International Journal of Transplantation Research and Medicine
Case Report and Review of the Literature: Resolution of Lithium-Induced Nephrogenic Diabetes Insipidus with Pre-Emptive Living Related Kidney Transplantation for End-Stage Renal Disease
B Daniel Campos1*, Natalia Velez-Ramos1, Stephanie M Smith2, Colin Lenihan2 and Marc L Melcher2
1Department of Surgery, University of Puerto Rico, USA
2Division of Abdominal Transplantation, Stanford University, USA
*Corresponding author:
B Daniel Campos, MD, Department of Surgery, Auxilio Mutuo Hospital, Transplant Center, University of Puerto Rico, PO BOX 191227, USA, Tel: 787-505-5074, Fax: 787-771-7416, E-mail: danielcampos@mac.com
Int J Transplant Res Med, IJTRM-3-024, (Volume 3, Issue 1), Case Report
Received: November 29, 2016 | Accepted: March 07, 2017 | Published: March 09, 2017
Citation: Campos BD, Velez-Ramos N, Smith SM, Lenihan C, Melcher ML (2017) Case Report and Review of the Literature: Resolution of Lithium-Induced Nephrogenic Diabetes Insipidus with Pre-Emptive Living Related Kidney Transplantation for End-Stage Renal Disease. Int J Transplant Res Med 3:024.
Copyright: © 2017 Campos BD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Long-term lithium therapy is known to cause renal dysfunction, including nephrogenic diabetes insipidus (nDI) and chronic tubulointerstitial nephropathy, which may progress to end-stage renal disease (ESRD) in approximately 1% of patients. We report a case of resolution of lithium-induced nDI following living related kidney transplantation for ESRD secondary to chronic lithium toxicity. A 63-year-old male presented with ESRD and a 22-year history of severe nDI following 11 years of oral lithium treatment for bipolar disorder. He underwent a preemptive 1-haplotype matched living related kidney transplant from his son. Prior to the transplant, he had a daily urine output of 10-14 L. One month following transplant, the patient's daily urine output decreased to 2-3 L. His kidney function stabilized with a serum creatinine of 1.4 mg/dl (6.4 mg/dl pre-operatively). Doppler ultrasound supports a functional shift from native kidneys to renal allograft. This case report demonstrates reversal of nDI with kidney transplantation in a patient with ESRD secondary to lithium nephropathy, and highlights our poor understanding of this functional shift.
Keywords
Kidney transplantation, Nephrogenic diabetes insipidus, Lithium nephrotoxicity, End-stage renal disease
List of Non-Standard Abbreviations Used in the Text
ESRD: End-Stage Renal Disease; nDI: Nephrogenic Diabetes Insipidus; CKD: Chronic Kidney Disease; LRKT: Living Related Kidney Transplant; GFR: Glomerular Filtration Rate; ENaC: Epithelial Na Channel
Introduction
Following lethal cases of lithium intoxication in the 1950s, lithium was removed from the market as a table salt substitute. However, to this day, it continues to be widely used in the treatment of bipolar disorder and refractory unipolar major depression [1]. As a monovalent cation, lithium is freely filtered through the glomeruli, and up to 80% of the filtered load is reabsorbed, mostly in the renal proximal tubule. In the distal nephron, lithium is absorbed through Epithelial Na Channels (ENaC) in the principal cells, where it may accumulate and induce nephrotoxic effects characterized by reduced urinary concentrating capacity [2,3].
The most common adverse effect of chronic lithium therapy is nephrogenic diabetes insipidus (nDI), which occurs in 40-50% of patients receiving lithium therapy [4,5]. Lithium-induced nDI appears to be reversible if exposure is limited to 1-2 years, but with chronic exposure, the process is irreversible or partially reversible at best [6]. Most patients with nDI compensate for urinary concentrating defects by increasing their water intake; however, the associated polydipsia and polyuria may profoundly impact quality of life. Furthermore, in situations where water intake is limited, the risk of hypernatremia and elevated serum osmolality increases, which may cause irritability, confusion, fatigue, lethargy and seizures.
A growing body of literature shows that long-term lithium use also leads to chronic kidney disease (CKD) in some patients, though this effect typically requires 10-20 years of exposure. Chronic tubulointerstitial nephropathy is the most common cause of chronic kidney disease (CKD) in lithium treated patients, and follows a slowly progressive course that results in end-stage renal disease (ESRD) in about 1% of these patients [7-9]. Although cessation of lithium treatment may slow the progression of CKD in some patients, those with more severe renal impairment may be less likely to benefit [9,10]. We present a case of severe nDI and ESRD secondary to lithium toxicity successfully treated by kidney transplantation.
Case Presentation
A 63-year-old Caucasian male with ESRD secondary to chronic lithium toxicity was seen in evaluation for kidney transplantation. The patient had a longstanding history of bipolar disorder treated with uninterrupted oral lithium for 11 years (1984 to 1995). Serum lithium levels were never monitored or used to adjust lithium dosing throughout this period. The patient first noticed progressively increasing urinary volume and thirst around 5 years after initiation of therapy. Lithium treatment was withdrawn after 11 years of therapy, without any improvement in the urine volume or thirst. At that time, the patient's serum creatinine (Cr) was 2.6 and estimated glomerular filtration rate (GFR) was 28 ml/min. Over the following 15 years, his CKD continued to progress. He was placed on the renal transplant waiting list. At the time of evaluation and listing at our institution for a LRKT, he had a serum Cr of 5.3 mg/dl and a GFR of 12 ml/min/m2. He had already had an ateriovenous fistula formed in anticipation of hemodialysis, but was not dialysis-dependent at the time of transplant. While he had a number of sequelae of CKD, including fatigue, anemia, and hyperphosphatemia, his dominant symptoms were polyuria and profound thirst. He reported a daily urine volume in excess of 10 L and an associated thirst that woke him hourly at night.
Our patient had clear clinical and biochemical evidence of nDI. At an evaluation approximately one year prior to transplantation, he had maximally dilute urine and inappropriately low urine osmolality for a high serum osmolality (urine specific gravity 1.005; serum Na 138 mmol/L; urine osmolality 211; serum osmolality 332). The same trend was seen one day pre-operatively (urine specific gravity 1.003; serum Na 139; urine osmolality 151; serum osmolality 308), as shown in figure 1.
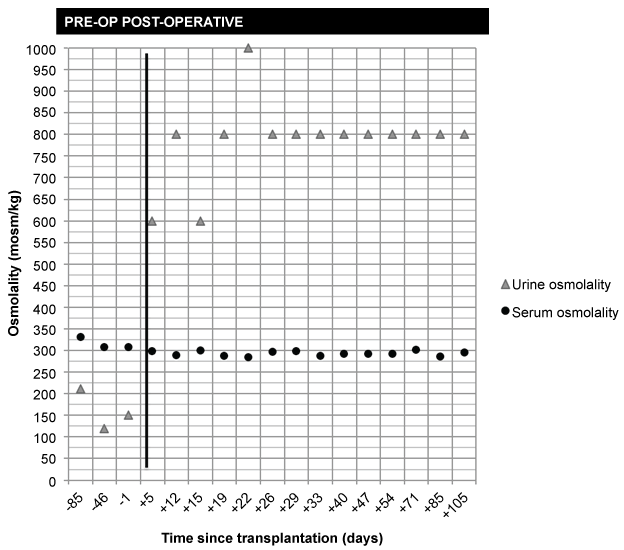
.
Figure 1: Serum and urine osmolality pre- and post-operatively.
Notes: Bold line denotes kidney transplantation (day 0); negative numbers denote number of days before transplantation; positive numbers denote number of days after transplantation. Urine osmolality estimated from the following: (urine specific gravity-1) *40000. Serum osmolality calculated from serum Na, glucose, and blood urea nitrogen: [serum osmolality = 2 Na + (glucose/18) + (BUN/2.8)].
View Figure 1
On July 6, 2011, the patient underwent a pre-emptive 1-haplotype matched living-related kidney transplant (LRKT) from his son. He was admitted overnight prior to transplantation to avoid dehydration, and required approximately 350 ml/hr of crystalloid solution to maintain his intravascular volume. Despite receiving 350 ml/hr of ½ normal saline while fasting overnight (corresponding to 175 ml/hr of free water), his serum Na rose from 139 mmol/L to 144 mmol/L the following day, supportive of significant inappropriate water excretion. Intra-operatively, 5,500 ml of crystalloid solution were given. A standard LRKT to the right external iliac vessels was conducted. Urinary drainage was achieved with a neo-ureterocystostomy with an anti-reflux mechanism over a double J stent. Prior to transplantation, his panel of reactive antibodies PRA (IgG) was 0 and his serologies for CMV and EBV were negative.
Tacrolimus, mofetil mycophenolate, and prednisone were given for maintenance immunosuppression. The patient had immediate graft function, and his post-operative course was uncomplicated. His fluid requirements and urine output decreased over the course of the hospitalization, and by post-operative day 3, he no longer required IV fluids and had a 24-hour urine output of 3.7 L. His kidney function also improved over this time period, with serum Cr decreasing from 6.4 mg/dl pre-operatively to 1.5 mg/dl on post-operative day 4, when he was discharged from the hospital (Figure 2).
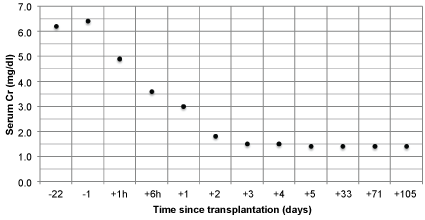
.
Figure 2: Serum creatinine pre- and post-operatively.
Notes: Kidney transplantation = day 0; two values were collected on day 0 (at 1 hour and 6 hours post-transplant); negative numbers denote number of days before transplantation; positive numbers denote number of days after transplantation.
View Figure 2
Three months post-transplant, the patient has stable renal function with a serum Cr of 1.4 mg/dl. At interval clinic appointments, urinalysis and blood work have demonstrated improved urinary concentrating ability in the setting of normal serum osmolality, beginning within one week of transplantation (Figure 1). The patient's intense thirst has gradually disappeared and his urine output has decreased to approximately 2-3 liters per day. He can now sleep uninterrupted for 4 to 5 hours at night. An ultrasound of his native kidneys documents atrophy and his transplanted kidney has normal inflow and outflow with a resistive index of 0.76.
Discussion
To our knowledge, this is the first published case of resolution of lithium-induced nDI following pre-emptive LRKT in a patient with ESRD secondary to chronic lithium nephropathy. Post-transplant, our patient's urine output decreased from 10-14 L/day to 2-3 L/day, and routine urine samples, which had been maximally dilute pre-operatively, became concentrated within one week of transplantation, providing clinical and biochemical support for resolution of nDI. Additionally, the patient's quality of life improved substantially in the absence of severe polydypsia and polyuria. This case report documents complete reversal of nDI as and additional benefit of kidney transplantation for patients with ESRD secondary to lithium therapy.
Among patients receiving long-term lithium treatment, 40-50% develop nDI [4,5] and 21-65% develop chronic renal insufficiency [8,11-14], with about 1% progressing to ESRD [7,8]. The mechanism of lithium-induced nDI is thought to involve ENaC-mediated entry into the principal cells of the collecting duct, which allows accumulation of lithium and a direct toxic effect of lithium on the principal cells. Lithium also down regulates expression of aquaporin-2 and aquaporin-3, which are thought to inhibit the actions of anti-diuretic hormone (ADH), contributing to urinary concentrating defects [2,3,15]. In addition, lithium inhibits glycogen synthase kinase type 3ß (GSK-3ß) activity, which may affect transcription of genes related to principal cell proliferation and aquaporin expression [5,15]. With chronic lithium exposure, the collecting duct undergoes structural remodeling and dysregulation of the cellular makeup, with an increased proportion of intercalated cells and proliferation of principal cells [5]. These processes damage the collecting tubules in a dose- and time-dependent fashion, leading to ADH resistance and nDI. Current medical treatment for nDI is limited to modest symptomatic relief with amiloride, thiazide diuretics, and, among patients without concurrent CKD, NSAIDs [5,16-19]. Salt restriction is thought to provide some benefit as well, but for the most part, the greatest symptomatic relief entails aggressive oral rehydration.
Concerning the mechanism by which LRKT reversed nDI in this patient, it is important to note that the patient's native kidneys were preserved. The patient had immediate graft function post-transplant, with serum Cr dropping to 1.4 mg/dl within 5 days post-operatively. The patient's urine output gradually decreased over the first 3 weeks post transplant. The renal allograft functionally replaced the lithium-damaged native kidneys. The functional transition from native kidneys to allograft has been previously documented among patients with resolution of pathologic proteinuria following preemptive kidney transplantation [20-22]. In a report of two cases with complete reversal of nephrotic syndrome post-transplant, renal scintiscan showed functional exclusion of native kidneys (contributing to 22% and 18% of overall function, respectively) [21]. In a subsequent series of 14 cases with resolution of proteinuria an average of 4.5 weeks post-transplant, 2 patients received pre- and post-transplant radioisotope renograms that showed decreased radioisotope uptake post-transplant, suggestive of reduced blood flow to the native kidneys [20]. The mechanism accounting for this shift from native kidneys to allograft has not been elucidated. Hypotheses include suppression of native kidney hypertrophy by factors released by the normal allograft [20]. In our patient, the normalization of the volume of urine produced in a 24-hour period further underscores our poor understanding of this phenomenon. Additional laboratory and clinical research is needed to understand the molecular mechanism responsible for this important functional shift in patients with renal transplantation.
Lithium nephrotoxicity is well documented in the literature. Renal biopsy samples from patients with lithium-induced CKD demonstrate chronic tubulointerstitial nephropathy, with elements of focal segmental glomerulosclerosis and global glomerulosclerosis [9]. Although some of the renal sequelae may be reversible with cessation of lithium treatment among those who have been treated short-term, the likelihood that the effects will be reversible decreases substantially with longer duration of lithium exposure [9,10]. Since the initiation of the United Network for Organ Sharing registry in 1988, there have been 494 transplants for lithium-induced ESRD in the United States, of which approximately one-third were pre-emptive [23]. Data is not available regarding the prevalence of nDI among these transplant recipients. In the same time frame there were 7 transplants where nDI was listed as a primary diagnosis for transplant. None of which are reported in the primary literature.
Information in the scientific literature regarding kidney transplant in patients with nephrogenic diabetes insipidus (NDI) is limited. The prevalence of nephrogenic diabetes insipidus among patients with end stage renal disease (ESRD) has not been reported, as current population analyses focus on dominant causes of ESRD such as diabetes mellitus, hypertension, and glomerulonephritis [24]. In the setting of congenital NDI, multiple anatomic changes occur in the urinary tract including dilation of the bladder and hydroureter [25]. It is postulated that prolonged backpressure of urine within this system can lead to hydronephrosis and renal damage, and subsequently ESRD [26]. After a primary literature search of PubMed, Web of Science, and EMBASE of all available papers, we were able to identify one paper that described kidney transplantation in the setting of NDI and ESRD. Hora, et al. describes a patient with congenital NDI who developed multiple anatomic changes including bilateral megaureters as a result of daily diuresis of up to 10 L [27]. The patient subsequently developed laboratory evidence of renal disease at age 18 with a serum creatinine level of 2.33 mg/dL which progressed to above 4.52 mg/dL at age 42 at which point he received a deceased donor renal transplant. We did not identify any reports of renal transplant in a patient with acquired NDI and end stage renal disease. Long term lithium has been implicated in causing acquired NDI by multiple reports, as lithium reduces the kidney's concentrating ability, with some patients progressing to NDI, even when stopping the drug [28].
As described previously in this paper, long term lithium use can lead to chronic tubulointerstitial nephropathy progressing to end stage renal disease in 1% of patients [7,8]. Although the relationship between lithium and ESRD has been established for multiple decades, the incidence of lithium related ESRD has been increasing in several parts of the world [29,30]. Data from the Australia and New Zealand Dialysis and Transplant Registry demonstrated a marked increase in patients on renal replacement therapy (both dialysis and transplant) with lithium related ESRD from 0.19% of all new renal replacement patients from 1992-1996 to 0.70% from 2007-2011 [29]. We conducted a primary literature search of PubMed, Web of Science, and EMBASE from 01/01/1994 to 08/20/2014 and were able to identify six papers describing renal transplantation for lithium related ESRD. There was only one paper identified describing a cohort of patients with kidney transplant and lithium ESRD, while five papers were individual case reports. Dube, et al. report a single center retrospective study of 14 patients who received a transplant for lithium related ESRD over the course of 9 years [31]. Patient survival at 5 years in this cohort was 80% while graft survival was 92.3% with one case of acute rejection. Moss, et al. also report a successful renal transplant in a 57-year-old women with ESRD after 37 years of lithium therapy [32]. This patient was restarted on her pretransplant dose of lithium, but subsequently switched to risperidone for mood symptoms after an episode of suspected lithium toxicity. Bora, et al. report a successful renal transplant in a 67 year old female with bipolar disorder and 35 years of lithium use [33]. The patient stopped lithium use 10 years before her transplant, but her renal failure continued to progress requiring transplant and her mood symptoms remained stable on lamotrigine and aripiprazole. Although these reports describe transplant in patients with relatively stable courses, multiple other reports describe cases where post-transplant recovery was complicated. Svedlund, et al. report a 67 year old patient who developed acute tubular necrosis following a deceased donor renal transplant for lithium related ESRD, who subsequently recovered and was stable on immunosuppressant and mood stabilizer medications [34]. Java, et al. presented a case of a 57 year old patient who underwent living related donor kidney transplant secondary to lithium related ESRD with a starkly different outcome: the patient developed idiopathic membranoproliferative glomerulonephritis type 3 leading to ESRD in the graft in spite of immunosuppressive therapy [35]. Lastly, the literature presents a case of a 62 year old female on lithium therapy who had to be retransplanted after a BK virus related collecting duct tumor formed in her original graft [36]. The authors implicated lithium therapy as an "additional hit" in the development of the tumor as lithium has been previously shown as a risk factor in the development of collecting duct tumors.
Data on the incidence of the development of ESRD in patients with NDI is virtually non-existent in the literature. Although the literature presents one previous report of a patient with congenital NDI who received a kidney transplant, this paper, to our knowledge, reports the first case of renal transplant for acquired NDI progressing to ESRD. Literature for renal transplant in patients with lithium related ESRD is also limited, but there are multiple case reports and one cohort study in the literature that describe relatively good post-transplant outcomes in these patients, with rare complications.
Thus, this case report describes LRKT as treatment for nDI in the setting of ESRD secondary to chronic lithium exposure. If supported by larger case series, renal transplantation could be considered a definitive treatment for nDI among patients with lithium-induced ESRD qualifying for renal transplant. Furthermore, this report provides additional support for the functional transition from native kidneys to renal allograft post-transplant. These findings also provide valuable information for physicians when discussing the benefits of kidney transplantation with this unique and rare patient population. This is especially important for both recipients and living donors, as it may factor into their decision to pursue transplantation or donation respectively. Pre-emptive transplantation in this setting, with the concomitant avoidance of dialysis, carries additional proven health benefits for patients in ESRD. In summary, this case report identifies reversal of lithium-induced nDI as an important advantage of kidney transplantation for patients with ESRD secondary to chronic lithium nephropathy.
Disclosures
The authors of this manuscript have no conflicts of interest to disclose.
References
-
Robert MA Hirschfeld, Charles L Bowden, Michael J Gitlin, Paul E Keck, Trisha Suppes, et al. (2010) Bipolar Treatment Guidelines. American Psychiatric Association.
-
Christensen BM, Zuber AM, Loffing J, Stehle JC, Deen PM, et al. (2011) alphaENaC-mediated lithium absorption promotes nephrogenic diabetes insipidus. J Am Soc Nephrol 22: 253-261.
-
Grunfeld JP, Rossier BC (2009) Lithium nephrotoxicity revisited. Nat Rev Nephrol 5: 270-276.
-
Stone KA (1999) Lithium-induced nephrogenic diabetes insipidus. J Am Board Fam Pract 12: 43-47.
-
Trepiccione F, Christensen BM (2010) Lithium-induced nephrogenic diabetes insipidus: new clinical and experimental findings. J Nephrol 23: S43-S48.
-
Garofeanu CG, Weir M, Rosas-Arellano MP, Henson G, Garg AX, et al. (2005) Causes of reversible nephrogenic diabetes insipidus: a systematic review. Am J Kidney Dis 45: 626-637.
-
Bendz H, Schon S, Attman PO, Aurell M (2010) Renal failure occurs in chronic lithium treatment but is uncommon. Kidney Int 77: 219-224.
-
Tredget J, Kirov A, Kirov G (2010) Effects of chronic lithium treatment on renal function. J Affect Disord 126: 436-440.
-
Markowitz GS, Radhakrishnan J, Kambham N, Valeri AM, Hines WH, et al. (2000) Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol 11: 1439-1448.
-
Presne C, Fakhouri F, Noel LH, Stengel B, Even C, et al. (2003) Lithium-induced nephropathy: Rate of progression and prognostic factors. Kidney Int 64: 585-592.
-
Bassilios N, Martel P, Godard V, Froissart M, Grunfeld JP, et al. (2008) Monitoring of glomerular filtration rate in lithium-treated outpatients--an ambulatory laboratory database surveillance. Nephrol Dial Transplant 23: 562-565.
-
Lepkifker E, Sverdlik A, Iancu I, Ziv R, Segev S, et al. (2004) Renal insufficiency in long-term lithium treatment. J Clin Psychiatry 65: 850-856.
-
Janowsky DS, Soares J, Hatch JP, Zunta-Soares G, Hu Q, et al. (2009) Lithium effect on renal glomerular function in individuals with intellectual disability. J Clin Psychopharmacol 29: 296-299.
-
McCann SM, Daly J, Kelly CB (2008) The impact of long-term lithium treatment on renal function in an outpatient population. Ulster Med J 77: 102-105.
-
Nielsen J, Kwon TH, Christensen BM, Frokiaer J, Nielsen S (2008) Dysregulation of renal aquaporins and epithelial sodium channel in lithium-induced nephrogenic diabetes insipidus. Semin Nephrol 28: 227-244.
-
Allen HM, Jackson RL, Winchester MD, Deck LV, Allon M (1989) Indomethacin in the treatment of lithium-induced nephrogenic diabetes insipidus. Arch Intern Med 149:1123-1126.
-
Khanna A (2006) Acquired nephrogenic diabetes insipidus. Semin Nephrol 26: 244-248.
-
Batlle DC, von Riotte AB, Gaviria M, Grupp M (1985) Amelioration of polyuria by amiloride in patients receiving long-term lithium therapy. N Engl J Med 312: 408-414.
-
Bedford JJ, Weggery S, Ellis G, McDonald FJ, Joyce PR, et al. Lithium-induced nephrogenic diabetes insipidus: renal effects of amiloride. Clin J Am Soc Nephrol 3: 1324-1331.
-
D'Cunha PT, Parasuraman R, Venkat KK (2005) Rapid resolution of proteinuria of native kidney origin following live donor renal transplantation. Am J Transplant 5: 351-355.
-
Piccoli GB, Mezza E, Picciotto G, Burdese M, Marchetti P, et al. (2004) The grafted kidney takes over: disappearance of the nephrotic syndrome after preemptive pancreas-kidney and kidney transplantation in diabetic nephropathy. Transplantation 78: 627-630.
-
Piccoli GB, Rossetti M, Marchetti P, Grassi G, Picciotto G, et al. (2004) Complete reversal of the nephrotic syndrome after preemptive pancreas-kidney transplantation: a case report. Transplant Proc 36: 589-590.
-
UNOS_registry.
-
U.S. Renal Data System, USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.
-
Knoers N (2000) Nephrogenic Diabetes Insipidus. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, et al. GeneReviews® [Internet], Seattle (WA), University of Washington, Seattle.
-
Colliver D, Storey R, Dickens H, Subramaniam R (2012) Nonobstructive urinary tractdilatation in children with diabetes insipidus. J Pediatr Surg 47: 752-755.
-
Hora M, Reischig T, Hes O, Ferda J, Klecka J (2006) Urological complications of congenital nephrogenic diabetes insipidus-long-term follow-up of one patient. Int Urol Nephrol 38: 531-532.
-
Bendz H, Sjödin I, Aurell M (1996) Renal function on and off lithium in patients treated with lithium for 15 years or more. A controlled, prospective lithium-withdrawal study. Nephrol Dial Transplant 11: 457-460.
-
Roxanas M, Grace BS, George CR (2014) Renal replacement therapy associated with lithium nephrotoxicity in Australia. Med J Aust 200: 226-228.
-
Aiff H, Attman PO, Aurell M, Bendz H, Schön S, et al. (2014) End-stage renal disease associated with prophylactic lithium treatment. Eur Neuropsychopharmacol 24: 540-544.
-
Dube G, Crew R, Tsapeppas D, Khorassani F, Wiener I (2013) Excellent outcomes of kidney transplant in patients with bipolar disorder. American Journal of Transplantation 5.
-
Moss MC, Kozlowski T, Dupuis R, Detwiler R, Lee RM, et al. (2014) Lithium use for bipolar disorder post renal transplant: is mood stabilization without toxicity possible? Transplantation 97: e23-e24.
-
Bora SS, Madan R, Sharma A (2012) A case of renal failure requiring a renal transplant secondary to lithium treatment. Prim Care Companion CNS Disord 14.
-
Svedlund J, Aiff H, Attman PO, Aurell M, Bendz H, et al. (2012) Renal failure caused by prophylactic lithium treatment-still a threat to the patients? International Clinical Psychopharmacology 28: e55-e56.
-
Java A, Gaut JP, Brennan DC (2012) De novo membranoproliferative glomerulonephritis III in a renal transplant patient: case report and review of the literature. Transpl Int 25: e58-e61.
-
Veldhuijzen NMH, Rookmaaker MB, Van Zuilen AD, Nquyen TQ, Boer WH, et al. (2014) BK virus (BKV) nephropathy, collecting duct cell proliferation and malignancy in a renal allograft: Case history and review of the literature. Nephrology Dialysis Transplantation.





