International Journal of Women's Health and Wellness
Survey of Breast Cancer Incidence in the Middle East and the United States
Barbara G Silverman1*, Hoda Anton-Culver2*, Freddie Bray3, Stevens Lisa4, Eser Sultan5, Nimri Omar6, Pavlos Pavlou7, Kevin Ward8, Haris Charalambous7, Anna Demetriou7, Argyrios Ziogas2, Ariana Znaor3, Lital Keinan-Boker1 and Chang Jenny2
1Israel National Cancer Registry, Israel Ministry of Health, Israel
2Department of Epidemiology, University of California, Irvine, USA
3International Agency of Research on Cancer, France
4Center for Global Health, National Cancer Institute, Maryland, USA
5Izmir Cancer Registry, Izmir & Hacettepe University, Ankara, Turkey
6National Cancer Registry of Jordan, Jordan
7Cyprus Cancer Registry, Cyprus
8Georgia Cancer Registry Emory University, USA
*Corresponding authors: Barbara G Silverman, Director, Israel National Cancer Registry, Israel Ministry of Health, HaShomer, Israel, Tel: 972-3-737-1516, E-mail: Barbara.silverman@moh.health.gov.il;
Hoda Anton-Culver, Director, Department of Epidemiology, University of California, Irvine, CA 92697-7550, USA, Tel: 949-824-7416, E-mail: hantoncu@uci.edu
Int J Womens Health Wellness, IJWHW-3-045, (Volume 3, Issue 1), Research Article; ISSN: 2474-1353
Received: November 26, 2016 | Accepted: February 18, 2017 | Published: February 20, 2017
Citation: Silverman BG, Anton-Culver H, Bray F, Lisa S, Sultan E, et al. (2017) Survey of Breast Cancer Incidence in the Middle East and the United States. Int J Womens Health Wellness 3:045. 10.23937/2474-1353/1510045
Copyright: © 2017 Silverman BG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Breast cancer is the most frequent cancer and the leading cause of cancer deaths in women worldwide. Regional comparisons of breast cancer incidence can shed light on population and health care factors that contribute to differences between countries. We surveyed breast cancer incidence in four population based cancer registries in the Middle East to identify factors associated with regional variations in breast cancer incidence.
Methods: The four participating registries (Izmir Turkey, Cyprus, Jordan and Israel) are members of the Middle East Cancer Consortium (MECC), and follow standard methods for cancer coding and staging. We identified all in situ and invasive breast cancer cases in women reported to the four participating registries for the period from 2005-2010. The WHO world standard population was used to calculate age-standardized incidence rates (ASR) for the four registries and for the US SEER program, for purposes of comparison.
Results: Breast cancer incidence varied widely among the four participating countries, with the highest rates occurring in Jewish Israeli women (100.4/100,000) and the lowest rates in Turkish women (50.7/100,000). SEER rates were similar to those of Israeli Jewish women and considerably higher than those observed in Israeli Arab, Turkish and Jordanian women. Age-standardized rates of in situ breast cancer were considerably higher in the SEER population than in the MECC registry population.
Conclusions: Differences in breast cancer incidence between countries in a single region most likely reflect a variety of lifestyle and genetic factors as well as the availability of and compliance with mammography screening in the different countries. Regional comparisons of cancer incidence offer neighboring countries opportunities to identify common risk factors and share strategies for cancer prevention and control.
Keywords
Breast cancer, Breast neoplasms, Epidemiology, SEER program, Middle East
Background
Breast cancer is the most frequent cancer in women, and the leading cause of cancer deaths in women worldwide [1]. Heredity, lifestyle factors such as diet, obesity and physical activity [2] reproductive factors and hormone replacement therapy [3,4] have all been associated with the incidence of breast cancer. Complicating efforts to study worldwide patterns in breast cancer incidence and the impact of various risk factors, is the considerable variation between countries in the use of screening mammography for early diagnosis of breast cancer. As part of a larger project examining cancer trends among Middle East Cancer Consortium (MECC) members [5] we analyzed breast cancer incidence in four member registries of the Middle East Cancer Consortium (Izmir, Turkey; Cyprus; Jordan and Israel) using cancer registry data in order to identify factors associated with differences in breast cancer incidence in this region.
Methods
Cancer registry data and population denominator data were provided by the four central cancer registries participating in the Middle East Cancer Consortium collaborative research projects:
The Cyprus Cancer Registry (CyCR) is a population-based registry, which started functioning under its new structure in May 1998. CyCR covers the population resident in the Government controlled area of Cyprus. The population resident in the area was 858,000 in 2013.
The Jordan Cancer Registry (JCR) is a national, population-based registry founded in 1996 in collaboration with MECC as an operational reporting system in the Jordanian Ministry of Health. The main objectives of JCR were to record the annual incidences of all cancers, overall and by gender, and to observe cancer incidence trend changes. The JCR covers the entire kingdom, including all resident population groups (approximately 9 million). Cancer cases are identified by the registry through a combination of passive and active data collection methods. Data are entered into a secure registry database according to coding standards of the International Classification of Disease for Oncology, Third edition. Registry operations include internal and external quality control activities.
The Izmir Cancer Registry (ICR) is a regional population-based registry covering the province of Izmir in Western Turkey. The province of Izmir covers an area of 11,973 km2 and is one of Turkey's most densely populated areas with 4.1 million inhabitants, out of which 91.4% live in urban areas. The ICR was established in 1993 and has become the core of the Cancer Registry system of Turkey. The ICR has functioned as the IARC Regional Hub for North Africa, West and Central Asia since October 2013. The estimated completeness of the registry is higher than 97% [6].
The Israel National Cancer Registry (INCR) is a population-based registry established in 1960. The registry covers the entire Israeli population, which numbers approximately 8 million (75% Jewish, 21% Arab, 4% other). Since 1982, hospitals, pathology and cytology laboratories and other health care providers have been required by law to submit reports of covered diseases to the registry. Completeness of the registry for solid tumors has been estimated at 94% [7]. For the period covered by the study, INCR data were entered and maintained in a purpose-built database system. All of the remaining registries used CanReg-5 [8] for data entry and maintenance processing.
For purposes of comparison, we also accessed breast cancer incidence data from the US Surveillance, Epidemiology and End Results (SEER) Program. The SEER program of the National Cancer Institute (NCI) contains approximately 97% of all incident cancer cases from tumor registries that covered 14% of U.S. population in 1995 to 28% for the time period of year 2005-2010 [9]. The SEER Program registries routinely collect data on patient demographics, primary tumor site, tumor morphology and stage, first course of treatment, and follow-up for vital status.
Analysis data sets prepared by the participating registries included all cases of in situ and invasive cancer arising in the breast (ICD-O-3 topography codes C50.0-C50.9 excluding lymphomas at these sites) in women included in the covered population of each registry for the period from 2005-2010. Most MECC countries follow the rules suggested by the International Association of Cancer Registries (IACR) and do not register second primary breast cancers when diagnosed in the contralateral breast [10]. SEER and the Israel National Cancer Registry register follow slightly different rules and consider each breast a primary site and therefore would register a second breast cancer case in the contralateral breast as a new primary. In order to enable comparisons of incidence rates between countries, we applied the IARC/IACR rules for multiple primaries to the SEER and Israeli data. We calculated annual age-specific breast cancer incidence for five year age categories as well as age-standardized incidence rates, using the WHO world standard population (grouping ages 75+ in a single category to match the age breakdown available for Israeli population data-see Appendix), for the following population groups: Izmir, Jordan, Cyprus, Israeli-Jewish and Israeli-Arab. Age-standardized rates and their 95% confidence intervals were calculated using the direct method [11] where in age-specific rates in the study population were applied to the corresponding age group in the WHO 2000-2025 world standard population [12]. Ninety-five percent confidence intervals of incidence rates were used to compare differences between rates. If the intervals for two incidence rates overlap, then there is no significant difference between them. Israeli cancer incidence figure were calculated separately for the Jewish and Arab populations to allow for comparison of cancer rates in the two groups, and comparison to the Muslim and non-Muslim populations in the other three participating countries.
Stage of disease at the time of diagnosis is determined according to criteria established by the Middle East Cancer Consortium (MECC) [13] and the 2000 SEER Summary Staging Manual [14]. In most registries stage determination is performed by tumor registrars on the basis of the available documentation. However, in Cyprus, a substantial proportion of cases are staged according to a clinical assessment completed by a physician.
For purposes of comparing distribution of morphologic diagnoses between countries, we divided breast cancer cases into 8 groups: ductal carcinoma in situ (ICD-O-3 morphology codes 8500-8503, fifth digit = 2), lobular carcinoma in situ (ICD-O-3 morphology code 85202), invasive ductal carcinoma (ICD-O-3 morphology codes 8500-8503, fifth digit = 3), invasive lobular carcinoma (ICD-O-3 morphology code range 85203), combination morphologies (ICD-O-3 morphology codes 85223, 85233, 85243), inflammatory breast cancer (ICD-O-3 morphology code 85303) and Paget's disease (ICD-O-3 morphology codes 85403-85433) and other (all other morphology codes).
The project was carried out as a part of the routine public health surveillance and reporting functions of the participating registries. Data analyses were performed using anonymized data and did not require patient contact or review of medical records.
Results
Age-adjusted invasive breast cancer incidence rates varied widely between the populations studied, with the highest rates for the total study period occurring in Jewish Israeli women (98.8/100,000) and the lowest rates in Turkish women (50.7/100,0000) (Table 1). The annual SEER rates were similar to those of Israeli Jewish women and considerably higher than those observed in Israeli Arab, Turkish and Jordanian women.
![]()
Table 1: Age standardized rates of invasive breast cancer in women, participating registries and SEER registries, overall and by years, 2005-2010.
View Table 1
![]()
Table 2: Age standardized rates of in situ breast cancer in women, participating registries and SEER registries, overall and by years, 2005-2010.
View Table 2
As was the case with invasive breast cancer, age-adjusted rates of in situ cancer varied considerably between the populations studied. Jewish Israeli women had the highest rate of in situ breast cancer (12.5/100,000) and Jordanian women, the lowest (1.4/100,000). The SEER rate of in situ cancer was approximately twice that of the rate in Israeli Jewish women (Table 2). The proportion of cases with unknown stage at diagnosis was high in all MECC registries and varied widely within registries by year of diagnosis (Table 3). Of cases with known stage, the proportion diagnosed as in situ ranged from 5% among Turkish women to 20.4% in the SEER population. The proportion with documented distant spread ranged from 3.6% in Israeli-Jewish women to 15.6% in Jordanian women (Figure 1 and Table 4). The proportion of cases with distant spread at diagnosis appeared to rise with age at diagnosis among Jordanian and Turkish women, but no clear trend with age was seen in the other participating registries (Table 5).
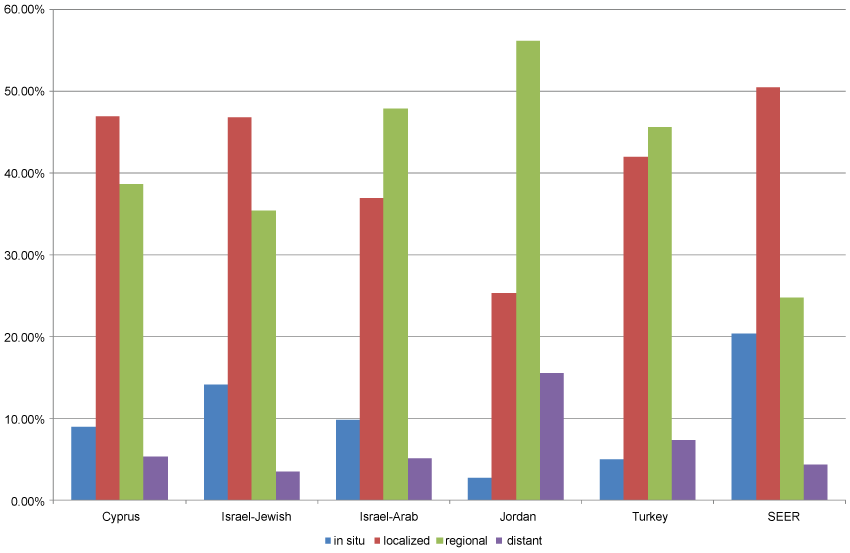
.
Figure 1: Distribution of summary stage at diagnosis*, breast cancer in women, participating registries and SEER registries, 2005-2010.
*Among cases with known stage at diagnosis.
View Figure 1
![]()
Table 3: Proportion of breast cancers in women with unknown stage at diagnosis, participating registries and SEER registries, by year, 2005-2010.
View Table 3
Invasive ductal carcinoma made up the overwhelming majority of cases for all age groups in most of the participating countries (66-80% depending on country and age group). A notable exception was Turkey, where invasive ductal carcinoma constituted only 52-56% of cases, and "mixed" histologies were more frequently reported (14-16%, compared to 1-4% in other MECC countries).
![]()
Table 4: Distribution of summary stage at diagnosis in breast cancer cases with known stage, participating registries and SEER registries.
View Table 4
![]()
Table 5: Distribution of summary stage at diagnosis in breast cancer cases with known stage, by age at diagnosis, participating registries and SEER registries.
View Table 5
Breast cancer incidence rose steeply with advancing age. In women under the age of 45, age-specific incidence of invasive breast cancer was highest in the SEER and Israeli Jewish populations, and lowest in the Jordanian population (Table 6).
![]()
Table 6: Age-specific rates of invasive breast cancer, per 100,000, participating registries and SEER registries.
View Table 6
Discussion
Comparison of patterns of breast cancer incidence in women in MECC member countries demonstrates considerable variation between the countries studied and offers opportunities for the transfer of information between countries that can improve cancer surveillance, prevention and treatment. Among Israeli Jewish and Cypriot women, adjusted incidence rates are similar to those of US women, while Israeli Arab, Jordanian and Turkish women have rates between half and two thirds those of US women. Furthermore, rates of in situ cancer were found to be considerably (two-fold to over 10-fold) lower in women in MECC member countries than in American women.
Differences in data collection, case definitions, and registry operations can account for variations in breast cancer incidence between countries. Given that MECC member registries operate using a standard set of criteria for identifying and coding cancer cases, it is likely that other factors known to be associated with variations in breast cancer risk are responsible for the observed variation between populations. These factors might include including screening practices, age at menarche and at menopause, parity, breastfeeding, oral contraceptives and hormone replacement therapy use, age at first and last pregnancy, genetic predisposition, alcohol consumption, smoking and socioeconomic status. While cancer mortality data are also useful for international comparisons, we limited our analysis to incidence data. The reasons for focusing on incidence data were as follows: 1) mortality data are dependent not only on the prevalence of risk factors and screening behaviors in the community but also on the quality and availability of medical care and local treatment practices, 2) mortality data are not routinely collected by all of the participating registries, and 3) given the long-standing cooperation between MECC registries and adherence to standards of coding, we believe that the coding of incidence data in the participating registries is more consistent than that of mortality, which is based on death certification.
Local variations in the use of mammography can have a considerable impact on the observed rate of breast cancer, particularly of early stage cancers [15]. It is therefore important to consider any potential differences in the availability and practice of nationwide screening between the four MECC member registries.
Cyprus implemented a national breast screening program targeting women ages 50-69 in 2003. While the national program provides biennial screening for the target population, screening in the private sector (outside the program) is recommended on a yearly basis by nearly all centers. The program initially started in the Nicosia district, with Larnaca and Paphos added in 2004, Famagusta district in 2006 and Limassol in 2007, thus achieving national coverage in 2007. Records for 2003-2008 show that the response rate of women who received a mammography invitation was 47% for that period, a figure that does not take into consideration women who undergo mammography screening in the private sector. According to public hospital records the number of mammograms conducted at public hospitals tripled in a decade, from 8,174 mammograms conducted in 2000, to 24,643 in 2010 [16]. A survey of 31 countries in Europe, Asia and North American reported that in 2006 there were 36 mammography units in Cyprus, translating into a mammography screening capacity of 84/1,000,000 women [17].
The Jordan Breast Cancer Program (JBCP) initiated nationwide breast cancer screening in 2006 [18]. However, a survey of Jordanian women indicated that compliance with recommended mammography screening guidelines is less than 10% [19].
In Israel, national guidelines are in place for biennial screening between the ages of 50 and 74. A national program of quality measures assesses compliance with mammography screening in the target population [20]. While past surveys have shown that participation in mammography screening of Arab Israeli women, particularly those in the Muslim and Druze community, lagged behind that of Jewish Israeli women [21,22], considerable progress has been made. The Israel National Breast Cancer Surveillance Program found that in 2013, compliance with mammography screening in the population of Arab women was nearly identical to that in Jewish women (69% and 70%, respectively) [23].
A population-wide breast cancer surveillance program has not been implemented for the population of Izmir, Turkey. However, as a result of Turkish health care system reform, utilization rates of both outpatient and inpatient breast cancer screening clinics have increased dramatically since 2003. The broadening of health service availability, in conjunction with public awareness campaigns, has offered increased opportunities for patient education and referrals for opportunistic screening [24].
The rate of in situ breast cancer diagnosis in the SEER population was considerably higher than that of the populations covered by the registries in this study. The most likely explanation for this finding is that until 2009, the USPSTF guidelines supported mammography screening every 1 to 2 years in women starting from the age of 40 [25]. In 2009, the recommendation was changed to biennial screening for women between the ages of 50 and 74, consistent with the applicable guideline in Europe [26]. Differences in implementation and compliance with mammography screening in MECC countries, as compared to mammography uptake in the US are also likely to account for some of the differences observed. In particular, women in traditional religious communities in MECC countries may be less likely to participate in screening [18].
While variations in mammography screening alone may contribute to apparent differences in breast cancer incidence due to enhanced case finding, between-country variation may also reflect true differences in disease incidence resulting from differences in the distribution of risk factors in the populations of the difference countries. Reproductive factors are of considerable importance in the pathogenesis of breast cancer, with nulliparity, early menarche and late menopause being associated with increased risk. Data from the World Bank indicate that in Jordan, fertility declined from 7.3 per woman in 1980 to 3.2 in 2013. Less dramatic declines were observed in Turkey and Cyprus (from 4.4 to 2.0, and 2.4 to 1.5 respectively [27]. In Israel fertility has remained stable since 1980, with rates in 2014 of 3.1 in the Jewish population and 3.2 in the Arab population [28]. In contrast, the fertility rate in the United States has been persistently low during the past three decades (1.8 in 1980 and 1.9 in 2013) [27]. Taking these data together would explain to some degree the current low incidence of breast cancer in the Turkish, Jordanian and the Israeli Arab populations compared to that of the US, since low parity in the 1980s in the US has translated into higher breast cancer rates while the relatively high parity in some MECC countries has acted as a protective factor. These data would indicate the potential for a future increase in breast cancer in these MECC countries subsequent to decreasing parity.
Postmenopausal hormone replacement therapy has been shown to be associated with an increased risk of breast cancer, although the magnitude of this effect may be modulated by other factors such as patient age, BMI and breast density [29]. Postmenopausal hormone replacement therapy use is apparently less widespread in MECC member countries for which data are available than among American women. A sharp drop in the use of oral estrogens in postmenopausal women (from 20% to 10%) was documented in a large Israeli health maintenance organization (HMO) subsequent to the publication of the Women's Health Initiative study findings in published in 2002. This finding is likely to be representative of national trends in postmenopausal hormone use in Israel [30]. A case control study of risk factors for breast cancer in Cypriot women found that 85% of cases and 71% of controls has never used hormonal replacement therapy [31]. Use of postmenopausal hormone replacement therapy is rare in Turkey [32].
The MASTOS case-control study carried out in Cyprus from 2002-2006 provided evidence for the role of diet in the pathogenesis of breast cancer. Participants donated blood samples for DNA extraction and completed a questionnaire, which included lifestyle and dietary information. The results showed that higher consumption of vegetables, fish and olive oil were independently associated with decreased risk of breast cancer [33]. Trichopoulou, et al. reported a negative association between adherence to a Mediterranean diet and breast cancer incidence in postmenopausal women in Greece [34]. Hence the low incidence rates of breast cancer in Jordan and Turkey, followed by Cyprus as compared to Israel and the SEER data, may be to a small degree due to differences in diet, with these countries having less westernized diets than Cyprus and Israel.
Despite the wide-spread perception that the incidence of invasive breast cancer is increasing in young women, longitudinal analysis of SEER data does not support this conclusion [35]. Among the MECC member registries, the incidence of invasive breast cancer in women under the age of 45 was highest among Israeli Jewish women (22.9/100,000, comparable to the SEER rate of 24.6 in the same age group). In the older age groups, in whom the majority of cancer cases occurred, patterns of age-specific incidence mirrored those of overall incidence.
The discovery of highly penetrant breast cancer susceptibility genes such as the BRCA genes in the mid 1990's [36] focused attention on genetic factors in the pathogenesis of breast cancer. Between 2% and 2.5% of Ashkenazi Jewish women are carriers of BRCA1 or BRCA2 mutations [37] and the lifetime risk of developing breast cancer among carriers has been estimated at over 80% [38]. About 10% of breast cancer cases in Ashkenazi women are associated with these mutations [38], but prevalence of the mutation is considerably more frequent in women under the age of 40 at the time of diagnosis [39]. The importance of genetic factors in breast and ovarian cancer incidence in Israel has led to calls for genetic testing of all women of Ashkenazi background, rather than limiting testing to those with a known family history [40]. In contrast, in Cyprus a study undertaking mutation analysis of the BRCA1 gene revealed very low frequency of mutations in the BRCA1 gene in breast cancer patients with a family history of breast cancer [41].
Conclusions
Age-standardized rates of breast cancer in women in the four participating Middle Eastern registries varied widely. Among the four, the rates of invasive breast cancer among Israeli Jewish women were closest to those of US women, as represented by the SEER database, while rates in Arab Israeli women more closely resembled those of women in Turkey and Jordan. In situ breast cancer showed a considerably lower incidence in all of the populations studied than in the SEER population. While mammography intake in Cyprus and Israel is a likely explanation for the higher cancer incidence rates observed in these countries, the difference in incidence between the Israeli-Jewish and Israeli-Arab populations points to the impact of lifestyle factors and genetics on breast cancer incidence. Regional comparisons of cancer incidence provide the opportunity for neighboring countries to identify common risk factors and share strategies for cancer prevention and control.
Highlights
• Breast cancer incidence varies widely among Middle East Cancer Consortium member countries.
• Invasive breast cancer rates among Israeli Jewish women were similar to those of the SEER population, while rates in Arab Israeli women more closely resembled those of women in Turkey and Jordan.
• In situ breast cancer incidence was considerably lower in the participating countries than in the SEER population.
• Regional comparisons of cancer incidence provide the opportunity for neighboring countries to identify common risk factors and share cancer prevention and control strategies.
References
-
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. (2015) Cancer Incidence and Mortality Worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359-E386.
-
Chlebowski RT (2013) Nutrition and physical activity influence on breast cancer incidence and outcome. Breast 22: S30-S37.
-
Chlebowski RT, Anderson GL (2015) Menopausal hormone therapy and breast cancer mortality: Clinical implications. Ther Adv Drug Saf 6: 45-56.
-
Grosse Y, Baan R, Straif K, Secretan B, El Ghissassi F, et al. (2009) WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens-Part A: pharmaceuticals. Lancet Oncol 10: 13-14.
-
Anton-Culver H, Chang J, Bray F, Znaor A, Stevens L, et al. (2016) Cancer Burden in Four Countries of the Middle East Cancer Consortium (Cyprus, Jordan, Israel, Izmir (Turkey) with Comparison to the United States Surveillance, Epidemiology and End Results Program. Cancer Epidemiol 44:195-202.
-
Eser S, Ozdemir R, Yakut C, Karakilinc H, Ozen E, et al. (2010) Estimating completeness of selected cancer registries data in Turkey; an evaluation using the capture-recapture Method. The 32nd Annual Meeting of International association of Cancer Registries, Yokohama, Japan.
-
Fishler Y, Shetrit A, Barchana M, Modan B (2003) Assessment of the completeness of the Israel Cancer Registry database-methods and findings [in Hebrew]. Israel Center for Disease Control.
-
Ervik MJ (2014) Can Reg5. International Agency for Research on Cancer.
-
Overview of the Surveillance, Epidemiology, and End Results (SEER) program.
-
International Association of Cancer Registries (2004) International rules for multiple primary cancers (ICD-O third edition). Lyon, International Agency for Research on Cancer Internal Report No 2004/02.
-
Breslow NE, Day NE (1987) Statistical methods in Cancer Research, Vol II-The design and analysis of cohort studies. IARC Scientific Publications No. 83. Lyon: International Agency for Research on Cancer.
-
https://seer.cancer.gov/stdpopulations/world.who.html.
-
Middle East Cancer Consortium MECC (2013) Manual of Coding and Staging. (5th edn), Version 5.1, July 2009, Israel.
-
John L Young, Steven D Roffers, Lynn A Gloeckler Ries, April G Fritz, Annette A Hurlbut (2001) SEER summary staging manual-2000: Codes and coding instructions. National Cancer Institute, NIH Pub. NO. 01-4969, Bethesda, MD.
-
Harding C, Pompei F, Burmistrov D, Welch HG, Abebe RBreast, et al. (2015) Breast cancer screening, incidence, and mortality across US counties. JAMA Intern Med 175: 1483-1489.
-
Paraskevi A Farazi (2014) Cancer trends and risk factors in Cyprus. Ecancermedicalscience 8: 389.
-
Autier P, Ouakrim DA (2008) Determinants of the number of mammography units in 31 countries with significant mammography screening. Br J Cancer 99: 1185-1190.
-
Donnelly TT, Khater AH, Al-Bader SB, Al Kuwari MG, Al-Meer N, et al. (2013) Arab Women's Breast Cancer Screening Practices: A Literature Review. Asian Pac J Cancer Prev 14: 4519-4528.
-
Othman A, Ahram M, Al-Tarawneh MR, Shahrouri M (2015) Knowledge, attitudes and practices of breast cancer screening among women in Jordan. Health Care Women Int 36: 578-592.
-
Jaffe DH, Shmueli A, Ben-Yehuda A, Paltiel O, Calderon R, et al. (2012) Community healthcare in Israel: quality indicators 2007-2009. Isr J Health Policy Res 1: 3.
-
Keinan-Boker L (2006) Performance of Breast Cancer Early Detection in Arab Women in Israel-Room for Improvement. J Womens Health (Larchmt) 15: 542-545.
-
Azaiza F, Cohen M (2006) Health beliefs and rates of breast cancer screening among Arab women. Journal of Women's Health 15: 520-530.
-
Rennert G (2013) National Program for Early Detection of Breast and Colon Cancer Annual Report, National Program for Breast Cancer Detection, Hebrew.
-
Baris E, Mollahaliloglu S, Aydin S (2011) Healthcare in Turkey: from laggard to leader. BMJ 342: c7456.
-
U.S. Preventive Services Task Force (2002) Screening for breast cancer: recommendations and rationale. Ann Intern Med 137: 344-346.
-
U.S. Preventive Services Task Force (2009) Screening for breast cancer: US preventive services task force recommendation statement. Ann Intern Med 151: 716-726.
-
http://data.worldbank.org/indicator/SP.DYN.TFRT.IN.
-
Israel Central Bureau of Statistics. Israel in Figures 2014.
-
Hou N, Hong S, Wang W, Olopade OI, Dignam JJ (2013) Hormone Replacement Therapy and Breast Cancer: Heterogeneous Risks by Race, Weight, and Breast Density. J Natl Cancer Inst 105: 1365-1372.
-
Barbara G Silverman, Ehud S Kokia, (2009) Use of hormone replacement therapy, 1998-2007: sustained impact of the Women's Health Initiative findings. Annals of Pharmacotherapy 43: 251-258.
-
Andreas Hadjisavvas, Maria A Loizidou, Nicos Middleton, Thalia Michael, Rena Papachristoforou, et al. (2010) An investigation of breast cancer risk factors in Cyprus: a case control study. BMC Cancer 10: 447.
-
Biri A, Bakar C, Maral I, Karabacak O, Bumin MA (2005) Women with and without menopause over age of 40 in Turkey: consequences and treatment options. Maturitas 50: 167-176.
-
Demetriou CA, Hadjisavvas A, Loizidou MA, Loucaides G, Neophytou I, et al. (2012) The Mediterranean dietary pattern and breast cancer risk in Greek-Cypriot women: a case-control study. BMC Cancer 12: 113.
-
Trichopoulou A, Bamia C, Lagiou P, Trichopoulos D (2010) Conformity to traditional Mediterranean diet and breast cancer risk in the Greek EPIC (European Prospective Investigation into Cancer and Nutrition) cohort. Am J Clin Nutr 92: 620-625.
-
Rosenberg SM, Newman LA, Partridge AH (2015) Breast cancer in young women: rare disease or public health problem. JAMA Oncol 1: 877-888.
-
Foulkes WD (2014) BRCA1 and BRCA2-update and implications on the genetics of breast cancer: a clinical perspective. Clin Genet 85: 1-4.
-
Warner E, Foulkes W, Goodwin P, Meschino W, Blondal J, et al. (1999) Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst 91: 1241-1247.
-
King MC, Marks JH, Mandell JB; New York Breast Cancer Study Group (2003) Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 302: 643-646.
-
Sade RB, Chetrit A, Figer A, Papa MZ, Flex D, et al. (2006) Hormone replacement therapy is more prevalent among Jewish BRCA1/2 mutation carriers. Eur J Cancer 42: 650-655.
-
Gabai-Kapara E, Lahad A, Kaufman B, Friedman E, Segev S, et al. (2014) Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A 111: 14205-14210.
-
Hadjisavvas A, Neuhausen SL, Hoffman MD, Adamou A, Newbold RF, et al. (2001) BRCA1 germline mutations in Cypriot breast cancer patients from 26 families with family history. Anticancer Res 21: 3307-3311.





