Journal of Clinical Gastroenterology and Treatment
Colonoscopy-assisted Fecal Microbiota Transplant for Outpatient Treatment of Recurrent or Refractory Clostridium Difficile Colitis; Long Term Follow-up of 58 Patients
Daniel Greenwald1*, Tarun Patel3, Daniel McQuillen2 and Amy Barto3
1Department of Internal Medicine, Lahey Hospital and Medical Center, USA
2Department of Infectious Disease, Lahey Hospital and Medical Center, USA
3Department of Gastroenterology, Lahey Hospital and Medical Center, USA
*Corresponding author:
Daniel Greenwald, MD, Department of Internal Medicine, Lahey Hospital and Medical Center, 41 Mall Road, Burlington, MA 01805, USA, E-mail: Daniel.Greenwald@lahey.org
J Clin Gastroenterol Treat, JCGT-2-013, (Volume 2, Issue 1), Research Article; ISSN: 2469-584X
Received: November 11, 2015 | Accepted: January 30, 2016 | Published: February 02, 2016
Citation: Greenwald D, Patel T, McQuillen D, Barto A (2016) Colonoscopy-assisted Fecal Microbiota Transplant for Outpatient Treatment of Recurrent or Refractory Clostridium Difficile Colitis; Long Term Follow-up of 58 Patients. J Clin Gastroenterol Treat 2:013. 10.23937/2469-584X/1510013
Copyright: © 2016 Greenwald D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Clostridium difficile infection (CDI) costs the national healthcare system billions of dollars annually, and is reaching epidemic proportions. The current study seeks to evaluate the efficacy and long term outcomes of outpatient colonoscopy-assisted fecal microbiota transplant (FMT) therapy in between 2011-2013 for recurrent or refractory CDI as part of a single center experience. Among 58 patients who underwent FMT and completed the telephone survey, 91.4% were disease free at the 3 month interval, 86.2% at the 6 and 12 month intervals, and 80.5% at the 18 month interval post FMT.
Purpose: The current study seeks to evaluate the efficacy and long term outcomes of outpatient colonoscopy-assisted fecal microbiota transplant (FMT) therapy in between 2011-2013 for recurrent or refractory CDI as part of a single center experience.
Keywords
Clostridium difficile colitis, Fecal microbiota transplant, Infectious colitis
Introduction
CDI costs the national healthcare system billions of dollars annually, and is reaching epidemic proportions [1,2] There are multiple risk factors for CDI. These most commonly include antibiotic use, prolonged hospitalizations, immunosuppression, and proton pump inhibitors [3,4]. Standard treatment options for CDI include antibiotics such as metronidazole, vancomycin as well as newer antibiotics such as fidaxomicin.
Recurrence rates for CDI are as high as 33% after one episode, and reach 65% after a second episode [5-7], FMT has emerged as a remarkably successful treatment option for patients with recurrent or refractory CDI despite standard antibiotic therapy [3,4,6,8-16]. In the randomized controlled trial conducted by van Nood et al. it was found that duodenal infusion of donor feces was more effective for treatement of CDI than vancomycin alone. Of the cohort that received an infusion of donor feces, 81% experienced a resolution of CDI. This was in stark contrast to only 31% of the cohort that received antibiotic therapy alone with vancomycin having resolution of CDI [16].
The current response rate quoted for FMT (when delivered as fresh stool via colonoscopy) as a treatment option for CDI is 89.4% (n = 326) based on a meta-analysis by Rossen et al. [17]. Currently, the largest single study of FMT outcomes that spans the longest follow-up time period for treatment of CDI is by Brandt and colleagues, and includes 77 patients for an average follow up time period of 17 months [18]. The case series presented here includes an 18 month follow up time period for a total of 58 patients.
We analyzed the outcomes of a case series of patients who underwent outpatient FMT over a two year period at Lahey Hospital and Medical Center. Our results support the efficacy of FMT for the treatment of recurrent or refractory CDI.
Statement of Methods
A total of 58 out of 79 patients who had previously undergone outpatient colonoscopy-assisted FMT with fresh patient-selected donor stool completed a telephone survey to obtain long term follow-up data regarding sustained response or relapse of infection. This was an IRB approved, informed consent waived case series that included a retrospective data review of 79 FMT recipients at a single center during the years 2011 to 2013. All subjects previously underwent a single FMT for recurrent or refractory CDI at Lahey Hospital and Medical Center with the same colonoscopy-assisted protocol using fresh patient-selected donor stool by a single endoscopist. Patients with recurrent or refractory disease were treated alike for the purpose of this study. CDI was diagnosed and recurrence confirmed by symptoms of diarrhea as well as PCR test at Lahey Hospital’s laboratory. Severity of CDI was graded according to the classificaton scheme by Sageer et al. for mild, moderate, and severe CDI [19].
Patients included in the study were contacted by telephone to complete a verbal survey. The questions included in the telephone survey included confirming the number of recurrences of CDI or that the patient had infection refractory to antibiotic treatment, the date of FMT, and success or relapse after the procedure (any relapse was corroborated by the medical record and PCR). The survey also included asking if the patient was taking probiotics prior to FMT and whether or not the patient had taken antibiotics for any indication after FMT. The interviewers also confirmed if the patients were on immunosuppressant medications or a PPI at the time of FMT, and whether or not they had any underlying gastroentestinal diseases such as Celiac disease, irritable bowel syndrome, or inflammatory bowel disease. The procedure records for each patient were reviewed to ensure that all colonoscopies were performed in a similar manner, had adequate bowel preparation, and that the donor stool was delivered consistently at the ileocecal valve in all patients. Exclusion criteria included a previous diagnosis of inflammatory bowel disease, hospitalization for any reason other than supervised bowel preparation at the time of FMT, or an inability to contact the subjects.
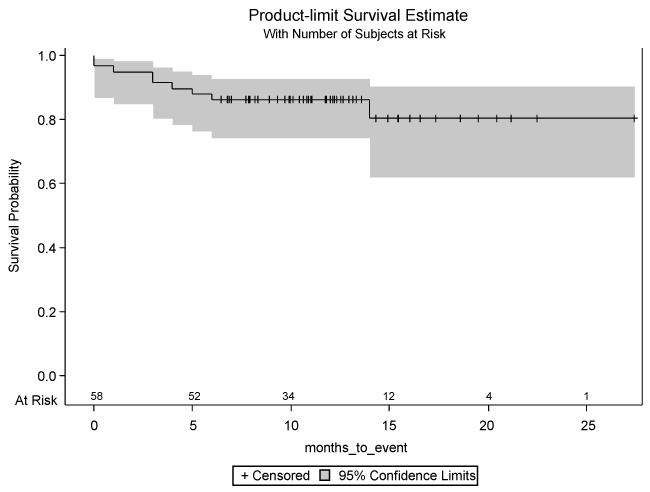
In terms of statistical analysis of the results, the investigators used a combination of the Chi-square test for categorical variables, the Fischer exact test for categorical variables with sparse data, student t-test for continuous variables, and Kruskal-Wallis test for count variables. In order to graphically represent the disease free time period for all patients post FMT, the investigators used a Kaplan-Meier plot (Figure 1) to display the disease free time period post FMT as analogous to the “survival” that is typically displayed on such a plot.
Summary of Results
With regard to demographic information, the 58 patients included 17 male patients and 41 female patients, with a median age of 69.5 years (Table 1). All patients had either recurrent or refractory CDI. All of the patients contacted were at least 18 months from the time of FMT and underwent a single FMT procedure. The cohort with recurrent CDI experienced a median of 4 relapses prior to FMT. There were 41 patients (71.9% of the cohort) who underwent FMT as an outpatient and the remaining 17 patients (29.3%) underwent the procedure as an inpatient, merely hospitalized for the purpose of a supervised bowel preparation. Among the 58 patients, 28.1% were classified as having mild CDI, 26.3% as moderate infection, and 45.6% as severe CDI [17,19].
![]()
Table 1: Patient descriptive statistics (n = 58).
View Table 1
Among 58 patients who underwent FMT and completed the telephone survey, 91.4% (n = 53) were disease free at the 3 month interval, 86.2% (n = 50) at the 6 and 12 month intervals, and 80.5% (n = 47) at the 18 month interval post FMT (Table 2). Within the entire cohort of 58 patients included in the present study, more of the patients who had a recurrence of CDI after FMT also had documented co-morbid gastrointestinal illnesses, such as celiac disease, compared to those who had resolution of their disease (Table 1). This was a statistically significant finding (p = 0.0186). Additionally, a subgroup difference was found among those patients who were on immunosuppressive medications prior to FMT, favoring relapse (p = 0.0186). A larger portion of patients who were disease free had used a probiotic supplement prior to FMT, when compared to patients who had recurrence at follow-up (p = 0.0186). The type of probiotic was variable among patients. There was also a trend observed towards relapse for those patients who had taken antibiotic therapy of any sort after FMT, with 55.6% of those patients experiencing a relapse of CDI, although this was not a statistically significant difference (p = 0.2358).
![]()
Table 2: Summary of disease free time period for n = 58 patients who underwent FMT for CDI, with 95% confidence intervals.
View Table 2
Lastly, there were no adverse events post FMT that were reported in our cohort of 58 patients, which would have included complications such as bowel perforation, sepsis, or death.
Discussion
For patients with recurrent or refractory CDI, our case series supports the efficacy of FMT as a treatment associated with a sustained disease free period as long as 18 months for over 80% of the patients reviewed. This may be an underestimation of the true disease free period post-FMT for the general population, as patients with co-morbid GI illness who were included in the present study were more likely to relapse. This may be related to innate differences in the bowel flora of those patients with gastrointestinal diseases, such as celiac or IBS. Regarding those patients who did not experience a sustained disease free period, there was a trend towards coincident use of antibiotic therapy post FMT as well as immunosuppressive medications pre-transplant. Although these were not statistically significant differences in our cohort, both of these characteristics are well established independent risk factors associated with susceptibility to CDI as well as risk of CDI relapse [4]. It was also shown that patients who remained disease free at the time of follow-up were more likely to have used probiotic therapy prior to FMT. Although this was not standardized across patients, this may support the role for adjunct probiotic supplementation of any sort prior to FMT. Larger studies are needed to examine whether this is a true association.
Limitations of the current study include the innate inaccuracies of patient report, which was used to confirm disease free period. While recurrences were confirmed by PCR at Lahey Hospital's laboratory, not all disease free patients had been seen at a follow-up appointment. The present study also used fresh patient selected donor stool for FMT, and therefore, the microbiota of the stool transplants was not standardized across recipients. Currently, there is a trend towards using frozen donor stool from a stool bank, which would standardize the donor flora that is delivered via FMT.
The current study represents the largest single center cohort of subjects who received colonoscopically administered FMT with the longest follow-up period (18 months) to date. With the data presented here, we further support the longevity of a sustained disease free period post FMT for the treatment of recurrent or refractory CDI.
Summary Box
What is known about the subject?
-- CDI costs the healthcare industry billions of dollars annually
-- Recurrence rates are high for CDI
-- CDI recurrence rates are as high as 65% after 2 episodes
What are the new findings?
-- Colonoscopy assisted FMT has a remarkable success rate
-- Sustained disease free rate as high as 91.4% at 3 months
-- Probiotic therapy before FMT was associated with better outcomes
How will it impact practice in the future?
-- FMT may become a first line treatment for recurrent or refractory CDI
-- FMT may be able to reduce morbidity and mortality from CDI
-- Supplementing probiotic therapy before FMT may emerge as a new standard
References
-
Ghantoji SS, Sail K, Lairson DR, DuPont HL, Garey KW (2010) Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect 74: 309-318.
-
McDonald LC, Owings M, Jernigan DB (2006) Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996-2003. Emerg Infect Dis 12: 409-415.
-
Aas J, Gessert CE, Bakken JS (2003) Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 36: 580-585.
-
Bakken JS (2009) Fecal bacteriotherapy for recurrent Clostridium difficile infection. Anaerobe 15: 285-289.
-
Pépin J, Routhier S, Gagnon S, Brazeau I (2006) Management and outcomes of a first recurrence of Clostridium difficile-associated disease in Quebec, Canada. Clin Infect Dis 42: 758-764.
-
Rohlke F, Surawicz CM, Stollman N (2010) Fecal flora reconstitution for recurrent Clostridium difficile infection: results and methodology. J Clin Gastroenterol 44: 567-570.
-
Shaughnessy MK, Amundson WH, Kuskowski MA, DeCarolis DD, Johnson JR, et al. (2013) Unnecessary antimicrobial use in patients with current or recent Clostridium difficile infection. Infect Control Hosp Epidemiol 34: 109-116.
-
Borody TJ (2000) "Flora Power"-- fecal bacteria cure chronic C. difficile diarrhea. Am J Gastroenterol 95: 3028-3029.
-
Borody TJ, Warren EF, Leis SM, Surace R, Ashman O, et al. (2004) Bacteriotherapy using fecal flora: toying with human motions. J Clin Gastroenterol 38: 475-483.
-
Eiseman B, Silen W, Bascom GS, Kauvar AJ (1958) Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 44: 854-859.
-
Garborg K, Waagsbø B, Stallemo A, Matre J, Sundøy A (2010) Results of faecal donor instillation therapy for recurrent Clostridium difficile-associated diarrhoea. Scand J Infect Dis 42: 857-861.
-
Gough E, Shaikh H, Manges AR (2011) Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 53: 994-1002.
-
Persky SE, Brandt LJ (2000) Treatment of recurrent Clostridium difficile-associated diarrhea by administration of donated stool directly through a colonoscope. Am J Gastroenterol 95: 3283-3285.
-
Silverman MS, Davis I, Pillai DR (2010) Success of self-administered home fecal transplantation for chronic Clostridium difficile infection. Clin Gastroenterol Hepatol 8: 471-473.
-
Tvede M, Rask-Madsen J (1989) Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet 1: 1156-1160.
-
van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, et al. (2013) Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 368: 407-415.
-
Rossen NG, MacDonald JK, de Vries EM, D'Haens GR, de Vos WM, et al. (2015) Fecal microbiota transplantation as novel therapy in gastroenterology: A systematic review. World J Gastroenterol 21: 5359-5371.
-
Brandt LJ, Aroniadis OC, Mellow M, Kanatzar A, Kelly C, et al. (2012) Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol 107: 1079-1087.
-
Sageer, M and Barto, A (2014) Recurrent Clostridium difficile infection: The scope of the problem and management decisions. Semin Colon Rectal Surg 25: 158-162.