Incidental Discovery of a Donor-Derived Urothelial Carcinoma Following Kidney Transplantation
Kathryn Milks1*, Sriyesh Krishnan1, Melanie Caserta1, Robert J Stratta2 and Hisham Tchelepi1
1Department of Radiology, Wake Forest Baptist Medical Center, USA
2General Surgery, Wake Forest Baptist Medical Center, USA
*Corresponding author:
Kathryn Milks, Department of Radiology, Wake Forest University Baptist Medical Center, Winston-Salem, North Carolina, USA, Tel: +614-307-4614, E-mail: ks.mueller@gmail.com
Int J Transplant Res Med,
Volume 1, Issue 2,
Case report,
IJTRM-1-006
Received: March 15, 2015: Accepted: June 17, 2015: Published: June 20, 2015
Citation: Milks K, Krishnan K, Caserta M, Stratta RJ, Tchelepi H (2015) Incidental Discovery of a Donor-Derived Urothelial Carcinoma Following Kidney Transplantation. Int J Transplant Res Med 1:006
Copyright: © 2015 Milks K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Urothelial carcinoma in renal transplant patients has been well described, particularly in the Asian literature, owing to the higher incidence of the disease in this part of the world, both in the native urothelial system and in the transplanted kidney. In the United States, however, urothelial carcinoma after kidney transplant is quite rare in the native urothelial system and extraordinarily rare in the renal allograft. It has been proposed in areas with a high incidence of urothelial carcinoma that patients undergo routine screening renal sonography after transplantation in order to monitor more closely for this often silent malignancy. It is yet unclear if this practice should also be considered in the Western world in the absence of clinical symptoms, given the relative rarity of disease. Herein we describe an unusual case of metastatic urothelial carcinoma originating in dually transplanted kidneys from a North America donor and recipient, incidentally discovered during evaluation for worsening renal allograft function. We surmise that the overall rate of urothelial carcinoma following kidney transplantation in the United States may be under-reported, which may offer a partial explanation for the striking geographic differences.
Keywords
Donor-transmitted malignancy, Kidney transplant, Urothelial carcinoma
Abbreviations
ATN: Acute Tubular Necrosis, CT: Computerized Tomography, TCC: Transitional Cell Carcinoma, UNOS: United Network for Organ Sharing
Introduction
Compared with the age- and gender-matched general population, kidney transplant recipients have at least a 3 to 4-fold higher risk of malignancy with the risk increasing up to 10-30 times depending on the type of malignancy [1]. Overall risk of malignancy may be as high as 15-20% at 10 years following kidney transplantation. It is well established that the requisite post-transplant immunosuppression in kidney transplant recipients contributes to their heightened cancer risk, most commonly non-melanoma skin cancers followed by lymphoma and post-transplant lymphoproliferative disorder [2,3]. Asian countries experience a higher rate of urothelial carcinoma in the general population and also post-transplantation [4]. Several prior studies have suggested routine native and allograft renal sonography to screen the post-transplant population [5,6]. In Western countries, the incidence of urothelial carcinoma following kidney transplantation is rareand is muchless common in the transplanted organ than in the native genitourinary system [7]. Kidney transplant recipients are at risk for 3 basic types of malignancies; pre-existing or recurrent tumors, de novo tumors occurring following transplantation, and donor-derived or transmitted tumors. In one study, the average time to development of any form of malignancy following transplantation was 9.4 years, and all-cancer rates continued to rise with increasing time following transplantation [2,3]. Conversely, in the case of occult or known donor-derived malignancy, average time to cancer discovery was 2 months (range 2 days to 38 months post-transplant) [4]. Limited literature on the development of urothelial carcinoma involving the renal allograft is available in North American renal transplant recipients. We report herein an unusual case of a high-grade urothelial carcinoma with sarcomatoid features in a dual kidney transplant recipient in the United States, diagnosed serendipitously by transplant renal biopsy for evaluation of worsening renal function.
Case Report
A 63 year-old white male with end stage renal disease secondary to lupus nephritis was on peritoneal dialysis for 9 months before undergoing ipsilateral dual kidney transplantation from a standard criteria donation after cardiac death donor. The donor was a 49-year-old white male with a history of smoking, hypertension, weakness, and unexplained weight loss. The donor sustained an irreversible brain injury secondary to a stroke but did not progress to brain death. Organ recovery was performed after voluntary withdrawal of life support with a warm ischemic time of 41 minutes. Donor kidney biopsy revealed 18% glomerulosclerosis and mild vascular changes; consequently, a decision was made to perform a dual kidney transplant. Both kidneys appeared anatomically normal except for atherosclerosis extending into the renal arteries and reperfusion biopsies of both allograft kidneys at the time of surgery showed acute tubular necrosis (ATN), parenchymal scarring, and donor-associated chronic vascular changes. The donor had no cross-sectional abdominal imaging prior to organ recovery.
The recipient received single dose alemtuzumab induction in combination with mycophenolate, steroids, and the delayed administration of tacrolimus. Multiple postoperative ultrasound examinations demonstrated decreased flow and high resistive indices in both allograft kidneys with small dual graft arteriovenous fistulas, presumably secondary to intra-operative biopsies. The kidneys otherwise appeared sonographically normal (Figure 1). The patient was followed closely, undergoing multiple ultrasound examinations during the first postoperative month, until the resistive indices normalized. A protocol-driven post-transplant biopsy at 1 month showed resolving ATN, scarring, and sequelae of longstanding hypertension. The serum creatinine level eventually stabilized in the 2.5-3.0mg/dl range with an estimated glomerular filtration rate of 25-30.
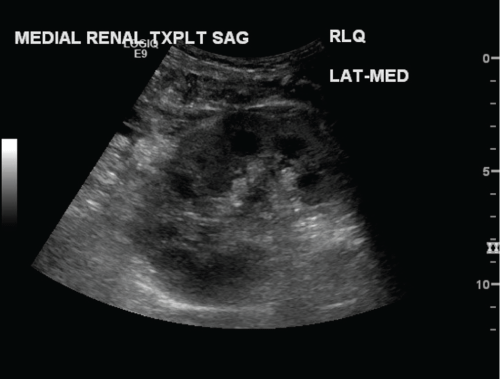
.
Figure 1: Sagittal grayscale image of the medial transplant kidney in a dual ipsilateral kidney transplant. Immediate postoperative ultrasound demonstrates a normal appearing kidney. No masses or hydronephrosis identified.
View Figure 1
The patient did well for the next 6 months until he presented with an elevated serum creatinine level in the 3.1-3.5mg/dl range. Urine cytology data was not performed but surveillance urine and blood BK polyomavirus serology was negative. Transplant renal ultrasound obtained at this time revealed a round, heterogeneous lesion adjacent to the medial allograft kidney (Figure 2). The collection was thought to represent an organized hematoma, but short term follow-up or computerized tomography (CT) evaluation was recommended. Subsequent ultrasound-guided percutaneous biopsy of the lateral kidney showed acute cell-mediated tubulo-interstitial rejection that was graded as Banff 1B as well as pronounced donor-transmitted vascular changes. The patient was treated with intravenous high-dose steroids with an initial clinical response as the serum creatinine level temporarily returned to the baseline range.
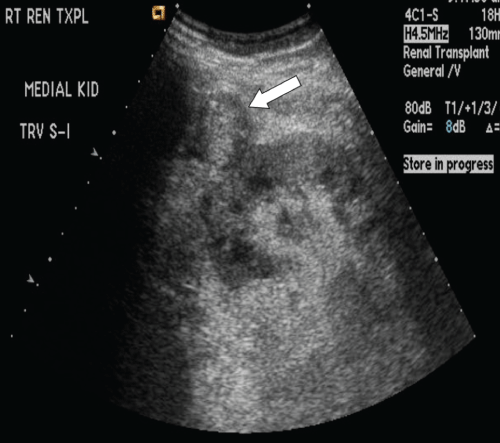
.
Figure 2: Sagittal grayscale sonogram of the medial transplant kidney eight months post-transplant. A new, ill-defined slightly hypoechoic rounded structure anterior to and contiguous with the kidney (white arrow) is identified, interpreted as a possible hematoma.
View Figure 2
Because of complaints of right lower quadrant fullness and pain, a non-contrast abdominal and pelvic CT scan was performedone month later and revealed enlarged and edematous allograft kidneys, stranding in the adjacent soft tissues, and a small amount of ill-defined perinephric transplant fluid (Figure 3). The collecting systems of both kidneys were mildly dilated. Thickening in the right lateral abdominal wall musculature and soft tissue stranding were also noted. Differential considerations included ATN, rejection, or infection, and the findings in the abdominal wall were favored to represent inflammatory or postoperative changes. Longitudinal monitoring of BK viremia and viruria continued to be negative.
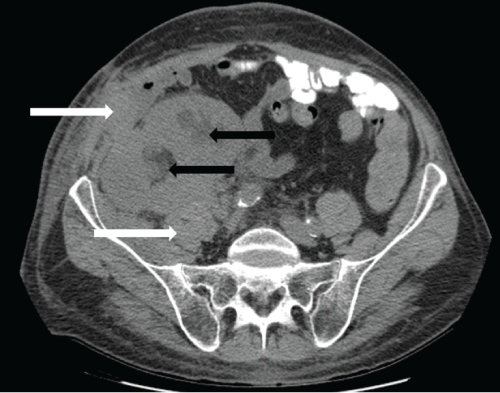
.
Figure 3: Non-contrast axial CT scan at the level of the dual transplanted kidneys, 9 months post-transplantation. The dual kidney transplants appear enlarged and edematous. Moderate hydronephrosis is present in both transplanted kidneys (black arrows). Perinephric soft tissue thickening and stranding extends to involve the right lower abdominal wall fascia and musculature (white arrows).
View Figure 3
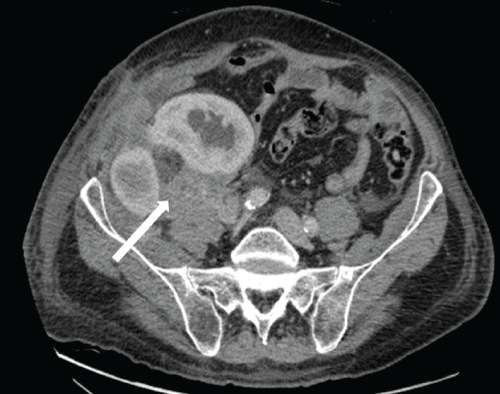
Repeat ultrasound showed progression of obstruction in both renal allografts and a persistent heterogeneous, pedunculated lesion anterior and contiguous with the medial allograft kidney, again interpreted as a probable hematoma. Given the short time interval from the prior ultrasound, no significant growth or shrinkage was appreciated. Repeat transplant biopsy for a rising serum creatinine level at this time (which was now eight months post-transplant)revealed a satisfactory response to anti-rejection therapy, resolving ATN, chronic parenchymal and vascular changes, and, surprisingly, high-grade invasive urothelial carcinoma with extensive squamous differentiation (Figure 4).Given the unexpected biopsy findings, further work-up included a contrast-enhanced CT scan, which demonstrated urothelial thickening in both kidneys with multiple enhancing soft tissue lesions in the perinephric tissue, vascular encasement, and abdominal wall muscular invasion (Figure 5). Evidence of metastatic urothelial carcinoma was present at diagnosis including extensive pelvic and retroperitoneal lymphadenopathy with borderline enlarged mediastinal nodes. Cystoscopy of the bladder and the native ureters showed no evidence of urothelial carcinoma.
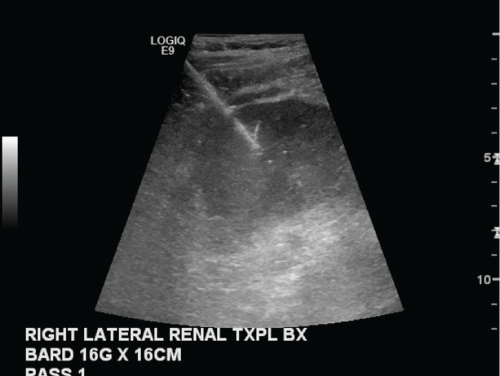
.
Figure 4: Grayscale image of the lateral kidney transplant obtained during a routine biopsy to evaluate for rejection.
View Figure 4
.
Figure 5: Contrast enhanced axial CT scan at the level of the dual transplanted kidneys 10 months post-transplantation. Abnormal, enhancing soft tissue tumor infiltrates and encases both transplanted kidneys, which have developed progressive hydronephrosis. Tumor extends into the abdominal wall musculature. Note posterior extension of tumor to involve the right psoas muscle (white arrow). Bulky intra-abdominal lymphadenopathy with encasement of the adjacent iliac vessels was also present.
View Figure 5
Results
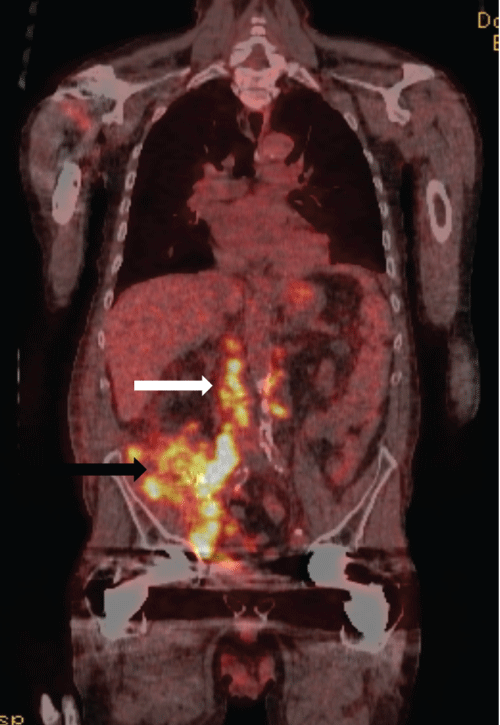
The patient underwent intentional pre-operative embolization of both kidneys followed by dual allograft nephro-ureterectomies at which time thick scar tissue and a large burden of extra-renal tumor were encountered; obvious tumor was left behind, encasing the iliac vessels and densely adherent to the retroperitoneum. Final pathology revealed high-grade urothelial carcinoma with sarcomatoid features and lymphovascular invasion with tumor at the margins of resection and satellite lesions in the renal parenchyma. Histocompatibility typing of the tumor demonstrated both host and donor histologic elements, suggesting a donor-derived malignancy. Positron emission tomography (PET) scanperformed one month following nephrectomies demonstrated extensive residual disease (Figure 6). In retrospect, the pedunculated lesion initially described on ultrasound emanating from the medial transplant kidney was tumor, and the most recent biopsy had serendipitously traversed the area containing malignancy. Following cessation of immunosuppression, the patient received chemotherapy with paclitaxel for six months. Three years following nephrectomies, the patient is alive on hemodialysis with no evidence of residual disease.
.
Figure 6: Coronal PET CT image in the same patient following dual transplant nephrectomies for biopsy proven invasive urothelial carcinoma. Hypermetabolic activity (black arrow) in the surgical bed and para-aortic lymph nodes indicates residual urothelial carcinoma by direct invasion of adjacent fascia and nodal metastases (white arrow).
View Figure 6
Discussion
Transmission of cancer from an organ donor to a recipient is an infrequent, but potentially devastating complication of transplantation. Early identification of a donor-transmitted malignancy (within 6 weeks of transplantation) is associated with better patient survival compared to late detection (ref). Diagnosis is often incidental and based on ultrasonographic imaging or kidney biopsy performed for other reasons, and survival is usually associated with allograft nephrectomy (in the absence of lymphoma) and cessation of immunosuppression, as reported by the United Kingdom transplant registry [8]. In 4 recipients that developed a late donor-transmitted cancer, the median duration of diagnosis was 10 months post-transplant, none were genitourinary malignancies, and 3 presented with metastatic disease (all of whom died) [8].
Donor-transmitted urothelial carcinoma in a renal allograft is distinctly rare in the United States, although this may be in part due to under-reporting. Unfortunately, in the absence of hematuria or other localizing symptoms, early detection of urothelial carcinoma in the donor or recipient is difficult if disease is microscopic.
The aggressive nature of urothelial carcinoma in this setting is likely multifactorial and related to the delay in diagnosis, squamous differentiation, probable donor origin, and the patient's chronically immunosuppressed state [9,10]. In addition, the patient was treated for biopsy-proven acute rejection just prior to the tissue diagnosis of urothelial carcinoma. The lack of malignant involvement of the urothelium of the native kidneys or bladder and relatively rapid time to cancer diagnosis following transplantation makes the probability of de novomalignancy unlikely. In addition, unexplained weight loss in the donor is highly suspect for occult malignancy. Typical independent predictors of urothelial carcinoma such as female gender, BK viral infection, use of Chinese herbal medicines, and transplant tourism do not apply in this case of donor-derived disease. Increased immunosuppression for the treatment of allograft rejection may have allowed a smoldering malignancy to become clinically manifest in our patient, which is supported by the time course of disease presentation. Although histocompatibility testing of the tumor was inconclusive, the time course of disease, lack of native urothelial involvement, and robust response to treatment make malignancy transmitted by the donated organs the most likely explanation. Transmission of high-grade urothelial carcinoma by solid organ or bone marrow transplantation is exceedingly rare. In some cases, as in our case of probable donor-transmitted malignancy, cessation of immunosuppression leads to "rejection" of the tumor by the recipient's recovering immune system, which portends an excellent response to therapy.
Because of the aggressive evolution and large burden of urothelial carcinoma in our patient, immediate dual allograft nephrectomy, cessation of immunosuppression, and chemotherapy were initiated. Our patient continues to do well, with documented regression of locally invasive and metastatic disease 3 years following nephrectomies.
Conclusion
Literature from Asia, where genitourinary malignancy following transplantation is more prevalent, suggests routine sonographic surveillance may detect such malignancies earlier [5]. Presumably, if our patient had received routine monthly native renal and transplant ultrasounds, documented growth of the mass mistaken for a hematoma may have led to earlier diagnosis and treatment. However, it is unlikely that such a strategy would be justified following renal transplantation in the United States, where this entity remains rare. However, if transplant urothelial carcinoma is under-reported in the western world, further research in this arena may elucidate a previously undetected disease burden. Additionally, this case study highlights the importance of comprehensive donor assessment and recipient surveillance, particularly in the setting of expanded donor and recipient acceptance policies.
Acknowledgements
A special thanks to Dr. Raymond Dyer for all his help.
References
-
Birkeland SA, Storm JJ, Lamm LU, Barlow L, Blohme I, et al. (1995) Cancer risk after renal transplantation in the Nordic countries. Int J Cancer 60: 183-189.
-
Vajdic CM, McDonald SP, McCredie MR, van Leeuwen MT, Stewart JH, et al. (2006) Cancer incidence before and after kidney transplantation. JAMA 296: 2823-2831.
-
Penn I (2000) Cancers in renal transplant recipients. Adv Ren Replace Ther 7: 147-56.
-
Buell JF, Beebe TM, Trofe J, Gross TG, Alloway RR, et al. (2004) Donor transmitted malignancies. Ann Transplant 9: 53-56.
-
Chiang YJ, Chu SH, Liu KL, Lai WJ, Wang HH (2006) Silent Urothelial Cancer Detected by Sonography after renal transplantation. Transplant Proc 38: 2084-2085.
-
Viart L, Surga N, Collon S, Jaureguy M, Elalouf V, et al. (2013) The high rate of de novo graft carcinomas in renal transplant recipients. Am J Nephrol 37: 91-96.
-
Tsaur I, Karalis A, Probst M, Blaheta RA, Scheuermann EH, et al. (2010) Development of urologic cancers in renal transplant recipients: 30-year experience at the Frankfurt Transplant Center. Cancer Sci 101: 2430-2435.
-
Desai R, Collett D, Watson CJ, Johnson P, Evans T, et al. (2012) Cancer transmission from organ donors-unavoidable but low risk. Transplantation 94: 1200-1207.
-
Penn I, Starzl TE (1972) Malignant tumors arising de novo in immunosuppressed organ transplant recipients. Transplantation 14: 407-417.
-
Barrett WL, First MR, Aron BS, Penn I (1993) Clinical course of malignancies in renal transplant recipients. Cancer 72: 2186-2189.





