International Journal of Women's Health and Wellness
Overview of Locally Advanced Breast Cancer: A Huge Challenge to Science
Francine Carla Cadona1*, Alencar Kolinski Machado1,2, Marco Aurélio Echart Montano3, Charles Elias Assmann1,4 and Ivana Beatrice Mânica da Cruz1,2,4
1Department of Morphology, Federal University of Santa Maria, Brazil
2Graduate Program in Pharmacology, Federal University of Santa Maria, Brazil
3Graduate Program in Bioscience and Health, University of the West of Santa Catarina, Brazil
4Graduate Program in Biological Sciences, Toxicological Biochemistry, Federal University of Santa Maria, Brazil
*Corresponding author:
Francine Carla Cadona, Biogenomics Laboratory, Department of Morphology, Health Science Center, Federal University of Santa Maria, Santa Maria, RS, Roraima Avenue 1000, Building 19, Santa Maria-RS, Brazil, Zip code: 97105-900, Tel: 55-55-32208736, Fax: 55-55-32208239, E-mail: fran.cine.bio@hotmail.com
Int J Womens Health Wellness, IJWHW-3-044, (Volume 3, Issue 1), Review article; ISSN: 2474-1353
Received: November 26, 2016 | Accepted: February 07, 2017 | Published: February 10, 2017
Citation: Cadona FC, Machado AK, Montano MAE, Assmann CE, da Cruz IBM (2017) Overview of Locally Advanced Breast Cancer: A Huge Challenge to Science. Int J Womens Health Wellness 3:044. 10.23937/2474-1353/1510044
Copyright: © 2017 Cadona FC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Cancer incidence and cancer-related deaths are dramaticallys rising worldwide. In women, current estimates show high incidence of breast cancer (BC). BC is considered the second most frequent cancer-related death causer in women worldwide, responsible for millions of deaths each year. However, BC early diagnosis improves treatment success and increase survival rates. Physical examination, mammography, ultrasound, magnetic resonance imaging are techniques that auxiliary BC diagnosis. On the other hand, BC relapse is a significant concern especially when the cancer spreads to the mammary and adjacent tissues and this leads to the development of locally advanced BC (LABC). Inflammatory BC (IBC), which is considered a subtype of LABC, is a rare and aggressive disease that causes swelling, redness, affecting third or more of the breast. Normally, chemotherapy is used in the first-line treatment of LABC, however multidrug resistance (MDR) is a severe problem found in the treatment. Studies are been performed to discover new alternative therapies, mainly in natural products, to solve this obstacle improving treatment responsiveness. In this review we will address aspects of LABC such as pathophysiology, diagnosis, current treatments and new therapeutic perspectives as well as LABC epidemiological parameters, emphasizing the worldwide incidence scenario.
Keywords
Breast cancer (BC), Locally advanced BC (LABC), Inflammatory BC (IBC), LABC epidemiology, LABC treatment, LABC future directions
Introduction
Breast cancer (BC) is considered the main type of cancer that occurs among women and the second most frequently responsible for cancer-related deaths in this gender worldwide [1]. High incidence of BC is observed in several different countries in terms of social and financial development. Recent statistical numbers showed 1.8 million estimated new BC cases in 2013 [2]. Moreover, current projections for 2050 estimate approximately 3.2 million new BC cases in women worldwide [3].
BC development starts in the mammary glands and ducts. Tumor growth initiates through several synergistic factors that contribute to genetic mutations, such as micro environmental and epigenetics changes [4]. Because of late detection and/or poor response to treatment, cancer cells can spread to other tissues via blood circulation, lymphatic system and/or by contiguity, developing distant tumor, in a process known as metastasis [5].
Different stages are observed in BC, following the classification between I to IV. In stage III, cancer has spread to the mammary and adjacent tissues, and cancers are classified as locally advanced BC (LABC). LABC is correlated with the development of architectural tissue alteration in the mammary tissue, because of the tumor growth. LABC includes advance primary tumors, frequently with lymph nodes involvement and Inflammatory BC (IBC) [6].
LABC is associated to poor survival and frequently patients with this condition die, despite of several treatments, such as chemotherapy and radiotherapy [7]. In this sense, LABC has been a common subset of all BC patients for the development of new therapies. In this review, we will focus on clinical trials in LABC, side effects of therapies used in this setting, and quality of life of patients enrolled in these trials. Moreover, the aim of this review is address update aspects of LABC, such as epidemiological parameters, pathophysiology, diagnosis, current treatments and new therapeutic perspectives.
Body
LABC epidemiological parameters
Worldwide, the incidence of BC has increased by over 20% and BC-related deaths have risen by 14% since 2008. Although developed countries still have the highest incidences of breast cancer, low to middle income countries (LMICs) still face a huge number of deaths related to the highest cancer mortality rates [8]. Figure 1 indicates the countries with the top 20 highest incidences of BC in 2012. Belgium had the highest rate of BC, followed by Denmark and France [9] (Figure 1).
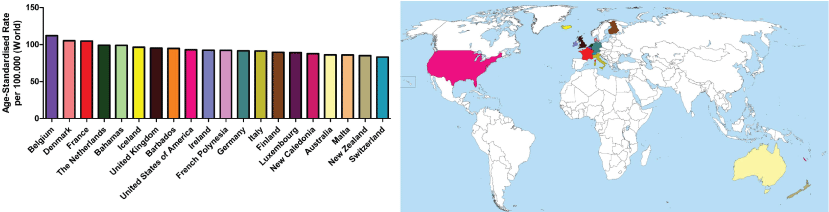
.
Figure 1: Representative image of the countries with the top 20 highest incidence of breast cancer in 2012.
View Figure 1
Moreover, current estimates show that approximately 8.5% of American and 4% of European patients with BC develop LABC [10]. Although these numbers seem to be low and despite LABC is confined to the breast and regional nodes, the high mortality rate in this condition is explained by the rapid onset of metastatic disease [11]. Fortunately, since the use of mammography became more usual and common in screening tests, LABC rates are decreasing. In the United States of America (USA) the percentage of patients with LABC that realized regular screening mammography is less than 5% [12].
IBC, a specific type of LABC, and rarer than other types of BC, is responsible for just a few cancers diagnosed cases. In the USA, for example, only 1 to 5% of BC diagnosed patients present with IBC. Additionally, IBC is more often found in younger patients compared to other cancer types, being more prevalent in African American women. Also, it seems to exist a relationship between IBC and body weight since this disease is more frequent in overweight subjects. IBC also can be diagnosed in men, however, differently than in women, this type of cancer occurs in advanced age [13].
According to the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program the average survival period after diagnosis for women with LABC is 4.9 years. However, this register cannot be extremely reliable since patients that developed IBC present less survival time [9].
LABC pathophysiology
LABC is related to bulky invasive tumor which spreads to breast skin and/or chest. LABC development may be followed by several consequences, such as skin ulceration and edema, tumor fixation into the chest wall, increase of the lymph nodes size and skin fixation of auxiliary nodes [14].
LABC includes large tumors, usually the tumor size is bigger than 5 centimeters in diameter. In this sense, the tumor mass can cause injury to breast skin, underlying muscles and multiple local lymph nodes [11].
Furthermore, the most aggressive LABC form, IBC, can cause swelling, redness, affecting third or more of the breast [15]. The edema, in this case known as peau d'orange, occurs because cancer cells block lymph vessels in the skin of the breast with lymphatic fluid extravasation. Other symptoms can be noted in this type of cancer, such as a rapid increase in breast size, sensation of inflammation as heaviness, aching, burning, or tenderness and moreover, patients can present with inverted nipple (facing inward) [16].
IBC has rapid progression, and high angiogenic and angioinvasive development. This ability to invade vessels confers to IBC an extremely high metastatic potential, making this illness the most lethal BC type [17]. Several molecules involved in this pathway were identified as overexpressed in IBC tissue samples when compared to non inflammatory LABC samples, such as vascular endothelial growth factor (VEGF) and its receptor VEGFR, an important signal protein that stimulates angiogenesis [18,19]. Also, overexpression of inflammatory cytokines were described in IBC tissue samples, including basic fibroblast growth factor (bFGF), interleukin (IL)-6, IL-8, IL-1, IL-12, and interferon gamma (IFN-γ). Further, some gene related to insulin-like growth factor-binding protein, for example HER-2, RhoC GTPase, and NF-KB are overexpressed in IBC [20-22].
Moreover, in comparison with non-IBC, IBC is frequently associated to negative estrogen receptor (ER) and progesterone receptor (PR). Previous reports suggested that ER-positive patients presented a better prognostic and a longer survival compared to those IBC ER-negative subjects (4 years versus 2 years, respectively) [17,23].
IBC has a very complex pathophysiology. Despite IBC being classified as a LABC, it seems to present a unique tumor pattern and specific characteristics. IBC has predominant growth, immortalized cells, tissue adhesion pathways and it is very aggressive. These tumor features are consequence of different genes and molecules expressions that provide the metastatic ability and tumor progression [24,25].
LABC diagnosis
Mammography screening is an essential evaluation for diagnosing tumoral BC tissue with less than 1 centimeter of size. Only mammography can detect the tumor at this size ensuring in this way approximately 95% of cure. Physical examination is important to find tumor, however just tumors bigger than 1 centimeter are identified in this way, decreasing the chance of cure. In this sense, bilateral mammogram screening is a significant tool to identify tumor in early stages and increase patient's survival rates. Although mammography helps to decrease breast cancer-related deaths, it is not recommended to young women [26]. Routine mammography screening is only suggested for women over the age of 40 years, since before that age mammography sensitivity is low [27].
In some cases, high mammary density can make difficult breast evaluation by mammography. In these subjects, ultrasound is a good alternative for evaluation. Ultrasound is helpful to determine tumor extensive breast involvement, nodal chains impairment, and to confirm mammography inconclusive results. Besides, ultrasound is used as an adjuvant to mammography in cases of abnormal results or it is applied as the first choice in special situations, for instance, in pregnancy, lactation, young women and inflammatory conditions in the breast tissue. In the presence of lesions found in the breast tissue by mammography, ultrasound auxiliaries in the biopsies collection as well as it is able to identify additional lesions in 14% of women with high mammary density [28].
Breast tissue biopsy is the most specific way to diagnose tumor development; however, breast surgery is an invasive procedure. In some cases, it is possible to perform a core needle biopsy that is essential to aid the diagnosis. The fine-needle aspiration biopsy has a high sensitivity and specificity in LABC. Moreover, the identification of several detectable biomarkers from tumor samples, such as hormone receptors and inflammatory molecules, could help in the decision of treatment and improve patient's survival [29].
Likewise, breast-specific magnetic resonance imaging (MRI) is frequently used in the diagnostic setting, mainly in young and high breast density women. Theoretically, routine screening is not suggested for women under 40 years old, however, annual breast MRI is indicated for women over 30-years-old that present a lifetime breast cancer risk at or exceeding 20%-25%. This group of population comprises women that suspect of a deleterious genetic mutation associated with BC or received radiation to treat lymphoma [30].
MRI is more sensitive to reveal LABC extension and staging of tumor than conventional methods, such as mammography and ultrasonography. Hence, MRI is a commonly used imaging modality in LABC. Pre-operative MRI is essential to LABC patients for surgical planning. MRI can classify the BC appearance by is morphology into different phenotypic categories. This information is involved with response of neoadjuvant chemotherapy and can offer breast-conserving surgery. Moreover, MRI can inform LABC in triple negative, Her 2 positive and hormone receptor negative tumors, especially in solid imaging phenotype. Moreover, MRI is able to detect tumor physiology by demonstrating contrast enhancement associated with tumor angiogenesis. In this sense, MRI can provide an earlier more accurate marker of tumor than conventional methods [31].
Therefore, MRI, in the same way to ultrasound, has high sensitivity, on the other hand presents low specificity for BC detection. Nevertheless, MRI similarly to ultrasound is considered more sensitive than mammogram for the dense breast tissue evaluation [32].
LABC current treatments
LABC treatment is the biggest challenge after diagnosis, since at BC stage III there is higher risk of metastasis. There are no standard therapy procedures conducted for LABC patients, particularly because this patient group is very heterogeneous. In this sense, the ideal therapy should be personalized and individual, considering the patient characteristics, as tumor size, ER, PR, human epidermal growth factor receptor-2 (HER2), local extension, and lymph nodes invasion state [33-36].
The most common methods for BC therapy, including LABC, are surgery [37], radiotherapy [38], and chemotherapy [39]. All these procedures are performed to control the locoregional disease and to eliminate distant metastasis. However, even with the treatment, there are a lot of patients that present tumor recurrences [40].
The surgery for complete breast removal is called mastectomy. First of all, to perform this type of surgery it is essential to evaluate if surgery is feasible. Second of all, it is necessary to consider three main aspects, including primary tumor area size, skin or thoracic involvement jury, and axillary lymph node impairment. Finally, gathering all this information it is possible to select the most effective mastectomy procedure for the patient. There are four different mastectomy methods: radical mastectomy, modified radical mastectomy, simple total mastectomy, and subcutaneous mastectomy. Currently, the most used mastectomy procedure is the modified radical mastectomy that differently from the radical mastectomy, consists of breast removal and axillary lymphadenectomy (surgery to remove axillary lymph nodes), preserving the breast muscle [39]. Radical mastectomy is not used commonly because of the high mortality rates, comorbidity, associated with this kind of method. On the other hand, simple total mastectomy and subcutaneous mastectomy are not indicated as an alternative way for LABC treatment [34,41].
Radiotherapy is another technique that can be used for LABC patients [34]. This procedure is able to destroy tumor cells through ionizing radiation beam [42]. The radiation dose is calculated considering the tumor tissue volume to kill all the neoplastic cells with less damage to normal cells than possible. Irradiated cells undergo water hydrolysis and DNA damage following vital system and proliferation capacity impairments. Radiotherapy is indicated for regional or local treatment, being used alone or in association with another procedure, before, during or after surgery and/or chemotherapy. Depending of the radiation dose and machine source, radiotherapy can be classified as superficial, semiprofound, profound teletherapy, profound therapy and brachytherapy. Unfortunately, radiotherapy has some immediate and late side effects, such asmucositis, while late side effects comprise fibrosis and tissue atrophies. In order to minimize side effects and damages to normal cells, the tolerance of normal area must be respected [39,43-45].
On the other hand, subjects with LABC can also be treated by chemotherapy [34,46]. Chemotherapy is a method based on chemical agents in the treatment of diseases caused by biological mediators. In the treatment of different cancer types, chemotherapy is used as antineoplastic to specially reduce cell proliferation [47]. Currently, there are several chemotherapeutic drugs being commercialized by the pharmaceutic industry and all these drugs perform their function acting in the cellular cycle. Chemotherapeutic drugs that act in cells at proliferative stage or not are called non-specific cell cycle. Drugs that damage cells only during the cellular proliferation are called cell cycle-specific. Additionally, there are some chemotherapeutics agents that perform their function at specific cellular cycle phases (synthesis, G2 or mitosis phases), and those drugs are nominated phase-specific agents [47-49]. Depending on the tumor development and stage of progression, the chemotherapy is a therapeutic alternative with curative purpose, however, in advanced situations, as for LABC, these drugs are used as neoadjuvant procedure or as palliative option to increase the quality of survival [50]. The biggest problem involving chemotherapy administration is that these agents are not specific for neoplastic cells. In this sense, usually chemotherapeutic drugs induce several side effects. These negative effects go from nausea, vomiting and agitation including also damages to the nervous central system related to the post period of administration being classified as early, immediate, late, or ultra-late side effects [37,47,51,52]. Regarding to this problem, several researches around the world look for the development of more specific chemotherapeutic agents for the tumor cells with the aim of reducing side effects which could compromise therapeutic attendance.
To achieve BC remission, patients with LABC can perform all three main treatment alternatives, mastectomy, radiotherapy, and chemotherapy, usually combined or in different therapeutic phases. However, systemic procedures and/or radiotherapy before surgery also can be perform by some institutes [37]. However, neoadjuvant chemotherapy, which is the administration of therapeutic agents before the main treatment procedure, has been an important alternative for LABC therapy [40,52,53].
Unfortunately, there are many patients with LABC that present resistance to chemotherapy [54]. In this sense, several researches are trying to understand the tumor cell pathway of drug resistance development to find new targets and propose new chemotherapic agents. There are some aspects in cancer cells that can promote chemotherapy resistance, such as absorption, distribution, transport, metabolism, and elimination. A study performed by Vishnukumar, et al. [55], for example, described that P-glycoprotein (P-gp), an ATP-dependent drug efflux pump which is able to transport anti-cancer drugs, is influenced by MDR1 gene. MDR1 gene, also called ABCB1 gene, is located at chromosome 7 (q21.1). MDR1 polymorphism directly affects P-gp expression, changing the anti-tumor agent transport.
The HER2 is a protein responsible for the development and growth of epithelial cells. However, HER2 gene is a proto-oncogene and an unspecific alteration in the sequence can activate this gene to an oncogene. Some LABC patients present HER2-positive receptors and it has been used as a new therapy target [40,56,57]. Trastuzumab emtansine is a recent chemotherapeutic drug which is an antibody loaded with the anti-tumor drug. The antibody binds specifically cells that present HER2 receptors expressed in the cellular membrane and release the drug inside of the tumor cell. This type of treatment has less side effects, is faster than common agents, and has been improving HER2-positive LABC treatment. On the other hand, some subjects still will develop recurrences post trastuzumab administration [57,58]. In this sense, several works are focusing in the identification of more effective procedures. A new proposal in this respect isneoadjuvant therapy in HER2-positive BC using pertuzumab associated with transtuzumab. The results showed higher percentage of patients achieving a pathological complete response with pertuzumab and trastuzumab than trastuzumab treatment alone [59]. In summary, the figure 2 shows a schematic representation of BC diagnosis and treatment.
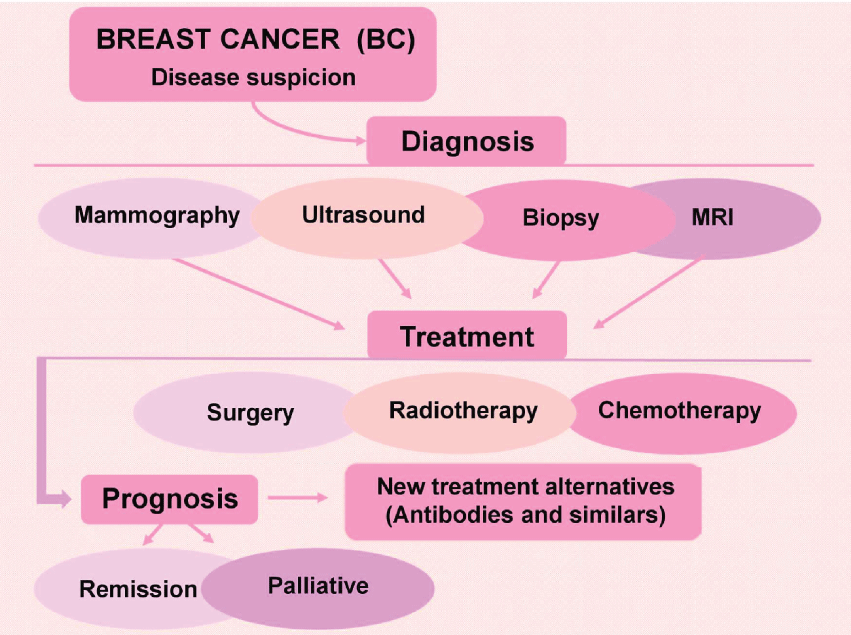
.
Figure 2: Schematic representation of breast cancer diagnosis and treatment approaches and disease progression (Tryfonidis, et al. 2015).
View Figure 2
Despite these neoadjuvant therapies and treatment improved, intensive clinical research in this field should be maintained. A promising area is the use of natural products that are composed by a bioactive chemical matrix, including different molecules that are able to positively modulate cellular homeostasis, especially by antioxidant capacity. New perspectives of treatment for LABC are discussed in the next section.
LABC new perspectives
In some cases, LABC can present pharmacological resistance and is difficult to treatment, resulting in poor prognosis. Normally, chemotherapy is the first-line way of therapy in LABC, however multidrug resistance (MDR) is a severe problem found during the treatment. Therefore, it is very important for patient's survival to detect MDR before initiate the first-line chemotherapy [54].
In this sense, Atalay, et al. (2006) reported that some genes are involved in LABC MDR, such as ATP-binding cassette sub-family B member 1 gene (ABCB1 - chromosome 7) and ATP-binding cassette sub-family C member 1 gene (ABCC1 - chromosome 16). The results found in this study, performed with 25 women with LABC before chemotherapy, suggested that ABCB1 gene expression during chemotherapy increased the resistance and treatment unresponsiveness. Nevertheless, ABCC1 gene expression did not contribute strongly with the clinical drug response [60].
Since LABC patients frequently present MDR during the pharmacological therapy, studies to improve this obstacle are been performed. A previous report indicated that guaraná (Paullinia cupana), a functional food native from Amazon, composed of high levels of caffeine, catechins and other bioactive molecules with several functional proprieties [61] presents antitumor activity against BC and increased the response to chemotherapeutic drugs [62]. Study performed by Hertz, et al. (2015) suggested that guaraná extract is able to decrease BC cells (MCF-7) proliferation and increase chemotherapy activity of several drugs commonly used for BC therapy [49].
Moreover, some bioactive molecules found in natural products have been studied as antitumor agents. Epigallocatechin-3-gallate, for example, found in huge concentrations in green tea, is capable of potentiating the antiproliferative activity of sunitinib by inhibiting Mitogen-activated Protein Kinase (MAPK) in the MCF-7 cell line, the most frequently altered pathway in this type of cancer [38].
Besides, caffeine also was tested in addition of cisplatin to analyze whether this molecule could be able to increase the chemotherapy response in MCF-7 cells. Niknafs (2011) reported that caffeine increased cisplatin response by apoptosis induction [62].
Conclusion
In summary, LABC is considered an aggressive type of BC and responsible for poor prognosis, resulting in low survival. LABC has the capacity to spread very fast and sometimes, this condition can complicate developing inflammatory injuries in the breast tissue, in case of IBC. Moreover, LABC is associated with difficult treatment, since LABC frequently present chemotherapy resistance. To solve this obstacle, new studies are being performed to find alternative therapies to improve treatment responsiveness. At this point, LABC is considered a huge challenge to science, since this condition is target for the development of new therapies to increase chemotherapy response and ensure patients survival and quality of life.
References
-
Siegel RL, Miller KD, Jemal A (2009) Cancer statistics. CA Cancer J Clin 59: 225-249.
-
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H, et al. (2015) The Global Burden of Cancer 2013. JAMA Oncol 1: 505-527.
-
Hortobagyi GN, de la Garza Salazar J, Pritchard K, Amadori D, Haidinger R, et al. (2005) The global breast cancer burden: variations in epidemiology and survival. Clin Breast Cancer 6: 391-401.
-
Polyak K (2007) Breast cancer: origins and evolution. J Clin Invest 117: 3155-3163.
-
Weigelt B, Peterse JL, van 't Veer LJ (2005) Breast cancer metastasis: markers and models. Nat Rev Cancer 5: 591-602.
-
International Union Against Cancer (2009) TNM classification of malignant tumors. (7th edn), Wiley-Blackwell, New Jersey, USA.
-
Tryfonidis K, Senkus E, Cardoso MJ, Cardoso F (2015) Management of locally advanced breast cancer-perspectives and future directions. Nat Rev Clin Oncol 12: 147-162.
-
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in globocan. Int J Cancer 136: E359-E386.
-
World Cancer Research Fund International (WCRFI) (2016).
-
Allemani C, Sant M, Weir HK, Richardson LC, Baili P, et al. (2013) Breast cancer survival in the US and Europe: a CONCORD high-resolution study. Int J Cancer 132: 1170-1181.
-
Onyinye D Balogun, Silvia C Formenti (2015) Locally advanced breast cancer - strategies for developing nations. Front Oncol 5: 89.
-
Giordano SH (2003) Update on locally advanced breast cancer. Oncologist 8: 521-530.
-
Anderson WF, Schairer C, Chen BE, Hance KW, Levine PH (2005) Epidemiology of inflammatory breast cancer (IBC). Breast Dis 22: 9-23.
-
Newman LA (2009) Epidemiology of locally advanced breast cancer. Semin Radiat Oncol 19: 195-203.
-
Chia S, Swain SM, Byrd DR, Mankoff DA (2008) Locally advanced and inflammatory breast cancer. J Clin Oncol 26: 786-790.
-
Merajver SD, Sabel MS (2004) Inflammatory breast cancer. In: Harris JR, Lippman ME, Morrow M, Osborne CK, Diseases of the Breast. (3rd edn), Lippincott Williams and Wilkins, Philadelphia.
-
Kleer CG, van Golen KL, Merajver SD (2000) Molecular biology of breast cancer metastasis. Inflammatory breast cancer: clinical syndrome and molecular determinants. Breast Cancer Res 2: 423-429.
-
Van der Auwera I, Van Laere SJ, Van den Eynden GG, Benoy I, van Dam P, et al. (2004) Increased angiogenesis and lymphangiogenesis in inflammatory versus noninflammatory breast cancer by real-time reverse transcriptase-PCR gene expression quantification. Clin Cancer Res 10: 7965-7971.
-
Shirakawa K, Furuhata S, Watanabe I, Hayase H, Shimizu A, et al. (2002) Induction of vasculogenesis in breast cancer models. Br J Cancer 87: 1454-1461.
-
Guérin M, Gabillot M, Mathieu MC, Travagli JP, Spielmann M, et al. (1989) Structure and expression of c-erbB-2 and EGF receptor genes in inflammatory and non-inflammatory breast cancer: prognostic significance. Int J Cancer 43: 201-208.
-
Kleer CG, Zhang Y, Pan Q, Gallagher G, Wu M, et al. (2004) WISP3 and RhoC guanosine triphosphatase cooperate in the development of inflammatory breast cancer. Breast Cancer Res 6: R110-R115.
-
Cohen EN, Gao H, Anfossi S, Mego M, Reddy NG, et al. (2015) Inflammation Mediated Metastasis: Immune Induced Epithelial-To-Mesenchymal Transition in Inflammatory Breast Cancer Cells. PLoS One 10: e0132710.
-
Wu M, Merajver SD (2005-2006) Molecular biology of inflammatory breast cancer: applications to diagnosis, prognosis, and therapy. Breast Dis 22: 25-34.
-
Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, et al. (2001) Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 7: 192-198.
-
Ishikawa M, Kitayama J, Kazama S, Nagawa H (2005) Expression of vascular endothelial growth factor (VEGF)-C in preoperative biopsy specimens and metastatic foci of regional lymph nodes in submucosal gastric carcinoma. World J Surg Oncol 3: 2.
-
Cuckle H (1991) Breast cancer screening by mammography: an overview. Clin Radiol 43: 77-80.
-
Foxcroft LM, Evans EB, Porter AJ (2004) The diagnosis of breast cancer in women younger than 40. Breast 13: 297-306.
-
Hlawatsch A, Teifke A, Schmidt M, Thelen M (2002) Preoperative assessment of breast cancer: sonography versus MR imaging. AJR Am J Roentgenol 179: 1493-1501.
-
Ragaz J, Baird R, Rebbeck P, Goldie J, Coldman A, et al. (1985) Neoadjuvant (preoperative) chemotherapy for breast cancer. Cancer 56: 719-724.
-
Saslow D, Boetes C, Burke W, Harms S, Leach MO (2007) American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 57: 75-89.
-
Elissa R Price, Jasmine Wong, Rita Mukhtar, Nola Hylton, Laura J Esserman (2015) How to use magnetic resonance imaging following neoadjuvant chemotherapy in locally advanced breast cancer. World J Clin Cases 3: 607-613.
-
Pediconi F, Catalano C, Roselli A, Dominelli V, Cagioli S, et al. (2009) The challenge of imaging dense breast parenchyma: is magnetic resonance mammography the technique of choice? A comparative study with x-ray mammography and whole-breast ultrasound. Invest Radiol 44: 412-421.
-
Hébert-Croteau N, Brisson J, Latreille J, Rivard M, Abdelaziz N, et al. (2004) Compliance with Consensus Recommendations for Systemic Therapy is Associated with Improved Survival of Women with Node-Negative Breast Cancer. J Clin Oncology 22: 3685-3693.
-
Rustogi A, Budrukkar A, Dinshaw K, Jalali R (2005) Management of locally advanced breast cancer: evolution and current practice. J Cancer Res Ther 1: 21-30.
-
McCutcheon S, Cardoso F (2015) Challenges in optimizing care in advanced breast cancer patients: Results of an international survey linked to the ABC1 consensus conference. Breast 24: 623-629.
-
Al-Khanbashi M, Caramuta S, Alajmi AM, Al-Haddabi I, Al-Riyami M, et al. (2016) MS Tissue and Serum miRNA Profile in Locally Advanced Breast Cancer (LABC) in Response to Neo-Adjuvant Chemotherapy (NAC) Treatment. PlosOne 1-22.
-
Brackstone M, Fletcher GG, Dayes IS, Madarnas Y, SenGupta SK, et al. (2015) Locoregional therapy of locally advanced breast cancer: a clinical practice guideline. Curr Oncol 22: S54-S66.
-
Zhou Y, Tang J, Du Y, Ding J, Liu JY (2016) The green tea polyphenol EGCG potentiates the antiproliferative activity of sunitinib in human cancer cells. Tumour Biol 37: 8555-8566.
-
Agrawal S, Banswal L, Saha A, Arun I, Datta SS, et al. (2016) Progesterone Receptors, Pathological Complete Response and Early Outcome for Locally Advanced Breast Cancer - a Single Centre Study. (PPLB - 01). Indian J Surg Oncol 7: 397-406.
-
Smith JW, Buyse ME, Rastogi P, Geyer CE Jr, Jacobs SA, et al. (2016) Epirubicin With Cyclophosphamide Followed by Docetaxel With Trastuzumab and Bevacizumab as Neoadjuvant Therapy for HER2-Positive Locally Advanced Breast Cancer or as Adjuvant Therapy for HER2-Positive Pathologic Stage III Breast Cancer: A Phase II Trial of the NSABP Foundation Research Group, FB-5. Clin Breast Cancer.
-
Huang NS, Liu MY, Chen JJ, Yang BL, Xue JY, et al. (2016) Surgical management of breast cancer in China: A 15-year single-center retrospective study of 18,502 patients. Medicine (Baltimore) 95: e4201.
-
42. Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Darby S, McGale P, Correa C, Taylor C, et al. (2011) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378: 1707-1716.
-
Hardenbergh PH, Bentel GC, Prosnitz LR, Marks LB (1999) Postmastectomy radiotherapy: toxicities and techniques to reduce them. Semin Radiat Oncol 9: 259-268.
-
Le Scodan R, Selz J, Stevens D, Bollet MA, de la Lande B, et al. (2012) Radiotherapy for stage II and stage III breast cancer patients with negative lymph nodes after preoperative chemotherapy and mastectomy. Int J Radiat Oncol Biol Phys 82: 1-7.
-
Liu J, Mao K, Jiang S, Jiang W, Chen K, et al. (2016) The role of postmastectomy radiotherapy in clinically node-positive, stage II-III breast cancer patients with pathological negative nodes after neoadjuvant chemotherapy: an analysis from the NCDB. Oncotarget 7: 24848-24859.
-
Nielsen DL (2004) Mechanisms and functional aspects of multidrug resistance in Ehrlich ascites tumor cells. Dan Med Bull 51: 393-414.
-
Rang HP, Dale MM, Ritter JM, Flower RJ, Henderson G (2012) Pharmacology. (7th edn), Elsevier, Amsterdam, Netherlands.
-
Spence R, AJ Jonhston, PG Oncology, Jonhsto PG, Longo DL (1996) Cancer chemotherapy and biotherapy. (2ª. edn), Oxford University Press, Oxford, Lippincott-Raven, Filadélfia.
-
Hertz E, Cadoná FC, Machado AK, Azzolin V, Holmrich S, et al. (2015) Effect of Paullinia cupana on MCF-7 breast cancer cell response to chemotherapeutic drugs. Mol Clin Oncol 3: 37-43.
-
Yeo B, Turner NC, Jones A (2014) An update on the medical management of breast cancer. BMJ 348: g3608.
-
Weiss J (2011) Cancer-related fatigue: prevalence, assessment and treatment strategies. Expert Rev Pharmacoecon Outcomes Res 11: 441-446.
-
Zhen Wang, Jun-Qiang Chen, Jin-Lu Liu, Xin-Gan Qin (2016) Serious neutropenia following neoadjuvant chemotherapy for locally advanced breast cancer: A case report. Oncol Lett 11: 1597-1599.
-
Nahleh ZA, Barlow WE, Hayes DF, Schott AF, Gralow JR, et al. (2016) SWOG S0800 (NCI CDR0000636131): addition of bevacizumab to neoadjuvant nab-paclitaxel with dose-dense doxorubicin and cyclophosphamide improves pathologic complete response (pCR) rates in inflammatory or locally advanced breast cancer. Breast Cancer Res Treat 158: 485-495.
-
Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN (2007) Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol 608: 1-22.
-
Vishnukumar S, Umamaheswaran G, Anichavezhi D, Indumathy S, Adithan C, et al. (2013) P-glycoprotein expression as a predictor of response to neoadjuvant chemotherapy in breast cancer. Indian J Cancer 50: 195-199.
-
Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, et al. (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365: 1273-1283.
-
Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, et al. (2014) Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol 32: 3744-3752.
-
Verma S, Miles D, Gianni L, Krop IE, Welslau M, et al. (2012) Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N Engl J Med 367: 1783-1791.
-
Loibl S, Gianni L (2016) HER2-positive breast cancer. Lancet in press.
-
Atalay C, Deliloglu Gurhan I, Irkkan C, Gunduz U (2006) Multidrug resistance in locally advanced breast cancer. Tumour Biol 27: 309-318.
-
Schimpl FC, da Silva JF, Gonçalves JF, Mazzafera P (2013) Guarana: revisiting a highly caffeinated plant from the Amazon. J Ethnopharmaco 150: 14-31.
-
Niknafs B (2011) Induction of apoptosis and non-apoptosis in human breast cancer cell line (MCF-7) by cisplatin and caffeine. Iran Biomed J 15: 130-133.





