Journal of Family Medicine and Disease Prevention
Preventing a Mass Disease: The Case of Gallstones Disease. Role and Competence for Family Physicians
Ignazio grattagliano*
Internal and general medicine, University of bari, Italy
*Corresponding author: Ignazio grattagliano, Internal and general medicine, University of bari, Italy, Tel: 0039-080-547823, Email: igratta@tin.it
J Fam Med Dis Prev, JFMDP-1-021, (Volume 1, Issue 4), Review Article; ISSN: 2469-5793
Received: October 23, 2015 | Accepted: November 20, 2015 | Published: November 23, 2015
Citation: Grattagliano I (2015) Preventing a Mass Disease: The Case of Gallstones Disease. Role and Competence for Family Physicians. J Fam Med Dis Prev 1:021. 10.23937/2469-5793/1510021
Copyright: © 2015 Grattagliano I. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Gallstone formation is due to a complex interaction between genetic and nongenetic factors. Genes are estimated to account for only about one-fourth of the overall gallstone risk, while metabolic/environmental factors are at least partially modifiable in stone-free risk groups, acting by primary prevention measures on diet, lifestyle and/or the environment or, in selected patients (i.e. rapid weight loss, bariatric surgery, somatostatin or analogues therapy, transient gallbladder stasis, hormone therapy). There is no specific recommendation for the secondary prevention of recurrent gallstones. Family physicians may contribute to achieve this specific goal, considering their capability of identifying and effectively managing several risk factors. Although further studies are needed to better explore the involvement of epigenetic factors regulating the effect of environment and lifestyle on gene expression in primary prevention of gallstone formation, preventive interventions are feasible and advisable in the general practice setting.
Keywords
Bile acids, Gallstones, Obesity, Primary prevention, Risk factors
Abbreviations
GSD: Gallstone Disease, LPAC: Low Phospholipid-Associated Cholelithiasis, TPN: Total Parenteral Nutrition, UDCA: Ursodeoxycholic Acid
Review Criteria
- GSD is one of the most frequent gastro-intestinal disorders in westernized countries.
- Due to the interplay of metabolic and lifestyle aspects in the pathogenesis of GSD, family physicians may play an important role in GSD prevention involving patients' education.
- Exhaustive literature was selected and final selection was based on papers relevant for clinical practice.
Message for the Clinician
- The presence of gallstones should be checked regularly in at risk individuals.
- Consider systemic alterations in patients with cholesterol gallstones.
- Healthy lifestyle including diet, regular physical activity and maintainance of an ideal body weight, might prevent cholesterol GSD.
- Only obese patients on rapid weight loss and patients on long-term therapy with somatostatin or analogues might benefit from temporary therapy with UDCA as for primary prevention.
Introduction
Gallstone disease (GSD) is one of the most frequent gastro-intestinal disorders in westernized countries [1-3], including Europe [4]. The 3 types of gallstones which develop in the gallbladder and bile ducts are distinguished by their chemical composition, and include cholesterol, pigment (black), and mixed (brown, containing small amounts of bilirubin salts and calcium). In industrialized countries 75% are cholesterol gallstones, about 20% are black and 5% are brown stones (Figure 1) [5-8]. Costs related to disease management are high due to diagnostic and surgical procedures involved [9]. Due to the interplay of metabolic and lifestyle aspects in the pathogenesis of GSD [2,10], family physicians (FPs) may play an important role in GSD prevention promoting appropriate patients' education.
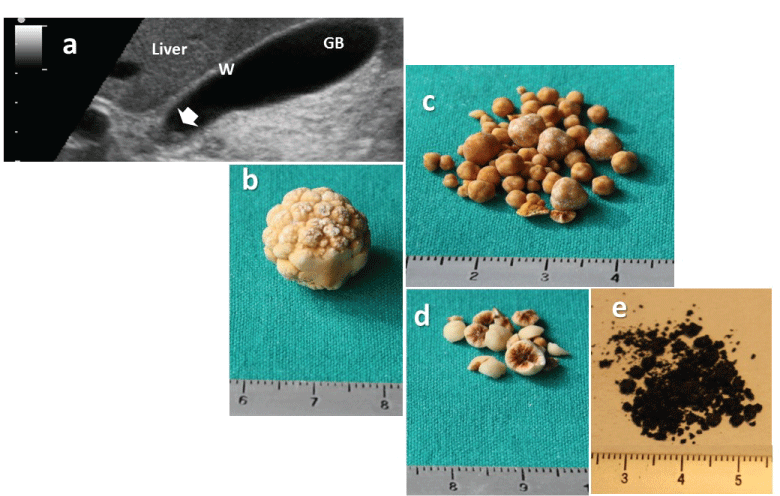
.
Figure 1: The burden of gallstone disease.
a) Ultrasonographic appearance of a single small gallstone (0.4 mm) within the gallbladder neck (arrow) seen on a longitudinal transabdominal scan. The hyperechogenic spot was mobile with decubitus and not associated with a posterior acoustic shadow. The gallbladder wall is not thickened (i.e. less than or equal to 3 mm in the fasting state) and the remaining lumen is anechoic. The 1 cm scale is shown on the left. Abbreviations: GB: gallbladder, W: wall.
b) Macroscopic appearance of a solitary pure cholesterol gallstone (≈12 mm) showing a yellowish morular surface
c) Multiple cholesterol gallstones (2-5 mm) showing a smooth surface
d) Multiple mixed cholesterol gallstones (≈5 mm) showing a pigment center on the cut surface;
e) Multiple black pigment gallstones, forming a largely friable sandy powder (≈1 mm).
Courtesy of P. Portincasa, MD
View Figure 1
This paper focuses on the essential issues related to the prevention of GSD and is intended to provide FPs with few helpful evidences during everyday practice.
Search Methodology
Exhaustive literature in the period ranging from 1974 to 2015 was selected by accessing PubMed (http://www.ncbi.nlm.nih.gov/pubmed). Keywords included the terms bile acids, biliary stones, choledocolitiasis, gallbladder, gallstones, prevention, obesity, metabolic syndrome, bariatric surgery, very low-calorie diet, and ursodeoxycholic acid or cholesterol-lowering drugs. Prospective, retrospective cohort studies, case-controlled studies and metanalyses published in English language in peer-reviewed international journals with impact factor were analyzed. Final selection was based on papers relevant for clinical practice.
Main Messages: The Essential Questions and the Answers
Who are the subjects at risk for and which are the most common modifiable factors in the prevention of GSD?
Several pathogenic mechanisms are identified for cholesterol GSD, namely genetic predisposition influencing cholesterol homeostasis (and possibly epigenetic changes), hepatic hypersecretion of cholesterol leading to supersaturated bile and accelerated precipitation of solid cholesterol crystals in a hypomotile gallbladder accomodating more mucins. Increased absorption of cholesterol from the intestine is another factor [2]. Although a positive family history suggests a role for genetic factors [11], genes are estimated to account for only about one-fourth of the overall gallstone risk, as suggested by the analysis of the Swedish twin registry [12]. In the majority of cases, a genetic background involving multiple pathways [13] determines an individual predisposition to develop cholesterol gallstones in response to a number of acquired unmodifiable and modifiable environmental factors (Table 1) [14]. As for other chronic metabolic diseases, also for GSD the gene-environment interactions and gene expression are possibly regulated by epigenetic mechanisms [14]. Approaches to preventive measures are especially effective in the case of cholesterol gallstones since modifiable pathogenic factors are often involved.
![]()
Table 1: Nongenetic risk factors for gallbladder stones including modifiable, potentially modifiable and non-modifiable factors.
View Table 1
Obesity and metabolic factors: The main risk factors for cholesterol gallstone (e.g. obesity, type 2 diabetes, dyslipidemia, hyperinsulinemia) are components of the metabolic syndrome [2]. Increased body mass index is a risk factor for gallstone formation and growth [2], and acts as an independent risk factor for symptomatic GSD particularly in women [15]. Correlation also exists with waist circumference and triglyceridemia [16]. Additional obesity related pro-lithogenic factors might intervene, and include gallbladder stasis [17], insulin resistance, reduced HDL-cholesterol [16]. Appropriate lifestyle interventions might influence the pathogenesis of cholesterol gallstones and should focus on ideal weight maintainance and weight loss among overweight and obese individuals [15]. The key mechanisms regulating this pathogenic process seem to involve the gene-environment interaction through epigenetic mechanisms also occurring during the fetal age and involving lifestyle, toxic agents and environmental pollutants [18].
Physical activity: People should be aware of the importance of performing a regular daily physical activity, whenever possible [19]. The overall beneficial effect goes beyond the simple protection for gallstone formation [20]. In the Epic-Norfolk prospective cohort study, energy expenditure and cardio-respiratory fitness [21] were investigated by questionnaire in 25,639 volunteers (40-74 years). Subjects were monitored over 14 years. After 5 years, 135 cases of symptomatic gallstones (70% women, 69% uncomplicated) were observed. After 14 years, 290 cases of symptomatic gallstones (68% women, 54% complicated) were recorded. The highest level of physical activity was associated with a 70% decreased risk of symptomatic gallstones in both sexes, with a likely causal effect seen after 5 years. Hyperinsulinemia promotes hepatic uptake of cholesterol [22] with increased secretion in bile [23] and decreased secretion of bile acids [24] (leading to supersaturated lithogenic bile). The regular exercise reduces insulin levels [25], insulin resistance [26], triglyceridemia [27], fatty acid-dependent hypersecretion of gallbladder mucin [28], and increases serum HDL-cholesterol [29]. HDL-cholesterol is the precursor of bile acids [30] and is inversely related to gallstone prevalence [31]. Also, physical activity promotes a cholecystokinin-dependent gallbladder contraction [32].
Dietetic factors: Long-term population-based prospective studies have shown difficulties in estimating the quantity and ingestion pattern of nutrients. High-fiber and high-calcium diets reduce biliary hydrophobic bile acids while a regular eating pattern decreases gallbladder stasis by increasing its regular emptying [19]. Both aspects play a preventive role for GSD. The likelihood of GSD is increased by westernized diets including meat intake [33] Fruit and vegetables [34] might be protective, but data remains controversial. Unsaturated fats [35] might protect against GSD. Coffee is reported to be protective in some [36,37], but not all epidemiological studies [38]. Although prospective epidemiological studies reported protective effects of alcohol consumption on gallstone formation [16] and a Danish Mendelian randomization study indicated that patients with symptomatic gallstones consumed less alcohol as compared to those with asymptomatic stones [15], the findings are controversial [39] while alcohol cannot be recommended for prevention of gallstones. Vitamin C supplementation might have a protective effect. Cholesterol conversion to bile acids requires 7α-hydroxylation and an appropriate hepatocyte content of vitamin C [40]. Vitamin C deficiency might therefore increase the risk of cholesterol gallstone formation [41]. Vitamin C supplementation (500 mg x 4 times/day) changed biliary bile acid composition, increased phospholipids, and prolonged the cholesterol crystallization time [42]. Observational studies have identified an association between low vitamin C consumption and risk of GSD [41]. In the EMIL observational population-based study (n = 2129 subjects, 18-65 years), gallstone prevalence was 4.7% vs. 8.2% in patients reporting regular use of vitamin C (n = 232) or not (n = 1897), respectively [43].
Thus, based on current levels of evidence, a regular eating pattern with high-fiber and high calcium content, vitamin C supplementation and a preference for unsaturated fats should be suggested in subjects at risk for gallstone formation.
How to screen people at risk?
Abdominal ultrasonography is the most convenient first-line screening test because of non-invasiveness, low costs, simple procedure, and high sensitivity and specificity in detecting the presence of gallstones (84% and 99%, respectively) [44]. The same procedure allows a detailed and simultaneous study of gallbladder morphology (wall thickness, presence of polyps, sludge) and kinetics (fasting and postprandial gallbladder volume with estimation of half-emptying time in response to a standard fatty meal [2,17]. Compared to ultrasonography, computed tomography (CT) will not show gallstones if the concretion is isodense with bile [45]. CT with quantitative assessment of stone density may help to select patients for oral bile acid litholysis (i.e. presence of small [< 5 mm], uncalcified [radiotransparent] gallstones) [46]. For choledocholithiasis, magnetic resonance cholangiopancreatography (MRCP) is the first choice approach, since it is noninvasive and has high sensitivity and specificity compared to ultrasonography [47]. Endoscopic ultrasonography (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) have high sensitivity (80-90%) and specificity (100%) but are invasive [47], and both are not free of complications.
Is any form of pharmacological prevention of gallstones possible in the general population?
No rationale exists for using pharmachological therapy as prevention of GSD. Studies are mainly experimental or incomplete with lack of clinical meaning. Investigated agents include the bile acid ursodeoxycholic acid (UDCA), omega-3 fatty acids [48], statins [18], ezetimibe [18,49,50], spirin [51] or liver nuclear receptor regulators of cholesterol metabolism, i.e. FXR agonists [2].
Are there specific subgroups of subjects in which a primary prevention is feasible and sustainable?
Approach to primary and sometime secondary preventive measures are especially effective in the case of cholesterol gallstones (Figure 2).
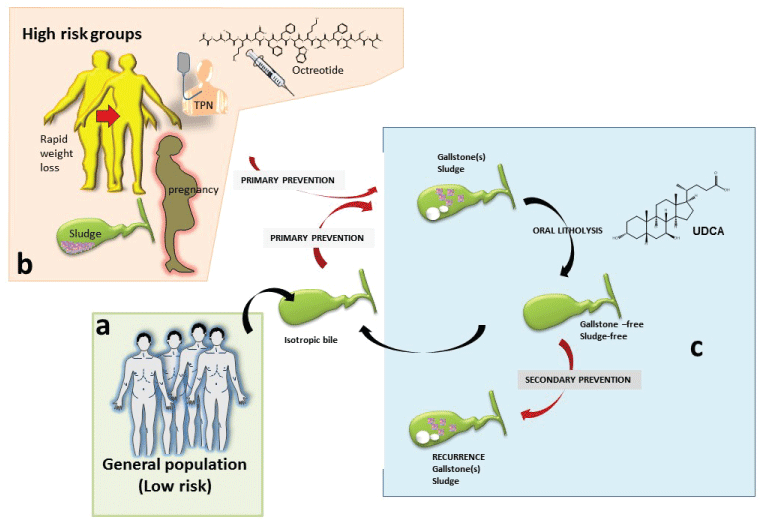
.
Figure 2: Approach to preventive measures is especially effective in the case of cholesterol gallstones.
a) Primary prevention of gallstones (which contributes to maintain the isotropic bile, meaning free of cholesterol crystals and stones), is indicated already in the general population (at low risk) by keeping general healthy lifestyles.
b) Tailored forms of preventions are indicated particularly in high risk groups (see text and Table 2). Patients with known biliary sludge, pregnant women, and patients undergoing rapid weight loss, patients on long-term octreotide, and those receiving long-term total parenteral nutrition (TPN) are at highest risk of developing gallstones and subsequent biliary symptoms and/or gallstone-related complications. Once gallstones/sludge has formed, oral litholysis (by ursodeoxycholic acid, UDCA) has a very limited role in a small subgroup of symptomatic patients with small, pure cholesterol stones in a functioning gallbladder.
c) If dissolution of concrements is achieved, the secondary prevention is indicated in subgroup of patients at risk of recurrent gallstones/sludge.
View Figure 2
Obese patients on rapid weight loss: If weight loss is rapid (i.e. over 1.5 kg/week) [52], the risk of gallstone formation, often asymptomatic, increases significantly. This is the case in patients starting very-low-calorie diets (i.e. < 800 kcal/day) [53] or bariatric surgery (up to 48% of patients for weight loss exceeding 25% of original weight) [53,54]. The overall risk decreases when body weight stabilizes, after approximately 24 months [53]. Weight cycling also represents an independent risk factor for gallstones [55]. Excessive de novo biosynthesis of cholesetrol and biliary cholesterol excretion are the two main pathogenic factors [56]. Some preventive measures are possible during weight-reducing programs.
It is advisable to keep the speed of weight loss to less than 1.5 kg/week [52,57]. The risk of developing symptomatic gallstones decreases if gallbladder motility is improved by appropriate fat content in diet (at least 7g/day) [53]. The litholytic hydrophylic UDCA greatly decreases the risk of cholesterol gallstone formation (< 10%) and that of becoming symptomatic [58] following rapid weight reduction [58,59]. A meta-analysis of five RCTs including 521 patients (322 on UDCA and 199 on placebo) concluded that UDCA 300-1, 200 mg/day effectively prevents gallstone formation after bariatric surgery [59] from 32% to 2% [60] with no severe side effects. The beneficial effect of (n-3) polyunsaturated fatty acids (11.3 g/day) on biliary cholesterol nucleation time and crystallization, and prevention of gallstone formation was confirmed in a randomized, double-blind placebo-UDCA (1,200 mg/day)-controlled trial in obese women on hypocaloric diet (1,200 Kcal/day, 20% energy as fat) [48]. The protective mechanism is probably mediated by replacement of biliary arachidonate by (n-3) PUFA [61], by increasing biliary phospholipids [62], change of intrahepatic cholesterol transport and hypersecretion of biliary cholesterol [63]. A concurrent prophylactic cholecystectomy has previously been recommended, based on the estimation that almost 19% of patients might require a cholecystectomy following bariatric surgery [64]. Data have not further supported since up to 97% of patients do remain asymptomatic, as confirmed by a recent decision analysis model [65].
Patients on long-term therapy with somatostatin or analogues: These patients exhibit biliary lithogenic changes and gastrointestinal motility changes, including delayed intestinal transit and gallbladder stasis [66,67]. Prophylactic therapy with UDCA has been suggested [67-69].
Patients with marked gallbladder stasis: Gallbladder stasis and changes of biliary composition are typical in pregnancy [70] or during prolonged fasting such as during total parenteral nutrition (TPN). Both sludge and small gallstones might disappear spontaneously in the postpartum period [70] and in TPN after restoration of oral diet [71]. Therefore there is no indication for oral litholysis in both conditions.
Patients on hormone therapy: A metanalysis [72] showed a significantly increased risk of GSD in women under hormone replacement therapy for controlling menopausal symptoms or osteoporosis prevention [73]. The possibility of any pharmachological preventive approach has not been addressed, and has no rationale, thus far.
Is there any form of recommendation for the (secondary) prevention of recurrent gallstones?
Recurrent gallstones: Dissolution rate of cholesterol gallstones is 37-60% [74] after 2 years of treatment with UDCA. Recurrence is high following dissolution (15% by 1 year and 45% by 5 years) [75-77]. Pharmacological prophylaxis of gallstone recurrence could be restricted to very high risk subgroups or to patients not fitting the criteria of a subsequent cholecystectomy.
Recurrent bile duct stones: There are no validated prophylactic measures for bile duct stones recurrence.
Patients with low phospholipid-associated cholelithiasis (LPAC): The heterozygous mutation of the gene ABCB4 encoding for the phospholipid-flippase is a rare form of monogenic predisposition for cholelithiasis associated with low biliary phospholipids and bile salt-mediated damage of the canalicular membrane. Before the age of 40, gallstones, intrahepatic sludge and microlithiasis develop, while biliary symptoms recurr after cholecystectomy [78,79]. As suggested by few studies, the prophylactic long term therapy with UDCA should be initiated as early as possible [78,79].
Conclusions, Future Perspectives and Messages in General Practice
The epidemiology of GSD (including complications) and the costs for its management makes all possible efforts worth towards primary prevention strategies. In general practice, a key role should be that of identifying general and specific modifiable risk factor for GSD and those subgroups of subjects put at risk of GSD. In this setting, educational interventions aiming to prevent GSD should focus on healthy lifestyles, maintainance of ideal body weight, regular physical activity, prevention of metabolic syndrome, all factors influencing glyco-lipid metabolism and ultimately biliary cholesterol saturation. Pharmachological interventions are restricted to a subgroup of patients (Table 2). Further studies will need to address the involvement of epigenetic factors regulating gene expression in response to environmental factors, to identify even better preventive measures.
![]()
Table 2: Current status on preventive approaches to cholesterol GSD.
View Table 2
References
-
Attili AF, Capocaccia R, Carulli N, Festi D, Roda E, et al. (1997) Factors associated with gallstone disease in the MICOL experience. Multicenter Italian Study on Epidemiology of Cholelithiasis. Hepatology 26: 809-818.
-
Portincasa P, Moschetta A, Palasciano G (2006) Cholesterol gallstone disease. Lancet 368: 230-239.
-
Everhart JE, Ruhl CE (2009) Burden of digestive diseases in the United States part I: overall and upper gastrointestinal diseases. Gastroenterology 136: 376-386.
-
Farthing M, Roberts SE, Samuel DG, Williams JG, Thorne K, et al. (2014) Survey of digestive health across Europe: Final report. Part 1: The burden of gastrointestinal diseases and the organisation and delivery of gastroenterology services across Europe. United European Gastroenterol J 2: 539-543.
-
Diehl AK (1991) Epidemiology and natural history of gallstone disease. Gastroenterol Clin North Am 20: 1-19.
-
Sherlock S, Dooley J (2002) Diseases of the liver and biliary system. Oxford: Blackwell Science 597-628.
-
Trotman BW, Ostrow JD, Soloway RD (1974) Pigment vs. cholesterol cholelithiasis: comparison of stone and bile composition. Am J Dig Dis 19: 585-590.
-
Attili AF, Carulli N, Roda E, Barbara B, Capocaccia L, et al. (1995) Epidemiology of gallstone disease in Italy: prevalence data of the Multicenter Italian Study on Cholelithiasis (M.I.COL.) Am J Epidemiol 141: 158-165.
-
Shaffer EA (2005) Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep 7: 132-140.
-
Portincasa P, Wang DQ (2012) Intestinal absorption, hepatic synthesis, and biliary secretion of cholesterol: where are we for cholesterol gallstone formation? Hepatology 55: 1313-1316.
-
Sarin SK, Negi VS, Dewan R, Sasan S, Saraya A (1995) High familial prevalence of gallstones in the first-degree relatives of gallstone patients. Hepatology 22: 138-141.
-
Katsika D, Grjibovski A, Einarsson C, Lammert F, Lichtenstein P, et al. (2005) Genetic and environmental influences on symptomatic gallstone disease: a Swedish study of 43,141 twin pairs. Hepatology 41: 1138-1143.
-
Wang DQ, Cohen DE, Carey MC (2009) Biliary lipids and cholesterol gallstone disease. J Lipid Res 50 Suppl: S406-411.
-
Di Ciaula A, Wang DQ, Bonfrate L, Portincasa P (2013) Current views on genetics and epigenetics of cholesterol gallstone disease. Cholesterol 2013: 298421.
-
Stender S, Nordestgaard BG, Tybjaerg-Hansen A (2013) Elevated body mass index as a causal risk factor for symptomatic gallstone disease: a Mendelian randomization study. Hepatology 58: 2133-2141.
-
Banim PJ, Luben RN, Bulluck H, Sharp SJ, Wareham NJ, et al. (2011) The aetiology of symptomatic gallstones quantification of the effects of obesity, alcohol and serum lipids on risk. Epidemiological and biomarker data from a UK prospective cohort study (EPIC-Norfolk). Eur J Gastroenterol Hepatol 23: 733-740.
-
Di Ciaula A, Wang DQ, Portincasa P (2012) Gallbladder and gastric motility in obese newborns, pre-adolescents and adults. J Gastroenterol Hepatol 27: 1298-1305.
-
Di Ciaula A, Portincasa P (2014) Fat, epigenome and pancreatic diseases. Interplay and common pathways from a toxic and obesogenic environment. Eur J Intern Med 25: 865-873.
-
Leitzmann MF, Giovannucci EL, Rimm EB, Stampfer MJ, Spiegelman D, et al. (1998) The relation of physical activity to risk for symptomatic gallstone disease in men. Ann Intern Med 128: 417-425.
-
World Health O (2010) Global recommendations on physical activity for health.
-
Banim PJ, Luben RN, Wareham NJ, Sharp SJ, Khaw KT, et al. (2010) Physical activity reduces the risk of symptomatic gallstones: a prospective cohort study. Eur J Gastroenterol Hepatol 22: 983-988.
-
Chait A, Bierman EL, Albers JJ (1979) Low-density lipoprotein receptor activity in cultured human skin fibroblasts. Mechanism of insulin-induced stimulation. J Clin Invest 64: 1309-1319.
-
Nepokroeff CM, Lakshmanan MR, Ness GC, Dugan RE, Porter JW (1974) Regulation of the diurnal rhythm of rat liver beta-hydroxy-beta-methylglutaryl coenzmye A reductase activity by insulin, glucagon, cyclic AMP and hydrocortisone. Arch Biochem Biophys 160: 387-393.
-
Subbiah MT, Yunker RL (1984) Cholesterol 7 alpha-hydroxylase of rat liver: an insulin sensitive enzyme. Biochem Biophys Res Commun 124: 896-902.
-
Kirwan JP, Kohrt WM, Wojta DM, Bourey RE, Holloszy JO (1993) Endurance exercise training reduces glucose-stimulated insulin levels in 60- to 70-year-old men and women. J Gerontol 48: M84-90.
-
Seals DR, Hagberg JM, Hurley BF, Ehsani AA, Holloszy JO (1984) Effects of endurance training on glucose tolerance and plasma lipid levels in older men and women. JAMA 252: 645-649.
-
Tran ZV, Weltman A, Glass GV, Mood DP (1983) The effects of exercise on blood lipids and lipoproteins: a meta-analysis of studies. Med Sci Sports Exerc 15: 393-402.
-
Mingrone G, Greco AV, Finotti E, Passi S (1988) Free fatty acids: a stimulus for mucin hypersecretion in cholesterol gallstone biles. Biochim Biophys Acta 958: 52-59.
-
Leon AS, Sanchez OA (2001) Response of blood lipids to exercise training alone or combined with dietary intervention. Med Sci Sports Exerc 33: S502-515.
-
Halloran LG, Schwartz CC, Vlahcevic ZR, Nisman RM, Swell L (1978) Evidence for high-density lipoprotein-free cholesterol as the primary precursor for bile-acid synthesis in man. Surgery 84: 1-7.
-
Petitti DB, Friedman GD, Klatsky AL (1981) Association of a history of gallbladder disease with a reduced concentration of high-density-lipoprotein cholesterol. N Engl J Med 304: 1396-1398.
-
Philipp E, Wilckens T, Friess E, Platte P, Pirke KM (1992) Cholecystokinin, gastrin and stress hormone responses in marathon runners. Peptides 13: 125-128.
-
Tsunoda K, Shirai Y, Hatakeyama K (2004) Prevalence of cholesterol gallstones positively correlates with per capita daily calorie intake. Hepatogastroenterology 51: 1271-1274.
-
Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL (2006) Fruit and vegetable consumption and risk of cholecystectomy in women. Am J Med 119: 760-767.
-
Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL (2004) The effect of long-term intake of cis unsaturated fats on the risk for gallstone disease in men: a prospective cohort study. Ann Intern Med 141: 514-522.
-
Leitzmann MF, Stampfer MJ, Willett WC, Spiegelman D, Colditz GA, et al. (2002) Coffee intake is associated with lower risk of symptomatic gallstone disease in women. Gastroenterology 123: 1823-1830.
-
Ruhl CE, Everhart JE (2000) Association of coffee consumption with gallbladder disease. Am J Epidemiol 152: 1034-1038.
-
Walcher T, Haenle MM, Mason RA, Koenig W, Imhof A, et al. (2010) The effect of alcohol, tobacco and caffeine consumption and vegetarian diet on gallstone prevalence. Eur J Gastroenterol Hepatol 22: 1345-1351.
-
Kratzer W, Mason RA, Kachele V (1999) Prevalence of gallstones in sonographic surveys worldwide. J Clin Ultrasound 27: 1-7.
-
Ginter E (1976) Chenodeoxycholic acid, gallstones and vitamin C. N Engl J Med 295: 1260-1261.
-
Simon JA, Hudes ES (2000) Serum ascorbic acid and gallbladder disease prevalence among US adults: the Third National Health and Nutrition Examination Survey (NHANES III). Arch Intern Med 160: 931-936.
-
Gustafsson U, Wang FH, Axelson M, Kallner A, Sahlin S, et al. (1997) The effect of vitamin C in high doses on plasma and biliary lipid composition in patients with cholesterol gallstones: prolongation of the nucleation time. Eur J Clin Invest 27: 387-391.
-
Walcher T, Haenle MM, Kron M, Hay B, Mason RA, et al. (2009) Vitamin C supplement use may protect against gallstones: an observational study on a randomly selected population. BMC Gastroenterol 9: 74.
-
Portincasa P, Di Ciaula A, Palmieri V, Vendemiale G, Altomare E, et al. (1994) Sonographic evaluation of gallstone burden in humans. Ital J Gastroenterol 26: 141-144.
-
Barakos JA, Ralls PW, Lapin SA, Johnson MB, Radin DR, et al. (1987) Cholelithiasis: evaluation with CT. Radiology 162: 415-418.
-
Pereira SP, Veysey MJ, Kennedy C, Hussaini SH, Murphy GM, et al. (1997) Gallstone dissolution with oral bile acid therapy. Importance of pretreatment CT scanning and reasons for nonresponse. Dig Dis Sci 42: 1775-1782.
-
Ledro-Cano D (2007) Suspected choledocholithiasis: endoscopic ultrasound or magnetic resonance cholangio-pancreatography? A systematic review. Eur J Gastroenterol Hepatol 19: 1007-1011.
-
Mendez-Sanchez N, Gonzalez V, Aguayo P, Sanchez JM, Tanimoto MA, et al. (2001) Fish oil (n-3) polyunsaturated fatty acids beneficially affect biliary cholesterol nucleation time in obese women losing weight. J Nutr 131: 2300-2303.
-
Portincasa P, Ciaula AD, Bonfrate L, Wang DQ (2012) Therapy of gallstone disease: What it was, what it is, what it will be. World J Gastrointest Pharmacol Ther 3: 7-20.
-
Wang HH, Portincasa P, de Bari O, Liu KJ, Garruti G, et al. (2013) Prevention of cholesterol gallstones by inhibiting hepatic biosynthesis and intestinal absorption of cholesterol. Eur J Clin Invest 43: 413-426.
-
Broomfield PH, Chopra R, Sheinbaum RC, Bonorris GG, Silverman A, et al. (1988) Effects of ursodeoxycholic acid and aspirin on the formation of lithogenic bile and gallstones during loss of weight. N Engl J Med 319: 1567-1572.
-
Weinsier RL, Wilson LJ, Lee J (1995) Medically safe rate of weight loss for the treatment of obesity: a guideline based on risk of gallstone formation. Am J Med 98: 115-117.
-
Johansson K, Sundstrom J, Marcus C, Hemmingsson E, Neovius M (2014) Risk of symptomatic gallstones and cholecystectomy after a very-low-calorie diet or low-calorie diet in a commercial weight loss program: 1-year matched cohort study. Int J Obes (Lond) 38: 279-284.
-
Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, et al. (2003) Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg 238: 467-484.
-
Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL (2006) Weight cycling and risk of gallstone disease in men. Arch Intern Med 166: 2369-2374.
-
Li VK, Pulido N, Fajnwaks P, Szomstein S, Rosenthal R, et al. (2009) Predictors of gallstone formation after bariatric surgery: a multivariate analysis of risk factors comparing gastric bypass, gastric banding, and sleeve gastrectomy. Surg Endosc 23: 1640-1644.
-
Everhart JE (1993) Contributions of obesity and weight loss to gallstone disease. Ann Intern Med 119: 1029-1035.
-
Wudel LJ Jr, Wright JK, Debelak JP, Allos TM, Shyr Y, et al. (2002) Prevention of gallstone formation in morbidly obese patients undergoing rapid weight loss: results of a randomized controlled pilot study. J Surg Res 102: 50-6.
-
Uy MC, Talingdan-Te MC, Espinosa WZ, Daez ML, Ong JP (2008) Ursodeoxycholic acid in the prevention of gallstone formation after bariatric surgery: a meta-analysis. Obes Surg 18: 1532-1538.
-
Sugerman HJ, Brewer WH, Shiffman ML, Brolin RE, Fobi MA, et al. (1995) A multicenter, placebo-controlled, randomized, double-blind, prospective trial of prophylactic ursodiol for the prevention of gallstone formation following gastric-bypass-induced rapid weight loss. Am J Surg 169: 91-96.
-
Booker ML, Scott TE, La Morte WW (1990) Effects of dietary fish oil on biliary phospholipids and prostaglandin synthesis in the cholesterol-fed prairie dog. Lipids 25: 27-32.
-
Mizuguchi K, Yano T, Kawano H, Abei M, Tanaka N (1997) Preventive effects of eicosapentaenoic acid (EPA) on cholesterol gallstone formation in hamsters. Nihon Yakurigaku Zasshi 110 Suppl 1: 50P-55P.
-
Smit MJ, Temmerman AM, Wolters H, Kuipers F, Beynen AC, et al. (1991) Dietary fish oil-induced changes in intrahepatic cholesterol transport and bile acid synthesis in rats. J Clin Invest 88: 943-951.
-
Tarantino I, Warschkow R, Steffen T, Bisang P, Schultes B, et al. (2011) Is routine cholecystectomy justified in severely obese patients undergoing a laparoscopic Roux-en-Y gastric bypass procedure? A comparative cohort study. Obes Surg 21: 1870-1878.
-
Worni M, Guller U, Shah A, Gandhi M, Shah J, et al. (2012) Cholecystectomy concomitant with laparoscopic gastric bypass: a trend analysis of the nationwide inpatient sample from 2001 to 2008. Obes Surg 22: 220-229.
-
Moschetta A, Stolk MF, Rehfeld JF, Portincasa P, Slee PH, et al. (2001) Severe impairment of postprandial cholecystokinin release and gall-bladder emptying and high risk of gallstone formation in acromegalic patients during Sandostatin LAR. Aliment Pharmacol Ther 15: 181-185.
-
Montini M, Gianola D, Pagani MD, Pedroncelli A, Caldara R, et al. (1994) Cholelithiasis and acromegaly: therapeutic strategies. Clin Endocrinol (Oxf) 40: 401-406.
-
Avila NA, Shawker TH, Roach P, Bradford MH, Skarulis MC, et al. (1998) Sonography of gallbladder abnormalities in acromegaly patients following octreotide and ursodiol therapy: incidence and time course. J Clin Ultrasound 26: 289-294.
-
Venneman NG, Besselink MG, Keulemans YC, Vanberge-Henegouwen GP, Boermeester MA, et al. (2006) Ursodeoxycholic acid exerts no beneficial effect in patients with symptomatic gallstones awaiting cholecystectomy. Hepatology 43: 1276-1283.
-
Maringhini A, Ciambra M, Baccelliere P, Raimondo M, Orlando A, et al. (1993) Biliary sludge and gallstones in pregnancy: incidence, risk factors, and natural history. Ann Intern Med 119: 116-120.
-
Marks JW, Stein T, Schoenfield LJ (1994) Natural history and treatment with ursodiol of gallstones formed during rapid loss of weight in man. Dig Dis Sci 39: 1981-1984.
-
Marjoribanks J, Farquhar C, Roberts H, Lethaby A (2012) Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev 7: CD004143.
-
Cirillo DJ, Wallace RB, Rodabough RJ, Greenland P, LaCroix AZ, et al. (2005) Effect of estrogen therapy on gallbladder disease. JAMA 293: 330-339.
-
Rubin RA, Kowalski TE, Khandelwal M, Malet PF (1994) Ursodiol for hepatobiliary disorders. Ann Intern Med 121: 207-218.
-
Villanova N, Bazzoli F, Taroni F, Frabboni R, Mazzella G, et al. (1989) Gallstone recurrence after successful oral bile acid treatment. A 12-year follow-up study and evaluation of long-term postdissolution treatment. Gastroenterology 97: 726-731.
-
Petroni ML, Jazrawi RP, Pazzi P, Zuin M, Lanzini A, et al. (2000) Risk factors for the development of gallstone recurrence following medical dissolution. The British-Italian Gallstone Study Group. Eur.J.Gastroenterol.Hepatol 12: 695-700.
-
Rabenstein T, Radespiel-Troger M, Hopfner L, Benninger J, Farnbacher M, et al. (2005) Ten years experience with piezoelectric extracorporeal shockwave lithotripsy of gallbladder stones. Eur J Gastroenterol Hepatol 17: 629-639.
-
Rosmorduc O, Poupon R (2007) Low phospholipid associated cholelithiasis: association with mutation in the MDR3/ABCB4 gene. Orphanet J Rare Dis 2: 29.
-
Marschall HU, Einarsson C (2007) Gallstone disease. J Intern Med 261: 529-542.





