Genes in Astrocytomas
Wallax Augusto Silva Ferreira1*, Mariana Diniz Araujo1, Nilson Praia Anselmo1, Maria Lucia Harada1 and Barbara do Nascimento Borges1,2
1Molecular Biology Laboratory, Biological Science Institute, Federal University of Para, Brazil
2Center of Agropecuary Technology, Socio-environmental and Hydric Resources Institute, Federal Rural University of Amazonia, Brazil
*Corresponding author:
Wallax Augusto Silva Ferreira. Molecular Biology Laboratory, Biological Science Institute, Federal University of Para, Rua Augusto Corrêa, 01, Zip Code: 66075-110, Bairro: Guama, Belém, Para, Brazil, E-mail: wallaxaugusto@gmail.com
J Genet Genome Res, JGGR-2-012, (Volume 2, Issue 1), Research Article; ISSN: 2378-3648
Received: February 06, 2015 | Accepted: February 25, 2015 | Published: February 27, 2015
Citation: Ferreira WAS, Araujo MD, Anselmo NP, Harada ML, Borges BN (2015) Analysis of the Methylation Pattern of SOX2 and OCT4 Genes in Astrocytomas. J Genet Genome Res 2:012. 10.23937/2378-3648/1410012
Copyright: © 2015 Ferreira WAS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Astrocytoma is a common aggressive intracranial tumor and a formidable challenge in clinic. Association of the altered DNA methylation pattern of the promoter CpG islands has been found in many human tumors. OCT4 and SOX2 are essential transcription factors for embryonic development and play key roles in determining the fate of stem cells. In this study, we aimed to investigate the methylation profiles of SOX2 and OCT4 genes in astrocytomas samples of Pará state. The methylation status of SOX2 and OCT4 genes was examined by methylation-specific polymerase chain reaction (MS-PCR) in 31 samples. At least in the investigated CpG island of SOX2 and OCT4 genes, we found that both promoters are methylated. Understanding these epigenetic mechanisms can lead to better prognostic tools and new drug targets for tumors of the central nervous system.
Keywords
Gliomas, MSP-PCR, Tumors of central nervous system
Introduction
Astrocytomas are malignant and prevalent intracranial tumours that comprise the majority of primary central nervous system tumors in adults, account for nearly 75% of neuroepithelial tumors [1]. They are classified according to the WHO malignancy scale, into low-grade astrocytoma (WHO Grade I and II, AI and AII), anaplastic astrocytoma (WHO Grade III, AIII), and glioblastomamultiforme (WHO Grade IV, GBM).
Epigenetic markers, as DNA promoter methylation, can regulate thegene expression without altering the gene coding sequence [2]. One of the features of carcinogenesis is the specific hypermethylation of CpG islands within the promoter of some genes, which commonly results in the silencing of these genes leading to cell growth, proliferation and ultimately to the formation of invasive tumor and metastasis [3,4].
To date, a number of genetics and epigenetics alterations have been correlated with astrocytic tumorigenesis [5-7], however a deep understanding of the molecular basis of this tumour is still far away, and the search for novel prognostic or predictive molecular indicators are still the primary goal for the improvement of its clinical management [8].
OCT4/POU5F1 (octamer DNA binding transcription factor 4) is an important member of the POU (Pit, Oct, Uncl) domain transcription factors encoded by POU5F1 gene (6p21.31), with, at least, three variants (A, B, and B1)produced by alternative splicing [9]. OCT4 performs an important role maintaining the cellular plasticity and promoting the self-renewal and the proliferation of pluripotent embryonic stem and germ cells in collaboration with other proteins, such as SOX2 (SRY-box 2), NANOG (Nanoghomeobox), and KLF4 (Kruppel-like factor 4) [Burdon, Niwa]. To date, many reports found that OCT4 is highly expressed in several tumors [10,11] and its expression profile has been correlated with tumor grade and disease progression and is associated with a worse prognosis [12-15]. Therefore, the high expression of OCT4 is considered as a hallmark of cancer stem cells [16,17].
SOX2 is a transcription factor belonging to the sex determining region Y-box family [18], which is expressed in a wide variety of tissues and play important roles in the regulation of organ development, cell type specificity [19], and in the pluripotency maintenance of cancer stem cells (CSCs) in self-renewal and differentiation [20]. Increased expression of SOX2 has been reported in a growing list of tumors, including lung cancer, esophageal carcinoma, pancreatic carcinoma, breast cancer, ovarian carcinoma, hepatocellular carcinoma and head and neck cancers [21-26]. In particular, the SOX2 expression is important for the maintenance and development of the central nervous system tumors [27,28], and some studies present evidences that SOX2 expression is positively correlated with the malignancy grade in brain tumors [29-31]. Recently, Jesse et al. [27] suggested that an increasing expression of SOX2 during brain tumor progression are likely to be closely linked with changes in other critical genes that work in concert with SOX2 to enhance the tumorigenicity of brain tumors.
Although in recent years a considerable number of studies have been carried out on the OCT4 and SOX2 expressionand methylation in various tumors and proposed as useful markers of these tumors [32], little is known about their methylation pattern in astrocytomas. In this study, we aimed to identify the SOX2 and OCT4 gene promoter methylation signatures in astrocytomasin a population in the northern Brazil (Belém, Para state) to verify the possible association between the methylation statusof these genes with clinicopathological features.
Material and Methods
This study involved 31 tissue samples from astrocitomas (Table 1), obtained by surgical resections from patients who underwent craniotomy at Ofir Loyola Hospital, from 2005 to 2009, in Belém (Para state). All samples were classified according to the WHO (World Health Organization) classification criteria [33]. All procedures were approved by the Ethics Committee of the involved hospital.All tissue specimens after dissection were snap-frozen and stored with RNAlater™ Storage Solution (Sigma-Aldrich) at -80°C until analysis. Genomic DNA was extracted from tissues using the phenol-chloroform protocol as described by Sambrook and Russell [34].
![]()
Table 1: Clinical characteristics of patient/tumor samples used for MSP-PCR
View Table 1
Bisulfite treatment of DNA samples was performed as previously described by Herman et al. [35]. The methylation and unmethylation-sensitive primers used in this study were previously described [36,37] (Table 2). 1μl of bisulfite-converted DNA was amplified in a 25μl reaction mixture containing 1.25mM dNTPs, 2.5μl of 1x reaction buffer, 2.5mM MgCl2, 0.5 mM forward and reverse of both genes primers, and 0.03U/μL of Taq DNA polymerase (Invitrogen). Universal methylated human male genomic DNA (Intergen, New York, NY, USA) was used as the positive control.
![]()
Table 2: Sequences of primers used in SOX2 and OCT4 methylation-specific PCR
View Table 2
The MS-PCR profile for both genes was conducted as following steps: pre-denatured for 4 min at 94°C, then at 94°C for 30 seconds, 55°C for 30 seconds, 72°C for 30 seconds for 40 cycles, and finally a 10-min extension at 72°C. Polymerase chain reaction products were separated on 3% Tris-borate EDTA agarose gels, stained with ethidium bromide and visualized under a UV transilluminator. Cases detected with the presence of methylated alleles were repeated once for confirmation.
For statistical analysis, we grouped the samples in data groups based on the histopathological classification of WHO, which were low-grades (I and II OMS grades) and high-grades (III and IV grades). Data were analyzed using Fisher's exact test, with p ≤ 0.05 being considered as statistically significant and performed with BioEstat 5.0 [38].
Results and Discussion
Of the 31 analyzed samples of astrocytomas patients, 13 were males and 18 females. The median age was 40.36 years (ranging from 3 to 71 years). Table 1 presents a summary of sex, age, tumor stage and histological grade.
For the SOX2 gene, our results show this gene is methylated in 70.96% of tumor tissues (22/31 cases) (Figure 1). There was no statistically significant difference in the frequencies of hypermethylated SOX2 gene promoter samples with clinicopathologic variables, age and sex (Table 3).
![]()
Table 3: Associations between demographic and clinical data of patients and methylation of SOX2 and OCT4 genes
View Table 3
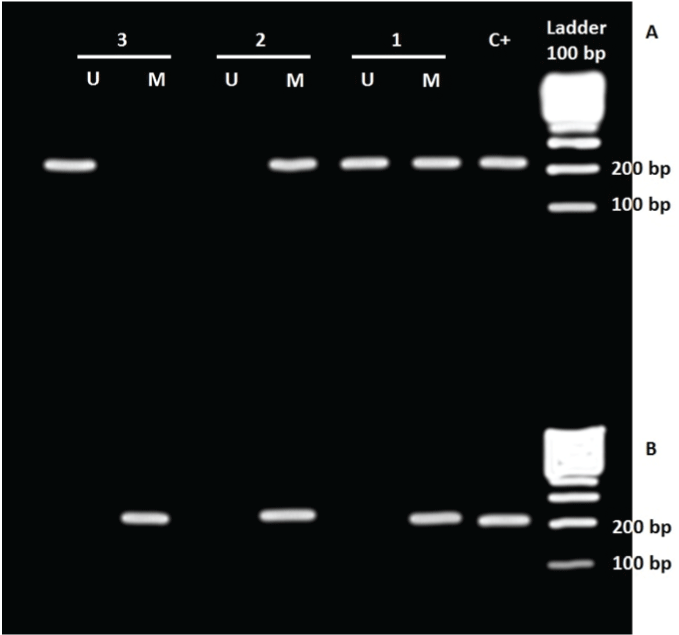
Figure 1: MSP analysis of the promoter CpG islands of SOX2 (A) and OCT4 (B) genes in astrocytomas. C+: positive control, U: unmethylated, M: Methylated.
Numbers above the figure represent patients 1=patient 3; 2=patient 9; 3=patient 23 (See Table 1)
View Figure 1
For the OCT4 gene, we detected that this gene was hypermethylated in 96.77% of tumor tissues (30 of 31 cases) (Figure 1). Similarly to the SOX2 gene, there was no statistically significant difference in the frequencies of methylated OCT4 gene promoter with clinicopathologic variables (Table 3).
Astrocytic tumors are the most common type of intrinsic brain tumors. They show a tendency for progression toward a more malignant phenotype [39], and the average survival of patients with aggressive forms of gliomas is less than 2 years [40]. Therefore, anadequate diagnosis and treatment of these brain tumors presents the major challenge in neuro-oncology today.
Promoter CpG methylation has an important role in controlling gene transcription and therefore contributes to the regulation of many biological processes. In cancer, aberrant DNA methylation is associated with initiation and progression of malignant disease. Therefore, the DNA methylation patterns could be used to improve cancer diagnosis and/or prognosis [41]. However, in spite of clinical research progress, there are few epigenetic biomarkers for astrocytoma diagnosis [42].
OCT4 (also known as Oct-3 and POU5F1), is a transcription factor involved in regulation of cell growth and differentiation [43,44]. OCT4, as well as SOX2 and Nanog, plays a pivotal role in the regulation and maintenance of pluripotency. In recent studies, OCT4 expression has been detected in various carcinomas including breast, prostate, bladder, head and neck squamous cell carcinomas and lung adenocarcinoma, which correlates with an unfavorable prognosis [15,45-47]. Furthermore, considerable studies indicate the DNA methylation of the OCT4 at the gene regulatory region is a key factor in OCT4 transcription [48].
Here, our results suggest, at least in the investigated CpG sites of OCT4 gene promoter, a persistent hypermethylation event in all astrocytomas analyzed. It is well-established that methylation of CpG dinucleotides is a common mechanism for the silencing of OCT4 expression within its promoter, the proximal enhancer and distal enhancer regions [49-51]. Lee et al. [51] showed that OCT4 gene is progressively methylated during the in vivo maturation of neural stem cells in the neuroepithelium of the central nervous system, coincident with the downregulation of its expression. It has also been shown previously that OCT4 expression can be induced by treatment of adult neural stem cells with the DNA methyltransferase inhibitor, 5-azacytidine and histone deacetylase inhibitor [52].
OCT4 promoter demethylationhasalreadybeenreportedtocontributetotumorigenesis [37,53]. In primary gliomas, the methylation levels of the OCT4 gene is notably reduced as compared to the normal group and is lower in high-grade gliomas than in low-grade ones [54]. Ontheotherhand, the difference between our results and those presented by Shi et al. [54] can be associated with different techniques employed, as well it is also possible that OCT4 was upregulated by hypomethylation of other CpG islands in the promoter regions of OCT4 that were not tested in this study.
Another gene evaluated was SOX2, a self-renewal transcription factor crucial to pluripotency maintenance in embryonic stem cells (ESCs) [55,56] expressed during various phases of embryonic development, which affects cell fate and differentiation. Increased expression of the SOX2 has been reported in several tumors and both epigenetic and genetic factors, particularly gene amplification, have been identified as frequent causes of SOX2 overexpression [57,58]. Schoenhals et al. [59] compared the expression of OCT4, SOX2, KLF4 and C-MYC in 40 human tumor types and their normal tissue counterparts using publicly available gene expression data, and found a significant overexpression of at least one of the pluripotency factors in 18 out of the 40 cancer types investigated. According this study, SOX2 was significantly overexpressed only in grade IV compared to grade II and III of gliomas. This pattern was corroborated by Alonso et al. [58], which evaluated the expression and methylation status of SOX2 in glioblastomamultiforme (GBM) and found that SOX2 promoter was hypomethylated in all the patient samples when compared to normal cell lines, correlating this data with high SOX2 protein levels and mRNA overexpression in 90% of the samples, suggesting that this gene could be used as a therapeutic target in GBM.
Nevertheless, our results suggest that SOX2 is hypermethylated in 86.36% of the samples, corroborating with other studies in several tumors. Wong et al. [60], who used the MSP-PCR technique to study the methylation profiles of SOX2 in endometrial carcinomas, observed that this gene was methylated in 37.5% (27/72) of the samples, and with a significant correlation between its mRNA expression, hypermethylation, and shorter survival of patients. SOX2 hypermethylation and downregulation has been reported in gastric cancers in association with effect on cell growth and patients' survival [61]. Moreover, the hypermethylation in the promoter region of SOX2 was demonstrated in hydatidiform moles and choriocarcinomas when compared with normal placentas in association with reduced RNA expression [36]
In conclusion, while no statistically significant changes between promoter methylation of both genes with clinicopathological features were found using methylation-specific PCR, we found that both genes are hypermethylated in samples of astrocytomas of the patients of Belém, of Para state - Brazil. It is clear, however, that more robust techniques such as pyrosequencing or promoter methylation array must be employed to be able to detect marginal but possibly meaningful differences in methylation.
Acknowledgments
This work was supported by the Fundação de Amparo à Pesquisa do Estado do Para (FAPESPA) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
-
Walker DG, Kaye AH (2001) Diagnosis and management of astrocytomas, oligodendrogliomas and mixed gliomas: a review. Australas Radiol 45: 472-482.
-
Hatada I, Fukasawa M, Kimura M, Morita S, Yamada K, et al. (2006) Genome-wide profiling of promoter methylation in human. Oncogene 25: 3059-3064.
-
Chik F, Szyf M, Rabbani SA (2011) Role of epigenetics in cancer initiation and progression. Adv Exp Med Biol 720: 91-104.
-
HanahanD, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646-674.
-
Chosdol K, Misra A, Puri S, Srivastava T, Chattopadhyay P, et al. (2009) Frequent loss of heterozygosity and altered expression of the candidate tumor suppressor gene 'FAT' in human astrocytic tumors. BMC Cancer 9:5.
-
Oji Y, Suzuki T, Nakano Y, Maruno M, Nakatsuka S, et al. (2004) Overexpression of the Wilms' tumor gene W T1 in primary astrocytic tumors. Cancer Sci 95: 822-827.
-
Shiraishi S, Tada K, Nakamura H, Makino K, Kochi M, et al. (2002) Influence of p53 mutations on prognosis of patients with glioblastoma. Cancer 95: 249-257.
-
Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, et al. (2007) Malignant astrocyticglioma: genetics, biology, and paths to treatment. Genes Dev 21: 2683-2710.
-
Gazouli M, Roubelakis MG, Theodoropoulos GE, Papailiou J, Vaiopoulou A, et al. (2012) OCT4 spliced variant OCT4B1 is expressed in human colorectal cancer. Mol Carcinog 51: 165-173.
-
Du Z, Jia D, Liu S, Wang F, Li G, et al. (2009) Oct4 is expressed in human gliomas and promotes colony formation in glioma cells. Glia 57: 724-733.
-
Friedman S, Lu M, Schultz A, Thomas D, Lin RY (2009) CD133+ anaplastic thyroid cancer cells initiate tumors in immunodeficient mice and are regulated by thyrotropin. PLoS One 4: e5395.
-
Huang P, Chen J, Wang L, Na Y, Kaku H, et al. (2012) Implications of transcriptional factor, OCT-4, in human bladder malignancy and tumor recurrence. Med Oncol 29: 829-834.
-
Rijlaarsdam MA, van Herk HADM, Gillis AJM, Stoop H, Jenster G, et al. (2012) Specific detection of OCT3/4 isoform A/B/B1 expression in solid (germ cell) tumours and cell lines: confirmation of OCT3/4 specificity for germ cell tumours. Br J Cancer 105: 854-863.
-
Zhao PP, Liu CX, Xu K, Zheng SB, Li HL, et al. (2012) Expression of OCT4 protein in bladder cancer and its clinicopathological implications. Nan Fang Yi Ke Da Xue Xue Bao 32: 643-656
-
Zhang X, Han B, Huang J, Zheng B, Geng Q, et al. (2010) Prognostic significance of OCT4 expression in adenocarcinoma of the lung. Jpn J Clin Oncol 40: 961-966
-
Liu D, Zhou P, Zhang L, Gong W, Huang G, et al. (2012) HDAC1/DNMT3A-containing complex is associated with suppression of Oct4 in cervical cancer cells. Biochemistry (Mosc) 77: 934-940.
-
Liu D, Zhou P, Zhang L, Wu G, Zheng Y, et al. (2011) Differential expression of Oct4 in HPV-positive and HPV-negative cervical cancer cells is not regulated by DNA methyltransferase 3A. Tumour Biol 32: 941-950.
-
Cavallaro M, Mariani J, Lancini C, Latorre E, Caccia R, et al. (2008) Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development 135: 541-557.
-
Li X, Wang J, Xu Z, Ahmad A, Li E, et al. (2012) Expression of sox2 and oct4 and their clinical significance in human non-small-cell lung cancer. Int J Mol Sci 13: 7663-7675.
-
Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, et al. (2014) SOX2 controls tumour initiation and cancer stem-cell functions in squamous- cell carcinoma. Nature 511: 246-250.
-
Cai YR, Zhang HQ, Qu Y, Mu J, Zhao D, et al. (2011) Expression of MET and SOX2 genes in non-small cell lung carcinoma with EGFR mutation. Oncol Repo 26: 877-885.
-
Dong Z, Liu G, Huang B, Sun J, Wu D (2014) Prognostic significance of SOX2 in head and neck cancer: a meta-analysis. Int J Clin Exp Med 7: 5010-5020.
-
Gen Y, Yasui K, Nishikawa T, Yoshikawa T (2013) SOX2 promotes tumor growth of esophageal squamous cell carcinoma through the AKT/ mammalian target of rapamycin complex 1 sig- naling pathway. Cancer Sci 104: 810- 816.
-
Lengerke C, Fehm T, Kurth R, Neubauer H, Scheble V, et al. (2011) Expression of the embryonic stem cell marker SOX2 in early-stage breast carcinoma. BMC Cancer 11: 42.
-
Sun C, Sun L, Li Y, Kang X, Zhang S, et al. (2013) Sox2 expression predicts poor survival of he- patocellular carcinoma patients and it pro- motes liver cancer cell invasion by activating Slug. Med Oncol 30: 503.
-
Yang Z, Pan X, Gao A, Zhu W (2014) Expression of Sox2 in cervical squamous cell carcinoma. J BUON 19: 203-206.
-
Jesse L. Cox, Phillip J. Wilder, Michelle Desler, Angie Rizzino (2012) Elevating SOX2 Levels Deleteriously Affects the Growth of Medulloblastoma and Glioblastoma Cells. PLoS One 7: e44087.
-
Wegner M, Stolt C (2005) From stem cells to neurons and glia: A soxist's view of neural development. Trends Neurosci 28: 583-588.
-
Eschbacher JM, Yeh RF, Smirnov I, Feuerstein B, Coons S (2008) SOX2: A glioma-specific marker and a potential target for therapy. FASEB J 22: 706.18.
-
Ma YH, Mentlein R, Knerlich F, Kruse ML, Mehdorn HM, et al. (2008) Expression of stem cell markers in human astrocytomas of different WHO grades. J Neurooncol 86: 31-45.
-
Schmitz M, Temme A, Senner V, Ebner R, Schwind S, et al. (2007) Identification of SOX2 as a novel glioma-associated antigen and potential target for T-cell-based immunotherapy. Br J Cancer 96: 1293-1301.
-
Yang S, Zheng J, Ma Y, Zhu H, Xu T, et al. (2012) Oct4 and Sox2 are overexpressed in human neuroblastoma and inhibited by chemotherapy. Oncol Rep 28: 186-192
-
David NL, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, et al. (2007) The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol 114: 97-109.
-
Sambrook J, Russell DW (2000) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press.
-
Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB (1996) Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 93: 9821-9826.
-
Li AS, Siu MK, Zhang H, Wong ES, Chan KY, et al. (2008) Hypermethylation of SOX2 gene in hydatidiform mole and choriocarcinoma. Reprod Sci 15: 735-744.
-
Zhang HJ, Siu MK, Wong ES, Wong KY, Li AS, et al. (2008) Oct4 is epigenetically regulated by methylation in normal placenta and gestational trophoblastic disease. Placenta. 29: 549-554.
-
Ayres M, Ayres JRM, Ayres DL, Santos AS (2007) BioEstat 5.0-Aplicações EstatísticasnasÁreas das Ciências Biológicas e Médicas: Sociedade Civil Mamirauá, Belém. CNPq, Brasília.
-
Kleihues P, Soylemezoglu F, Schauble B, Scheithauer BW, Burger PC (1995) Histopathology, classification, and grading of gliomas. Glia 15: 211-221.
-
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, et al. (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987-996.
-
Dehan P, Kustermans G, Guenin S, Horion J, Boniver J, et al. (2009) DNA methylation and cancer diagnosis: new methods and applications. Expert Rev Mol Diagn 9: 651-657.
-
Yu J, Zhang H, Gu J, Lin S, Li J, et al. (2004) Methylation profiles of thirty four promoter-CpG islands and concordant methylation behaviours of sixteen genes that may contribute to carcinogenesis of astrocytoma. BMC Cancer 4: 65.
-
Freberg CT, Dahl JA, Timoskainen S, Collas P (2007) Epigenetic reprogramming of OCT4 and NANOG regulatory regions by embryonal carcinoma cell extract. Mol Biol Cell 18: 1543-1553.
-
Babaie Y, Herwig R, Greber B, Brink TC, Wruck W, et al. (2007) Analysis of Oct4-dependent transcriptional networks regulating self-renewal and pluripotency in human embryonic stem cells. Stem Cells 25: 500-510.
-
Ezeh UI, Turek PJ, Reijo RA, Clark AT (2005) Human embryonic stem cell genes OCT4, NANOG, STELLAR, and GDF3 are expressed in both seminoma and breast carcinoma. Cancer 104: 2255-2265.
-
Kastler S, Honold L, Luedeke M, Kuefer R, Moller P, et al. (2010) POU5F1P1, a putative cancer susceptibility gene, is overexpressed in prostatic carcinoma. Prostate 70: 666-674.
-
Lim YC, Oh SY, Cha YY, Kim SH, Jin X, et al. (2010) Cancer stem cell traits in squamospheres derived from primary head and neck squamous cell carcinomas. Oral Oncol 47: 83-91.
-
Cantz T, Key G, Bleidissel M, Gentile L, Han DW, et al. (2008) Absence of OCT4 expression in somatic tumor cell lines. Stem cells 26: 692-697.
-
Feldman N, Gerson A, Fang J, Li E, Zhang Y, et al. (2006) G9a-mediated irreversible epigenetic inactivation of Oct-3/4 during early embryogenesis. Nat Cell Biol 8: 188-194.
-
Li JY, Pu MT, Hirasawa R, Li BZ, Huang YN, et al. (2007) Synergistic function of DNA methyltransferases Dnmt3a and Dnmt3b in the methylation of Oct4 and Nanog. Mol Cell Biol 27: 8748-8759.
-
Lee SH, Jeyapalan JN, Appleby V, Mohamed Noor DA, Sottile V, et al. (2010) Dynamic methylation and expression of Oct4 in early neural stem cells. J Anat 217: 203-213.
-
Ruau D, Ensenat-Waser R, Dinger TC, Vallabhapurapu DS, Rolletschek A, et al. (2008) Pluripotency associated genes are reactivated by chromatin-modifying agents in neurosphere cells. Stem Cells 26: 920-926.
-
Hoffmann MJ, Müller M, Engers R, Schulz WA (2006) Epigenetic control of CTCFL/BORIS and OCT4 expression in urogenital malignancies. Biochem Pharmacol 72: 1577-1588.
-
Shi J, Shi W, Ni L, Xu X, Su X, et al. (2013) OCT4 is epigenetically regulated by DNA hypomethylation of promoter and exon in primary gliomas. Oncol Rep 30: 201-206.
-
Rao RR, Calhoun JD, Qin X, Rekaya R, Clark JK, et al. (2004) Comparative transcriptional profiling of two human embryonic stem cell lines. Biotechnol Bioeng 88: 273-286.
-
Wang J, Rao S, Chu J, Shen X, Levasseur DN, et al. (2006) A protein interaction network for pluripotency of embryonic stem cells. Nature 444: 364-368.
-
Pietanza MC, Ladanyi M (2012) Bringing the genomic landscape of small-cell lung cancer into focus. Nat Genet 44: 1074-1075.
-
Alonso MM, Diez-Valle R, Manterola L, Rubio A, Liu D, et al. (2011) Genetic and epigenetic modifications of Sox2 contribute to the invasive phenotype of malignant gliomas. PLoS One. 6: e26740.
-
Schoenhals M, Kassambara A, De Vos J, Hose D, Moreaux J, et al. (2009) Embryonic stem cell markers expression in cancers. Biochem Biophys Res Commun 383: 157-162.
-
Wong OG, Huo Z, Siu MK, Zhang H, Jiang L, et al. (2010) Hypermethylation of SOX2 Promoter in Endometrial Carcinogenesis. Obstet Gynecol Int pii: 682504.
-
Otsubo T, Akiyama Y, Yanagihara K, Yuasa Y (2008) SOX2 is frequently downregulated in gastric cancers and inhibits cell growth through cell-cycle arrest and apoptosis. Br J Cancer 98: 824-831.





