Journal of Clinical Nephrology and Renal Care
Henoch-Schonlein Purpura after Tetanus Toxoid Vaccination: A Case Report
Wei-Yen Kong*, Wan Zaharatul Ashikin Wan Abdullah, Halim Gafor, Rozita Mohd and Rizna Abdul Cader
Universiti Kebangsaan Malaysia Medical Centre, Kuala Lumpur, Malaysia
*Corresponding author:
Wei-Yen Kong, Universiti Kebangsaan Malaysia Medical Centre, Jalan Yaacob Latif, Bandar Tun Razak, Kuala Lumpur, Malaysia, Tel: 60176663477, E-mail: wykong1979@yahoo.com
J Clin Nephrol Ren Care, JCNRC-2-018, (Volume 2, Issue 2), Case Report
Received: October 25, 2016 | Accepted: December 19, 2016 | Published: December 21, 2016
Citation: Wei-Yen K, Abdullah WZAW, Gafor H, Mohd R, Cader RA (2016) Henoch-Schonlein Purpura after Tetanus Toxoid Vaccination: A Case Report. J Clin Nephrol Ren Care 2:018.
Copyright: © 2016 Wei-Yen K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Henoch-Schönlein purpura (HSP), also known as IgA vasculitis, is the most common form of small-vessel systemic vasculitis in children. The diagnosis of this condition is usually based on clinical presentations of the disease. The etiology of HSP is not entirely clear, but it has been frequently associated with infections and vaccinations.
We present here the first reported case of a young boy who developed classic features of HSP, i.e. palpable purpura, arthralgia, abdominal pain with bloody diarrhea and kidney involvement, following a tetanus vaccine. The diagnosis of HSP was confirmed by skin biopsy and also biopsies obtained from esophagogastroduodenoscopy and colonoscopy. He was initially treated with pulsed intravenous methylprednisolone. He then developed acute kidney injury and renal ultrasonography was negative for renal vein thrombosis. Kidney biopsy showed crescentic glomerulonephritis and he was treated with plasmapheresis, followed by intravenous cyclophosphamide. He responded well with almost complete normalization of his serum creatinine and urine protein.
Background
Henoch-Schönlein purpura (HSP), or IgA vasculitis [1], is the most common form of small-vessel systemic vasculitis in children, with 90% of cases occurs in the pediatric group. It is usually diagnosed based on clinical presentations of the disease [2], and a variety of classification criteria for HSP have been proposed, including the American College of Rheumatology (ACR) criteria [3,4]. It is thought to be an immune-mediated process, associated with deposition of IgA immune complexes within the affected organs. Although a variety of infections (bacterial, viral, parasitic), chemical triggers (vaccines and various medications), malignancies (both solid organ tumors and hematological malignancies) and autoimmune conditions have been implicated as the possible causative agents [5], the underlying cause of HSP remains largely unknown. Immunologic, genetic, and environmental factors all seem to play a role [6-8].
The annual incidence of HSP varies geographically, from 6.2 to 70.3 per 100,000 in children less than 17 years of age with slight male predominance (M:F = 1.2:1.0) [9]. Peak age incidence is 4-6 years and 90% of HSP cases occur before the age of 10 years. In adults, the incidence varies between 3.4-14.3 per million population. Its true incidence may be under-reported [10].
In 1990, the American College of Rheumatology (ACR) developed criteria for the diagnosis of HSP [3]. The criteria require the presence of two out of four features, and yield a diagnostic sensitivity of 87.1 percent and specificity of 87.7 percent. The criteria are: (1) patient 20 years or younger at onset, (2) palpable purpura (without thrombocytopenia), (3) bowel angina (diffuse abdominal pain or diagnosis of bowel ischemia), and (4) histological changes showing granulocytes in small walls of arterioles and venules (leukocytoclastic vasculitis). In 2006, the criteria were revised: palpable purpura was made a mandatory feature, the age criterion was removed, arthritis was added as a criterion, and granulocytes in biopsy specimens was replaced with IgA deposition [11,12]. These criteria have been generally accepted by expert organizations.
We present here a case of HSP which developed in a 15-year-old boy, seven days after he received tetanus toxoid vaccination. This is believed to be the first reported case of HSP probably triggered by this vaccine.
Case Report
A 15-year-old boy with no known past medical history came to us in March 2013 with a five-day history of purpuric rashes over bilateral lower limbs and painful, swollen joints over bilateral elbows, hands and ankles, which worsen progressively. He denies any history of recent infection or new medication. He did receive a dose of tetanus toxoid intramuscular injection approximately seven days prior to the onset of these symptoms, and the vaccine was given because he sustained a small open wound at his right thigh during a sporting event. Physical examination revealed palpable purpuric rashes distributed mainly at the extensor aspect of both lower limbs, from the feet extended up to bilateral inguinal and gluteal areas. There were also minimal purpuric rashes at the abdomen and upper back. His bilateral elbows, ankles and a few small joints of the hands are swollen and tender.
A diagnosis of vasculitis was made and he was started on oral prednisolone at 0.5 mg/kg of body weight daily. Connective tissue screening including ANA, ds-DNA, rheumatoid factor, C3 and C4 levels, and ASOT were all unremarkable. ESR was not raised and CRP was only slightly elevated at 1.5 mg/dl (NR < 0.5 mg/dl). Hepatitis B, hepatitis C and HIV serologies were negative. However, despite initiation of oral prednisolone therapy, he had no clinical improvement but started to have severe colicky abdominal pain associated with bloody diarrhea. He also began to have frothy urine and periorbital edema. Urinalysis showed blood 3+ and protein 3+. His 24-hour urine protein was about 8 grams. Serum creatinine and albumin were 41 umol/L (0.46 mg/dL) and 21 g/L respectively at this point.
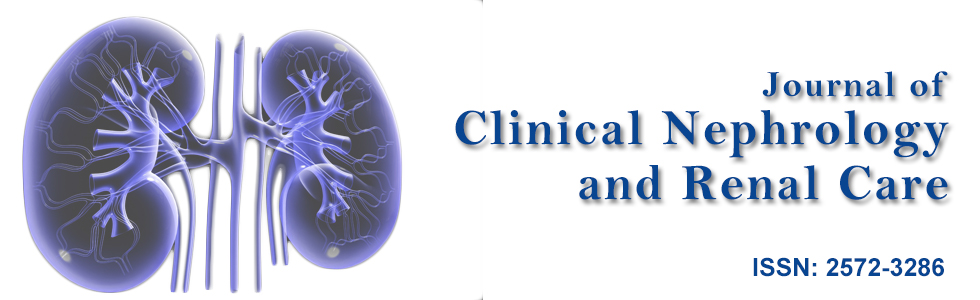
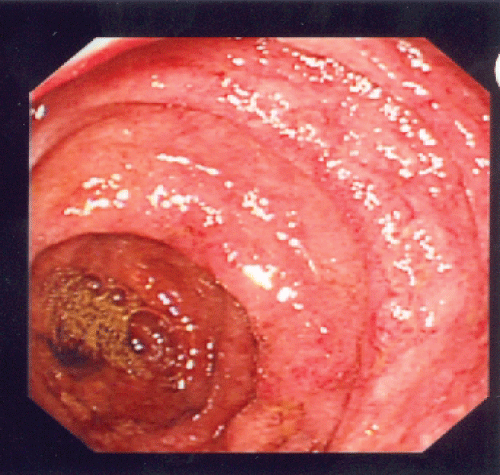
Urgent esophagogastroduodenoscopy (EGD) and colonoscopy were performed. Gross examination on EGD showed vasculitic lesions at stomach antrum, stomach body and duodenum with edematous mucosa (Figure 1). Gross examinations on colonoscopy demonstrated multiple vasculitic lesions and ulcers at ileocecal valve, terminal ileum and throughout the large bowel (Figure 2). Biopsies were taken, and results were consistent with leucocytoclastic vasculitis. In view of these findings as well as his clinical deterioration, he was given pulsed intravenous methylprednisolone 500 mg daily for 3 days, followed by high dose oral prednisolone at 1 mg/kg of body weight daily.
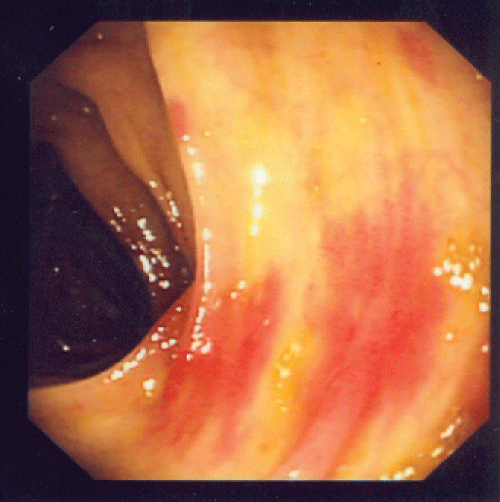
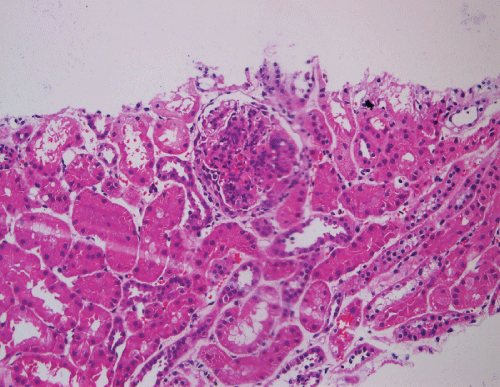
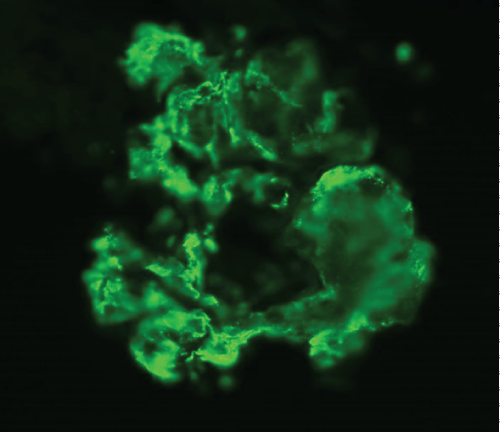
A renal biopsy was performed as he also started to have acute kidney injury, with creatinine rising from baseline of 41 umol/L (0.46 mg/dL) to a peak of 198 umol/L (2.24 mg/dL). A total of 8 glomeruli were obtained with cellular crescents demonstrated in 4/8 glomeruli (Figure 3). Most glomeruli also had features of mesangial expansion with segmental endocapillary proliferation. There was no tubular atrophy or interstitial fibrosis. Immunofluorescence studies showed mesangial deposition of IgA in granular pattern (Figure 4). These renal biopsy findings were consistent with renal involvement in Henoch-Schonlein purpura. Renal vein thrombosis was ruled out.
.
Figure 3: Mesangial expansion with segmental endocapillary proliferation and cellular crescent.
View Figure 3
.
Figure 4: Immunofluorescence studies showing mesangial deposition of IgA in granular pattern.
View Figure 4
We were initially planning to treat his HSP-related crescentic glomerulonephritis (GN) by intravenous cyclophosphamide but we had to withhold this treatment as the patient developed hospital acquired pneumonia (HAP), of which he was started on intravenous antibiotics. With active infection and further worsening of renal function, concerning of a rapidly progressive GN, plasmapheresis was initiated. A total of seven plasmapheresis treatments were given while he was also receiving treatment for HAP. His renal function improved remarkably. His HAP also resolved upon adequate treatment with antibiotics. IV cyclophosphamide was started and continued for 4 months, followed by maintenance Cyclosporine A and tapering dose of oral prednisolone. In January 2014, his serum creatinine level almost normalized and his 24-hour urine protein had dropped to about 0.8 grams.
Discussion
The diagnosis of HSP was made as the patient not only presented with the classic tetrad of this condition (i.e. palpable purpura, arthralgia, abdominal pain with bloody diarrhea and kidney involvement), he also fulfilled ACR criteria for the diagnosis of HSP, as outlined above.
The correlations between HSP and many types of vaccinations have been reported. However, we believe this is the first known reported case of HSP following a tetanus vaccine in the literature. HSP has been observed following influenza vaccination (including H1N1 influenza) [13,14], measles vaccination [15], and also typhoid, cholera and yellow fever vaccinations [16]. Other types of vasculitides have also been described after pneumococcal [17], hepatitis B [18] and BCG [19] vaccinations. However, a recent systemic literature review concluded that "existing literature does not allow establishing a causative link between vaccination and vasculitides" [20].
In HSP, IgA immune complexes (antigen-antibody complexes) form as a result of infections, chemical triggers (including vaccinations and drugs), malignancies and autoimmune mechanisms [21]. These antigen-antibody complexes deposit in the walls of small vessels and activate the alternate complement pathway, leading to neutrophil accumulation resulting in inflammation and vasculitis without a granulomatous reaction. This sequence of events can involve multiple organs particularly skin, joints, gastrointestinal tract and the kidneys. The short term morbidity of HSP is usually due to gastrointestinal complications (e.g. intussusceptions, bowel ischemia or perforations), whereas the long term morbidity is mainly secondary to insults to the kidneys.
The majority of patients with HSP present with rather classic clinical manifestations. In patients with atypical presentations, biopsies of the affected organs (i.e. skin or gastrointestinal tract) demonstrating leukocytoclastic vasculitides with predominance of IgA depositions can confirm the diagnosis of HSP. A kidney biopsy is also helpful to establish the diagnosis. However, it is an invasive procedure and is generally reserved for patients with uncertain diagnosis, or more commonly, for patients who have more severe renal involvement such as significant proteinuria and/or acute kidney injury, as illustrated in our case. Kidney biopsy will not only guide the management, but will also give prognostic value. The long term prognosis of renal involvement in HSP correlates with the severity of initial renal presentations and histological changes seen in renal biopsy [22]. Worse renal prognosis is seen with nephrotic range proteinuria, elevated serum creatinine, hypertension during the acute phase [22], crescentic GN [23] and tubule-interstitial fibrosis on renal histopathology. In general, the outcome of renal disease in HSP is favorable in most patients, with complete recovery reported in 94% of children and 89% of adults [24]. The risk of chronic renal disease is increased in adults [25].
Fortunately, in contrast to other forms of systemic vasculitis, HSP is usually self-limited, and care is primarily supportive, i.e. adequate hydration, rest and symptomatic relief of pain. Acetaminophen and non-steroidal anti-inflammatory drugs will be sufficient in treating arthralgia. In cases with severe rash and arthralgia/arthritis, severe abdominal pain, bowel ischemia, gastrointestinal bleeding, renal and/or other organ involvements, glucocorticoids therapy is indicated. Immunosuppressive drugs (i.e. cyclophosphamide, azathioprine, cyclosporine A, and mycophenolate mofetil), in combination with glucocorticoids, are recommended for severe disease not responsive to glucocorticoids alone. Plasmapheresis has also been used in patients with crescentic GN [26,27], clinically presenting as rapidly progressive glomerulonephritis (RPGN). Its efficacy is uncertain, partially due to concurrent administration of glucocorticoids. However, there is some data suggesting that even plasmapheresis alone may be curative in some patients [27].
In the case of our patient, his renal function continued to deteriorate rapidly despite glucocorticoid therapy. Renal biopsy showed cellular crescents in 4/8 glomeruli. Renal vein thrombosis, as a potential complication of severe nephrotic syndrome, was ruled out. Although the acute kidney injury might be partially contributed by possible acute tubular necrosis in the setting of active infection, in this case HAP, it was behaving more like RPGN. Since potent immunosuppressive drug is contraindicated in the setting of active infection, the initial plan for intravenous cyclophosphamide was changed to plasmapheresis for the treatment of RPGN. He received a total of seven plasmapheresis treatments, with marked improvement in his renal function and proteinuria. Upon resolution of HAP, treatment was continued with intravenous cyclophosphamide briefly, followed by maintenance Cyclosporine A and tapering dose of oral prednisolone.
References
-
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, et al. (2013) 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 65: 1.
-
McCarthy HJ, Tizard EJ (2010) Clinical practice: Diagnosis and management of Henoch-Schönlein purpura. Eur J Pediatr 169: 643.
-
Mills JA, Michel BA, Bloch DA, Calabrese LH, Hunder GG, et al. (1990) The American College of Rheumatology 1990 criteria for the classification of Henoch-Schönlein purpura. Arthritis Rheum 33: 1114.
-
Calabrese LH, Michel BA, Bloch DA, Arend WP, Edworthy SM, et al. (1990) The American College of Rheumatology 1990 criteria for the classification of hypersensitivity vasculitis. Arthritis Rheum 33: 1108.
-
Levy M, Broyer M, Arsan A, Levy-Bentolila D, Habib R, et al. (1976) Anaphylactoid purpura nephritis in childhood: natural history and immunopathology. Adv Nephrol Necker Hosp 6: 183.
-
Rigante D, Castellazzi L, Bosco A, Esposito S (2013) Is there a crossroad between infections, genetics, and Henoch-Schonlein purpura? Autoimmun Rev 12: 1016.
-
Yang YH, Yu HH, Chiang BL (2014) The diagnosis and classification of Henoch-Schönlein purpura: an updated review. Autoimmun Rev 13: 355.
-
Trnka P (2013) Henoch-Schönlein purpura in children. J Paediatr Child Health 49: 995.
-
Sohagia AB, Gunturu SG, Tong TR, Hertan HI (2010) Henoch-Schonlein Purpura - A Case Report and Review of the Literature. Gastroenterol Res Pract 2010: 597648
-
Roberts PF, Waller TA, Brinker TM, Riffe IZ, Sayre JW, et al. (2007) Henoch-Schonlein purpura: a review article. Southern medical journal 100: 821-824.
-
Dillon MJ, Ozen S (2006) A new international classification of childhood vasculitis. Pediatr Nephrol 21: 1219-1222.
-
Dillon MJ (2007) Henoch-Schonlein purpura: recent advances. Clin Exp Rheumatol 25: S66-S68.
-
Patel U, Bradley JR, Hamilton DV (1988) Henoch-Schonlein purpura after influenza vaccination. Br Med J (Clin Res Ed) 296: 1800.
-
Pimentel MI, Vasconcellos Ede C, Cerbino-Neto J (2011) Henoch-Schonlein purpura following influenza A H1N1 vaccination. Rev Soc Bras Med Trop 44: 531.
-
Mastroiacovo P (1976) Measles vaccination and Schonlein-Henoch purpura. Minerva pediatrica 28: 1591.
-
Szer IS (1994) Henoch-Schonlein purpura. Current opinion in rheumatology 6: 25-31.
-
Fox BC, Peterson A (1998) Leukocytoclastic vasculitis after pneumococcal vaccination. American journal of infection control 26: 365-366.
-
Le Hello C, Cohen P, Bousser MG, Letellier P, Guillevin L (1999) Suspected hepatitis B vaccination related vasculitis. J rheumatol 26: 191-194.
-
Watson DA (1992) Pustular vasculitis complicating BCG vaccination. Tuber Lung Dis 73: 126.
-
Bonetto C, Trotta F, Felicetti P, Alarcon GS, Santuccio C, et al. (2016) Vasculitis as an adverse event following immunization – Systematic literature review. Vaccine 34: 6641-6651.
-
Tizard EJ, Hamilton-Ayres MJ (2008) Henoch Schonlein purpura. Arch Dis Child Educ Pract Ed 93: 1-8.
-
Narchi H (2005) Risk of long term renal impairment and duration of follow up recommended for Henoch-Schonlein purpura with normal or minimal urinary findings: a systematic review. Arch Dis Child 90: 916.22. Narchi H (2005) Risk of long term renal impairment and duration of follow up recommended for Henoch-Schonlein purpura with normal or minimal urinary findings: a systematic review. Arch Dis Child 90: 916.
-
Habib R, Niaudet P, Levy M (1993) Schönlein-Henoch purpura nephritis and IgA nephropathy. In: Tisher CC, Brenner BM, Renal Pathology with Clinical and Functional Correlations. Lippincott, Philadelphia, 472.
-
Blanco R, Martinez-Taboada VM, Rodriguez-Valverde V, Garcia-Fuentes M, Gonzalez-Gay MA (1997) Henoch-Schonlein purpura in adulthood and childhood: two different expressions of the same syndrome. Arthritis and rheumatism 40: 859-864.
-
Lu S, Liu D, Xiao J, Yuan W, Wang X, et al. (2015) Comparison between adults and children with Henoch-Schonlein purpura nephritis. Pediatr Nephrol 30: 791.
-
Kauffmann RH, Houwert DA (1981) Plasmapheresis in rapidly progressive Henoch-Schoenlein glomerulonephritis and the effect on circulating IgA immune complexes. Clin Nephrol 16: 155.
-
Hattori M, Ito K, Konomoto T, Kawaguchi H, Yoshioka T, et al. (1999) Plasmapheresis as the sole therapy for rapidly progressive Henoch-Schönlein purpura nephritis in children. Am J Kidney Dis 33: 427.