Journal of Clinical Nephrology and Renal Care
Does Pre-Eclampsia Predispose Patients to the Development of Focal Segmental Glomerulosclerosis? "The Chicken or the Egg?"
Nicole Lioufas1, Jonathan Ling1, Juli Jaw2, Mathew Mathew2, Matthew Jose1 and Richard Yu1*
1Department of Nephrology, Royal Hobart Hospital, Australia
2Department of Nephrology, Launceston General Hospital, Australia
*Corresponding author:
Yu Richard, Department of Nephrology, Royal Hobart Hospital, Australia, E-mail: richard.yu@ths.tas.gov.au
J Clin Nephrol Ren Care, JCNRC-2-012, (Volume 2, Issue 2), Case Series
Received: June 14, 2016; Accepted: August 29, 2016; Published: August 31, 2016
Citation: Lioufas N, Ling J, Jaw J, Mathew M, Jose M, et al. (2016) Does Pre-Eclampsia Predispose Patients to the Development of Focal Segmental Glomerulosclerosis? "The Chicken or the Egg?". J Clin Nephrol Ren Care 2:012.
Copyright: © 2016 Lioufas N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Pre-eclampsia is the most common medical complication of pregnancy affecting 3-5% of pregnancies worldwide. Traditional teaching has generally maintained that the natural history of Pre-eclampsia is one of resolution of renal pathology and other clinical features- some days to weeks after delivery of the placenta. Renal injury is mediated by both endothelial and podocyte injury in pre-eclampsia. In some women however, the renal injury does not resolve and proteinuria persists following pregnancy. The development of further glomerular lesions, notably focal segmental glomerulosclerosis (FSGS) following pre-eclampsia, has previously been described, and is an association that is increasingly recognized.
Here we describe a heterogeneous case series of four women seen in our unit over a five year period who were diagnosed with FSGS some months to years following a pregnancy complicated by pre-eclampsia. These cases illustrate the complex and individual relationship that might exist between pre-eclampsia and FSGS lesions.
We also discuss current concepts in our understanding of the pathophysiology behind the complex relationship between podocyte injury in pregnancy and subsequent FSGS lesions in some women.
Keywords
Pre-eclampsia, FSGS, Podocyte, Podocytopathy, Pregnancy, Focal, Segmental, Glomerulosclerosis
Introduction
Pre-eclampsia is the most common medical complication of pregnancy affecting 3-5% of pregnancies worldwide [1]. Clinically, it is characterized by the development of hypertension at more than 20 weeks gestation, as well as the demonstration of hepatic, haematological, neurological or renal involvement. Most commonly, renal involvement in pre-eclampsia is defined as the development of proteinuria (quantified as proteinuria of ≥ 0.3 g/day). In other iterations, the definition is also met by a rising serum creatinine or development of oliguria [2]. Although renal biopsy is rarely ever required in diagnosing the clinical syndrome of pre-eclampsia, the classic glomerular lesion associated with pre-eclampsia is well described in the literature- characterized histologically by endotheliosis and podocyte swelling [3].
Traditional teaching has generally maintained that the natural history of Pre-eclampsia is one of resolution of renal pathology and other clinical features- some days to weeks after delivery of the placenta. Renal injury is mediated by both endothelial and podocyte injury in pre-eclampsia. In some women however, the renal injury does not resolve and proteinuria persists following pregnancy. The development of further glomerular lesions, notably focal segmental glomerulosclerosis (FSGS) following pre-eclampsia, has previously been described [4].
It is postulated that the podocytopathy seen in FSGS in these women post-partum can be a progression of the initial podocyte-induced damage in pre-eclampsia- once again contravening the traditional concept of pre-eclampsia as a disease with clinical ramifications limited to pregnancy and the peri-partum period. Podocyturia has been noted in patients with pre-eclampsia, although its utility remains uncertain. However, the relationship between FSGS lesions and Pre-Eclampsia can be both complex and individual in each patient- all of which may raise the age old question of which is the 'chicken and which is the egg'? Although, in a subset of women, the initial podocyte injury of pre-eclampsia in pregnancy can be a trigger for ongoing slit diaphragm dysfunction, cell death and scarring that converges eventually upon the histological endpoint of FSGS post-partum, it should also be recognised that in others, subtle features of FSGS may already exist going into pregnancy- but be unmasked and possibly progress far more rapidly in the setting of further podocyte injury from pre-eclampsia.
Here we describe a heterogeneous case series of three women seen in our unit over a five year period who were diagnosed with FSGS some months to years following a pregnancy complicated by pre-eclampsia. These cases illustrate the complex and individual relationship that might exist between pre-eclampsia and FSGS lesions.
A) A 17-year-old Caucasian woman, G1P0, was initially referred to a nephrology service at 26 weeks gestation for nephrotic range proteinuria. She had no prior comorbidities of note, including no history of hypertension or diabetes, and had a BMI of 29 kg/m2. Her urine dipstick at time of antenatal booking was negative for protein. At 22 weeks gestation, she was found to be hypertensive with a blood pressure of 160/100, with neurological features and proteinuria consistent with a diagnosis of pre-eclampsia. Her protein creatinine ratio at this time was > 900 mg/mol creat, and 24 hour urinary protein collection revealed proteinuria of 19.6 g/day, with benign sediment and a normal creatinine of 46 umol/L. This patient underwent renal biopsy to clarify the cause of her nephrotic range proteinuria, which revealed endothelial swelling with sub-endothelial deposits and IgM staining, which was consistent with glomerular endotheliosis and pre-eclampsia. Her delivery was expedited at this period of 26 weeks gestation due to foetal distress, and she delivered a healthy child via caesarean section.
However, sixteen months following this presentation, despite improvement in proteinuria indices, the patient still continued to experience hypertension and mild proteinuria (0.8 gr am/day). At this stage her renal function remained normal with a creatinine of 50 umol/L. To investigate this further, the patient underwent a second renal biopsy which revealed features consistent with FSGS affecting 2 of 14 glomeruli. At this time there were also some changes consistent with hypertensive nephrosclerosis, with hyalinosis of afferent and intralobular arterioles.
B) A 35-year-old woman, G1P1, was referred to the renal clinic one month after the birth of her son. Her pregnancy was complicated by pre-eclampsia at 37 weeks gestation, requiring emergency caesarean section. She had no significant past medical history of note, had a BMI of 21 kg/m2. Prior to her pregnancy she was documented as having normal renal function, with a creatinine of 60 umol/L and no proteinuria.
At four months post-partum, her serum creatinine was raised at 123 umol/L, with a urinary albumin-creatinine ratio of 310 g/mol creat. Her autoimmune screen was unremarkable. She progressed to renal biopsy, which revealed FSGS with 7 of 21 glomeruli sclerosed and 5% of the surface area affected by interstitial fibrosis. She was commenced on perindopril 2.5 mg daily, and currently has a creatinine of 143 mcmol/L.
C) A 30-year-old woman, G1P0, was referred to the renal unit 4 years ago for macroscopic haematuria and proteinuria first detected at 23 weeks gestation. She had a normal renal tract ultrasound, and no history of urinary tract infections or family history of renal disease. She had no history of hypertension or diabetes, and had a BMI of 18 kg/m2. She was a current smoker. Upon presentation, she had a urine protein-creatinine ratio of 252 g/mol creat, a 24 hour urinary protein of 1.2 gram/day, a mid-stream urine which showed 90 erythrocytes. Her autoimmune screen was unremarkable.
During pregnancy, the patient's blood pressure was managed with nifedipine and labetalol, and a decision was made to delay renal biopsy and observe. Unfortunately during her pregnancy, at 31 weeks gestation, she developed severe pre-eclampsia, placental abruption, culminating in a foetal death-in-utero.
At 10 weeks post-partum, her 24 hour urinary protein remained elevated at 1.05 grams/day and serum creatinine 89 umol/L. She proceeded to have a renal biopsy showing FSGS with 6 of 11 glomeruli sclerosed with 50% interstitial fibrosis. Considering her original booking urinary results, the concern was that this patient had underlying reflux nephropathy, which was confirmed on DMSA scan. Following this time, this young woman was keen to conceive a second time, and conceived later that year. She gave birth to a successful live-born child, however, this was with the cost of progression of her CKD. Over the following year, she was commenced on perindopril and amlodipine to manage her hypertension. However, her creatinine progressed over the course of two years from 125 umol/L postpartum, to 168 umol/L over 8 months, and subsequently to 337 umol/L over the following year. She progressed to dialysis dependence in the next year and is currently on Peritoneal Dialysis whilst awaiting the prospect of kidney transplantation (Figure 1 and Table 1).
.
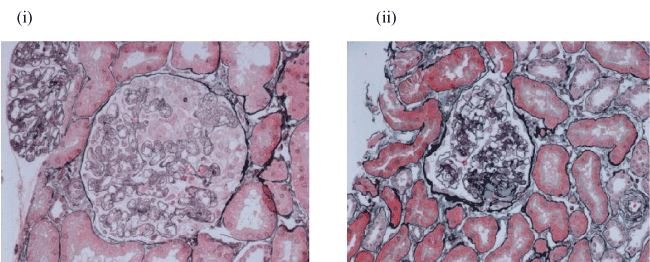
Figure 1: Case A's renal biopsy.
(i) Biopsy 1 (25 weeks gestation). Endotheliosis - characterised by endothelial swelling with sub-endothelial deposits and IgM staining; (ii) Biopsy 2 (15 months post-partum) FSGS - 2 out of 14 glomeruli sclerosed.
View Figure 1
![]()
Table 1: Summary of the details of the three cases.
View Table 1
Discussion
Pregnancy is a physiological stress test for the maternal kidney. There are a number of physiological changes in renal perfusion and filtration that occur, including the need for 'supra-physiological' levels of GFR in the course of a normal pregnancy. GFR rises to 150% of normal by the second trimester in physiological pregnancy and renal plasma flow increases by 50-85% [5]. In women with subtle or more significant pre-existing renal impairment, going into pregnancy, potential loss of renal function has long been a well described risk.
Pre-eclampsia affects 3-5% of pregnancies worldwide [1] and in recent years, has been demonstrated to be a powerful independent marker of increased CKD and ESRF risk in subsequent decades for affected women [6].
The time between a distant recorded history of pre-eclampsia and the increased risk of chronic kidney disease over decades has made that relationship more difficult to uncover; but in recent years, that relationship has been clearly recognised. In a population of Norwegian women, Vikse, et al. demonstrated that over a span of greater than four decades, a history of pre-eclampsia during first pregnancy was associated with a relative risk of ESRF of 4.7 (95% CI, 3.6 to 6.1). Pre-eclampsia during a second pregnancy in the same Scandinavian cohort was associated with a relative risk of 6.7 (95% CI, 4.1 to 9.9). All of this provides epidemiological insights into the observation that pre-eclampsia and its sequelae may not be limited to pregnancy alone [6].
The factors that underpin this long term increase in renal disease risk remain poorly elucidated. While some of this risk may be driven by overlapping risk factors for maternal pre-eclampsia and CKD in later life, other mechanisms including the possibility of pre-eclampsia being a trigger for long term de novo renal disease in some women has also been raised by recent literature.
Suzuki, et al. demonstrated in a Japanese renal biopsy case series that of 127 renal biopsies performed on women between the ages of 35-65 over a 160 year period for clinical indications, 32 women had a past history of pre-eclampsia. Of these 32 women, 12 demonstrated FSGS on renal biopsy. In contrast, of the 95 women with no history of pre-eclampsia in this Japanese cohort, no cases of FSGS were recorded as the final biopsy diagnosis [7,8].
Although the mechanism of this strong relationship between FSGS and past pre-eclampsia is unclear, these observations allude to the heightened risks, not only of kidney disease in later life among affected women- but more specifically, a dramatically increased risk of podocycopathy in later life, in the form of clinically relevant FSGS.
These observations at the epidemiological and clinical level have tended to span decades. Our small Case Series however concentrates on the more unusual scenario where clinically relevant kidney disease emerges in a relatively short space time (months to years) following a diagnosis of pre-eclampsia. While this occurrence in a short space of time is far less common, our Case Series highlights the potential role of pre-eclampsia in unmasking or setting off a cascade of renal injury that potentiates FSGS lesions. Furthermore, it needs to be recognised that for a small group of women, that relationship can manifest dramatically over months to a few years- and not only over the decades that is shown in epidemiological data and larger case series to date.
These cases represent a heterogeneous view of renal disease in this subset of women. Two of the above cases (A and B) appear to have resulted in the development of secondary FSGS following an initial pre-eclamptic insult. However, case C appears to have already had underlying renal pathology, the progression of which was likely potentiated by a pregnant and pre-eclamptic state. These cases also illustrate the potentially complex relationship between intrinsic renal disease and pre-eclampsia as well as the emerging evidence that pre-eclampsia and associated podocytopathy may well pre-dispose some women to de novo renal disease by triggering long term injury that increases the risk of FSGS lesions.
The latter is perhaps best illustrated by Case A which offers both histological and clinical insights into this potential relationship. In this 17 yo primigravid, renal biopsy at 25 weeks pregnancy for investigation of nephrotic range proteinuria (quantified at 19.6 g/day) demonstrated pathognomic histological findings of pre-eclampsia. This was followed by a second biopsy 15 months post-partum when mild proteinuria persisted (quantified at 0.8 g/day at the time of second biopsy). Previous endotheliosis characterizing pre-eclampsia had evolved into FSGS renal lesions in the intervening 16 months between the two biopsies.
The relatively short interval between clinically overt FSGS and recent pre-eclampsia in Cases A and B crystallises the same observations of significantly increased CKD and FSGS risks reported over decades in larger observational reports to date. It also begs the unanswered question of how pre-eclampsia may lead to FSGS in a subset of affected women over a highly variable span of time.
In analyzing patients with pre-eclampsia, the most common renal biopsy finding at the time is that of endotheliosis and podocyte effacement [3,9]. There are a number of complex pathological mechanisms underlying pre-eclampsia, including endothelial damage, placental ischaemia via decreased perfusion through the spiral arteries, and cytokine dysregulation resulting in a process similar to a thrombotic microangiopathy [10]. More recently there have been advances revealing dysregulation of podocyte related proteins and podocyte swelling [11]. Certainly other causes of glomerular scarring have been documented to cause this glomerular pathology when there is a failure of the auto-regulatory mechanisms and replacement of damaged podocytes [12]. This possibility is highlighted by Case A's serial biopsies, where endotheliosis progressed to FSGS over the course of one year.
However, we must also note that pre-eclampsia in itself is increased among patients with intrinsic renal disease. Potentially women that have silent renal pathology present for the first time during pregnancy, and therefore from their previously undiagnosed renal disease have an increased risk of pre-eclampsia. More recent studies reveal that pre-eclampsia is associated with the long-term development of end stage kidney disease [6]. Currently, the guidelines for the follow-up of pre-eclampsia recommend for women to initially be seen at 6 weeks postpartum, and subsequently have annual screening of their blood pressure and urinary protein indices [2]. This 6 week postpartum visit is essential to ensure the resolution of proteinuria and normalisation of blood pressure, to allow for further referral and evaluation of renal disease.
Overall, this case series highlights that pre-eclampsia is a systemic stressor which does not necessarily have an entirely benign course post pregnancy. The observation of long term CKD and ESRF risk among women with a history of Pre-eclampsia has been highlighted at epidemiological level.
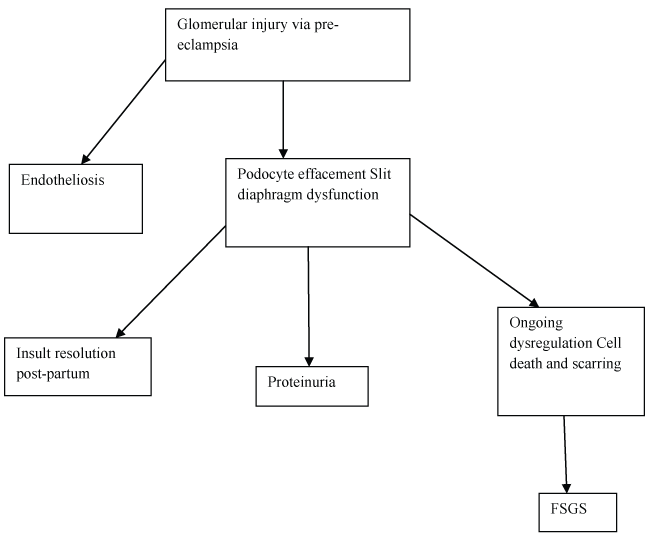
Furthermore, pre-eclampsia can potentially predispose women to the development of FSGS via a mechanism of podocyte induced injury that extends well beyond the pregnancy milieu to which traditional clinical models of pre-eclampsia have been limited (Figure 2).
.
Figure 2: Proposed pathophysiological mechanism for pre-eclampsia to predispose to FSGS.
View Figure 2
Our understanding of the long term sequelae of pre-eclampsia, in particular with regards to the risk and relationship to FSGS lesions remains incomplete. At a clinical level, however, this Case Series highlights the absolute importance of adhering to current guidelines [2] in following up all women post-partum, who have experienced pre-eclampsia- in order to document complete resolution of proteinuria and to ensure that there is no development of intrinsic renal pathology (at least in the short term) as a sequelae of pre-eclampsia.
References
-
Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R (2014) Pre-eclampsia Part 1: current understanding of its pathophysiology. Nat Rev Nephrol 10: 466-480.
-
Task Force for Hypertension in Pregnancy (2013) Hypertension in Pregnancy. The American College of Obstetricians and Gynecologists, Women's Health Care Physicians
-
Penning M, Bloemenkamp K, Zon T, Zandbergen M, Schutte J, et al. (2014) Association of Preeclampsia with Podocyte Turnover. Clinical Journal of the American Society of Nephrology 9: 1377-1385.
-
Garovic V (2014) The Role of the Podocyte in Preeclampsia. Clinical Journal of the American Society of Nephrology 9: 1337-1340.
-
Jeyabalan A, Conrad KP (2007) Renal Function During Normal Pregnancy and Pre-eclampsia. Front Biosci 12: 2425-2437.
-
Vikse B, Irgens L, Leivestad T, Skjaerven R, Iversen B (2008) Pre-eclampsia and the risk of end-stage kidney disease. N Engl J Med 359: 800-809.
-
Suzuki H (2013) Close association between pre-eclampsia and chronic kidney disease in middle aged women. 2nd International conference on Nephrology and Therapeutics, July 29-31, Embassy Suites Las Vegas, USA.
-
Suzuki H, Inoue T, Kikuta T, Okada H (2014) Renal diseases associated with pre-eclampsia in post menopausal women. J Clin Nephrol Res 1: 1011.
-
Han L, Yang Z, Li K, Zou J, Li H, et al. (2014) Antepartum or immediate postpartum renal biopsies in preeclampsia/eclampsia of pregnancy: new morphologic and clinical findings. Int J Clin Exp Pathol 7: 5129-5143.
-
Craici I, Wagner S, Weissgerber T, Grande J, Garovic V (2014) Advances in the pathophysiology of pre-eclampsia and related podocyte injury. Kidney Int 86: 275-285.
-
Aita K, Etoh M, Hamada H, Yokoyama C, Takahashi A, et al. (2009) Acute and Transient Podocyte Loss and Proteinuria in Preeclampsia. Nephron Clin Pract 112: c65-70.
-
D'Agati V, Frederick M, Kaskel F, Falk R (2011) Focal Segmental Glomerulosclerosis. N Engl J Med 365: 2398-2411.