International Journal of Stem cell Research and Therapy
Central Odontogenic Fibroma of the Mandible-Revisiting Pathogenesis of Benign Tumor of the Jaw
Steven Wang1,2, Qilin Xu1, Faizan Alawi3, Qunzhou Zhang1, Lee R Carrasco1,2, Brian P Ford1,2, Prem Patel1,2 and Anh D Le1,2*
1Department of Oral and Maxillofacial Surgery and Pharmacology, University of Pennsylvania School of Dental Medicine, Pennsylvania, United States of America
2Department of Oral & Maxillofacial Surgery, Penn Medicine Hospital of the University of Pennsylvania, Pennsylvania, United States of America
3Department of Pathology, University of Pennsylvania School of Dental Medicine, Pennsylvania, United States of America
*Corresponding author: Anh D Le, DDS, PhD, Department of Oral & Maxillofacial Surgery and Pharmacology, University of Pennsylvania School of Dental Medicine, 240 South 40th Street, Philadelphia, PA 19104, United States of America, Tel: 215-898-8933; Fax: 215-573-5032, E-mail: Anh.Le@uphs.upenn.edu
Int J Stem Cell Res Ther, IJSCRT-3-043, (Volume 3, Issue 2), Case Report; ISSN: 2469-570X
Received: September 15, 2016 | Accepted: December 05, 2016 | Published: December 07, 2016
Citation: Wang S, Xu Q, Alawi F, Zhang Q, Carrasco LR, et al. (2016) Central Odontogenic Fibroma of the Mandible-Revisiting Pathogenesis of Benign Tumor of the Jaw. Int J Stem Cell Res Ther 3:043. 10.23937/2469-570X/1410043
Copyright: © 2016 Wang S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Central odontogenic fibroma (COF) is an uncommon benign tumor of the jaw, which clinically presents as a slow-growing neoplasm with cortical bony expansion. It has been postulated that COF might be derived from the inactive-like small nests or islands of odontogenic epithelium. However, the pathophysiology of this rare benign tumor remains largely unknown. Herein, we reported a case of simple type of COF in the mandible in a 24-year-old man who presented with progressive mandibular swelling and worsening pain. The lesion extended from the first molar to the coronoid notch displacing both second and third molars to the mandible angle. The coronoid was entirely excavated and surgical specimen was analyzed histologically and immunohistochemically. The COF is composed of fragmented small and scant nests or islands of odontogenic epithelium embedded in a mature fibrous connective stroma, the major component of tumor mass of COF. Interestingly, the epithelial islands expressed several stem cell-related genes, such as ALDH1, Bmi-1, Oct4, CD44 and CD29. Meanwhile, they displayed epithelial-mesenchymal transition (EMT) phenotypes characterized by the expression of vimentin, a mesenchymal cell marker, a loss in the expression of E-cadherin, a marker of epithelial cells, and the expression of EMT-regulatory genes, such as Slug, Zeb1, and β-catenin. Additionally, the isolated and ex vivo cultured primary epithelial cells from COF maintained the expression of several stem cell-related genes, such as Bmi-1, Oct4, ALDH1, CD44, CD29 and CK14. These findings support the hypothesis that the epithelial rests of COF harbor a unique subpopulation of epithelial stem-like cells, which may undergo an active EMT process, thus contributing to the overgrowth of mesenchymal stromal tissues and consequently, the aggressive progress of COF.
Keywords
Central odontogenic fibroma, Epithelial-mesenchymal transition, Stem cells
Introduction
The odontogenic fibroma (OF) is an uncommon benign tumor of odontogenic ectomesenchymal origin, and one of the most poorly characterized types of all odontogenic neoplasms [1-3]. The lesion is usually considered to originate from either periodontal ligament, dental follicle, or dental papilla. According to the 1992 World Health Organization (WHO) classification, OF is defined as a benign odontogenic neoplasm of fibroblastic origin characterized by relatively mature fibro-collagenous connective tissue and varying amounts of odontogenic epithelium with the potential to occur either in an intraosseous (central, COF) or an extraosseous (peripheral, POF) location. COF is an extremely rare tumor, accounting for about 0.1% of all odontogenic tumors [4-6], whereas POF is relatively common. Histologically, central odontogenic fibroma (COF) is classified into two types, the epithelium-poor (simple) type and the epithelium-rich (complex) type (or WHO type). The simple type consists of a delicate fibrous connective tissue with a fibromyxoid quality and is usually confused with hyperplastic dental follicle of an impacted tooth because both are characterized by the absence of stellate reticulum and the presence of rare rests of unpolarized odontogenic epithelium and dystrophic calcifications [7].
There have been a few reports describing the immunogenic findings from immunohistochemical studies on the epithelial components of COF [8], whereby the positive expression of cytokeratin (CK) 14 and CK19 verified the lack or abundance of epithelial components in epithelium-poor and epithelium-rich COFs, respectively, and excluded the diagnosis of other type of central fibrous tumors [8,9]. Of note, several lines of evidence have implicated that ameloblastoma, odontogenic carcinoma, or sarcoma might be derived from recurring or aggressive COFs [10-13]. Most recently, one study has reported a unique case of epithelium-rich COF with unusual epithelial extension into nerves and vessels, which is unrelated to peripheral odontogenic fibroma, a reactive fibroepithelial hyperplasia with epithelium [14]. These findings are interesting, but the molecular mechanisms underlying the pathophysiology of COF are still poorly understood, thus making it one of the most poorly defined odontogenic tumors.
Cancer stem cells (CSCs) or Tumor initiating cells (TICs) represent a unique subpopulation of tumor cells that possess self-renewal and tumor-initiating capabilities, thus may play a critical role in the recurrence, relapse, and metastasis of malignant tumors [15,16]. TICs also exhibit plasticity by reversibly transitioning between the stem and non-stem cell states, whereby epithelial-mesenchymal transition (EMT) may play a critical role in governing this process [15,16]. However, the role of EMT process and tumor epithelial stem-like cells in the pathogenesis of benign tumors, including odontogenic fibromas, remains largely unknown. Here, we reported a case of a 24-year-old man with a central odontogenic fibroma in right posterior mandible, which harbored a subpopulation of epithelial cells with EMT phenotypes and positive expressions of some epithelial stem cell-related genes. These findings support the notion that EMT process and tumor epithelial stem-like cells may play an important role in the aggressive progress of COF.
Materials and Methods
Collection of COF specimens
The fresh tumor sample was obtained from surgical procedures after obtaining informed consent from the patient according to the protocol approved by the Institutional Review Boards of the University of Pennsylvania (IRB#817407). Fragments of the surgical specimen not required for histopathologic diagnosis were stored in a sterile tube containing PBS supplemented with 10% heat-inactivated fetal bovine serum (FBS), 200 U/mL penicillin, and 200 μg/mL streptomycin (all from Life Technologies, Carlsbad, CA, USA) for further processing in the lab.
Histological, Immunohistochemical (IHC), and Immunocytochenical (ICC) studies
The tumor tissue sample was fixed in 4% paraformaldehyde (PFA) and embedded in either paraffin or O.C.T. Paraffin-embedded (5 μm) and frozen (8 μm) sections were cut for hematoxylin-eosin (H&E) staining, immunohistochemical (IHC) or immunofluorescence (IF) studies. For IHC staining, paraffin-embedded sections were deparaffinized, unmasked with 1 × IHC Antigen Retrieval Solution (E13399-110, eBioscience, CA, USA), and blocked with 2.5% goat serum in PBS for 1h at room temperature. Then, sections were immunostained overnight at 4 °C with specific primary antibodies for human E-cadherin (Millipore, Temecula, CA, USA), Vimentin, Bmi-1, Oct4 (Santa Cruz Technology, Santa Cruz, CA, USA), CD44, CD29 (BioLegend, San Diego, CA, USA), and ALDH1 (Cell Signaling Technology, Danvers, MA, USA) (all in 1:200). After extensively washing with PBS, the ABC reagents (R.T.U. VECTASTAIN Kit, VECTOR, CA, USA) were applied to the sections, followed by color development using Vector NovaRED substrate Kit (Vector NovaRED SUBSTRATE KIT, VECTOR, CA) and counterstained with hematoxylin. Images were observed and photographed under a microscope (Olympus, IX73).
For dual-color IF studies, frozen sections or cultured cells in 8-well chamber slides (Millicell® EZ SLIDES, Millipore) were fixed with 4% PFA, permeabilized in 0.5% triton X-100 in PBS and blocked with 2.5% goat serum in PBS for 1h at room temperature. Then, sections or cells were immunostained with a primary antibody (mouse IgG; 1:200) for human E-cadherin, CD44, or CD29 in combination with another antibody (Rabbit IgG; 1:200) for Vimentin, ALDH1, Bmi-1, Slug, Zeb1 (Cell Signaling Technology), β-Catenin (Abcam, Cambridge, UK), CK14 (Abcam), or CK19 (Sigma-Aldrich, St. Louis, MO, USA). An isotype IgG was used as a negative control. Nuclei were counterstained with 4', 6-diamidino-2-phenylindole (DAPI) (Life Technologies, Carlsbad, CA, USA) and slides were observed under a fluorescence microscope (Olympus IX-73).
In vitro culture of primary epithelial cells from COF specimens
Surgical COF samples were washed with sterile PBS and cut into 2-4 mm pieces followed by enzymatic digestion with 0.1% collagenase (Sigma-Aldrich) and 50 U/mL dispase (Life Technologies) at 37°C for at least 2 h in a shaking incubator. Single cell suspension was filtered through a 70-μm nylon mesh cell strainer and centrifuged at 1200 rpm for 6 min. The cell pellet was resuspended at a concentration of 1 × 106 cells/mL in complete keratinocyte growth medium (KGM-2 kit; Lonza, Basel, Switzerland) and seeded into 10-cm tissue culture dishes pre-coated with 0.1% gelatin and cultured in complete KGM-2 medium (2 × 105 cells/mL) in a humidified incubator at 37°C with 5% CO2. Fresh media were changed twice a week and cell growth was observed and photographed under a microscope. Cells at 75% to 100% confluence were subcultured following trypsinization with 0.05% trypsin and 0.02% EDTA (Life Technologies). Early passages of primary cells were frozen in 90% FBS and 10% DMSO and stored in liquid nitrogen and cells less than 6 passages were used for further experiments.
Flow cytometry
Cells were harvested and washed twice with PBS containing 2% heat-inactivated FBS and resuspended in cell staining buffer (BioLegend) at a concentration of 107/ml, followed by incubation with FITC-conjugated antibodies (1:100) for human CD44, CD29, E-Cadherin, and EpiCam (BioLegend) or an isotype-matched mouse IgG control at 4°C for 30 min. Then, cell samples were washed twice with PBS/2% FBS and submitted to flow cytometric analysis (BD LSRII).
Karyotyping
Cytogenetic analysis was performed by Cell Line Genetics, Inc. (Madison, WI, USA) on 20 G-banded metaphase cells in COF-derived primary epithelial cells.
Results
Clinical summary of the case
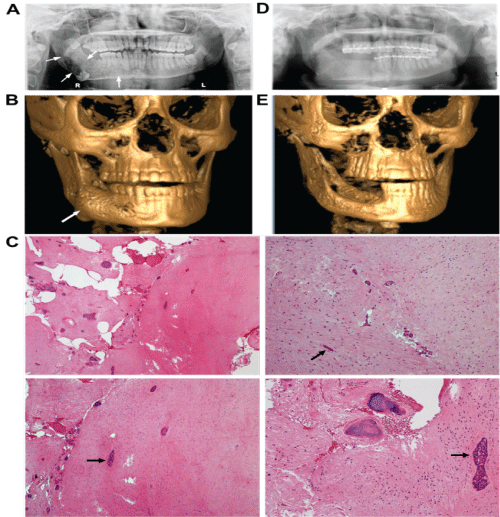
A 24-year-old male presented with progressive mandibular swelling and worsening pain. On clinical examination, he had mild right mandibular facial swelling. Intraoral exam revealed a significant bony expansion in the right posterior mandibular body, a moderate mobility of the right posterior mandibular dentition, and a normal overlying mucosa without evidence of inflammation or infection. A panoramic radiograph showed a mixed radiolucent-radiopaque multilocular lesion of the right posterior mandible from the 1st premolar to the ramus and coronoid region, with inferior displacement of a molar tooth to the inferior border, and superior displacement of a molar tooth into the coronoid process (Figure 1A and Figure B). A maxillofacial CT confirmed the findings on the panoramic radiograph with expansion of buccal and lingual cortices. Incisional biopsy was performed under local anesthesia and the specimen was submitted for pathological examination. The histopathology revealed a haphazard proliferation of spindle- and stellate-shaped cells with benign, round to vesicular-shaped nuclei, showing a fibro-collagenous connective tissue stroma containing scattered areas of myxoid change (Figure 1C). Small and scant islands of odontogenic epithelium were immersed in loose connective tissue with a fibromyxoid appearance and dystrophic calcifications in some areas of the lesion, while no inflammatory cells and mitotic activity were observed (Figure 1C). These findings fulfilled the histologic criteria of epithelium-poor COF [8,17]. The entire lesion was then removed under general anesthesia, whereby the procedure entailed enucleation and curettage, extraction of the involved teeth, coronoidectomy, peripheral ostectomy, primary closure of the oral mucosa, and maxillo-mandibular fixation with Erich arch bars for three weeks. Histological examination of the surgical specimen revealed histopathological properties similar to those as described for the biopsied specimen. Based on clinical, radiological, and histopathological findings, two pathologists, including a Board-certified oral and maxillofacial pathologist, independently arrived at the same diagnosis of epithelium-poor (simple) type of COF. The patient had an uneventful post-operative course. On a 5-month follow-up, the patient did well and radiographic examination showed healing as expected with adequate bone fill at the surgical site (Figure 1D and Figure 1E).
.
Figure 1: Clinical and histopathological images.
A) Preoperative orthopantogram of right mandibular lesion (arrowheads); B) Preoperative 3D reconstruction of right mandibular lesion (arrowhead); C) H&E staining. Upper left: low magnification (Scale bar, 200 μm); upper right: fibro-collagenous stroma with haphazard proliferation of fibroblasts; lower left: calcifications scattered throughout the tumor tissue; lower right: small and scant islands and strands of epithelial rests (arrowhead) immersed in the fibro-collagenous stroma. Scale bars, 100 μm; D) Postoperative orthopantogram; E) Postoperative 3D reconstruction.
View Figure 1
Stem cell-related genes are highly expressed in epithelial rests in odontogenic fibroma
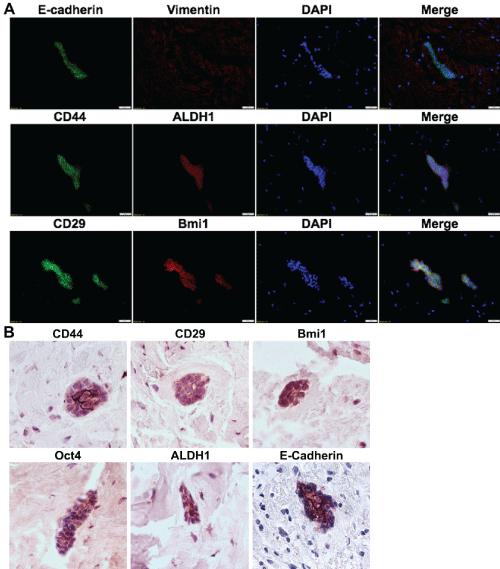
In addition to our histopathological findings (Figure 1), we performed immunohistochemical and immunofluorescence studies to examine the expression of epithelial stem cell-related genes, such as CD44, CD29, ALDH1, Bmi-1, and Oct-4 in this COF lesion. As expected, the epithelial rests in COF were positive for E-cadherin, a specific marker for epithelial cells. More importantly, they also highly expressed the aforementioned epithelial stem cell-related genes (Figure 2A and Figure 2B).
.
Figure 2: Stem cell-related genes are highly expressed in epithelial rests of odontogenic fibroma.
A) The combined expression of E-cadherin, CD44 or CD29 (Green) with Vimentin, Bmi-1 or ALDH1 (Red) in epithelial rests of the odontogenic fibroma tissue was determined by immunofluorescence (IF) staining and observed under a fluorescence microscope; the nuclei were counterstained with DAPI. Scale bars, 100 μm: B) The expression of E-cadherin, CD44, CD29, Bmi1, Oct4, and ALDH1 in epithelial rests of odontogenic fibroma tissue was determined by IHC staining. Scale bars, 50 μm.
View Figure 2
EMT-regulatory genes are expressed in some epithelial rests in odontogenic fibroma
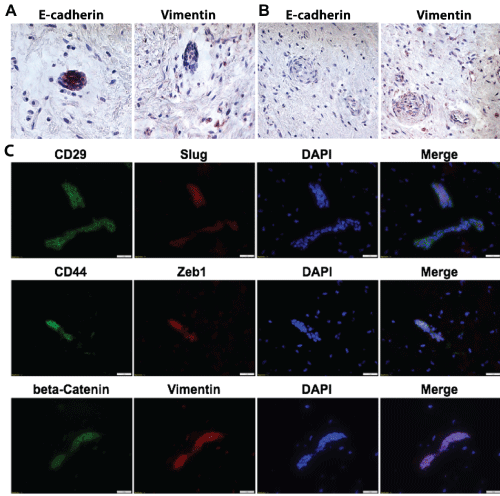
Of note, we also found that some of the epithelial islands or rests lost the expression of E-Cadherin and simultaneously expressed Vimentin, a mesenchymal marker (Figure 3A and Figure 3B). In addition, we showed that certain epithelial-mesenchymal transition (EMT)-related transcription factors, such as Zeb1 and Slug, were co-expressed with CD29 or CD44 in the epithelial rests (Figure 3C). β-catenin, another important EMT-associated transcriptional factor, was co-expressed with vimentin in the epithelial rests (Figure 3C). Taken together, these findings support the existence of a unique subpopulation of epithelial stem-like cells conferred with EMT phenotypes within this COF lesion.
.
Figure 3: EMT- regulatory genes are highly expressed in epithelial rests of odontogenic fibroma.
A), B) The expression of E-cadherin and Vimentin in epithelial rests of the odontogenic fibroma tissue was determined by IHC staining. Scale bars, 50 μm; C) The combined expression of CD44, CD29, or beta-Catenin (Green) with Slug, Zeb1, or Vimentin (Red) in epithelial rests of the odontogenic fibroma tissue was determined by immunofluorescence (IF) staining and observed under a fluorescence microscope; the nuclei were counter stained with DAPI. Scale bars, 100 μm.
View Figure 3
In vitro cultured epithelial cells derived from odontogenic fibroma expressed stem cell-related genes
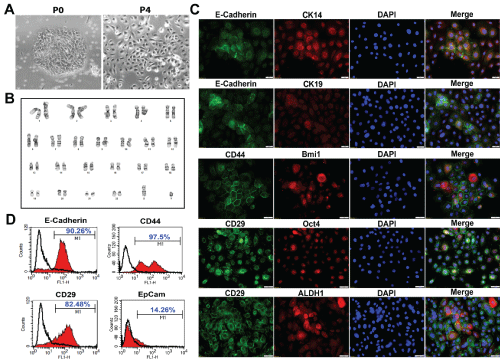
We then isolated and cultured primary epithelial cells from this COF, whereby the primary cells formed single cell-derived colonies and exhibited typical cuboidal morphology and cobblestone growth characteristic of epithelial cells (Figure 4A). Further G-banded metaphase karyotyping analysis revealed an apparently normal male karyotype (Figure 4B). Immunocytochemical studies showed that the ex vivo cultured cells were all positive for E-cadherin (Figure 4C), and the purity of epithelial cells was further confirmed by flow cytometric analysis (Figure 4D). In addition, we showed that this COF-derived primary epithelial cells positively expressed some epithelial stem cell-related genes, such as CD29, CD44, Bmi-1, Oct4, and ALDH1 (Figure 4C). Meanwhile, those cells are positive for CK14, another epithelial stem cell marker, but negative for CK19, a duct epithelial lineage marker (Figure 4C). All together, these results suggest that a subpopulation of epithelial stem-like cells within this COF lesion could be isolated and maintain their stem cell properties during ex vivo propagation.
.
Figure 4: Stem cell-related genes are expressed in odontogenic fibroma-derived primary epithelial cells.
A) The morphology of odontogenic fibroma-derived primary epithelial cells; B) Metaphase karyotyping of odontogenic fibroma-derived primary epithelial cells; C) The combined expression of CD44, CD29, or E-Cadherin (Green) with CK14, CK19, Bmi-1, Oct4, or ALDH1 (Red) in odontogenic fibroma-derived primary epithelial cells was determined by immunofluorescence (IF) and observed under a fluorescence microscope; the nuclei were counter stained with DAPI. Scale bars, 50 μm; D) The expression of E-Cadherin, CD44, CD29, and EpCam in odontogenic fibroma-derived primary epithelial cells was determined by flow cytometry.
View Figure 4
Discussion
Odontogenic fibroma (OF) is a rare odontogenic tumor of mesodermal origin in the jawbone, possibly originating from either periodontal ligament, dental follicle, or dental papilla [2,18]. Different studies have reported a variable incidence rate of OF, from 3% to 23% of all odontogenic tumors [18]. OF can be further divided into peripheral or extraosseous (POF) type and central or intraosseous (COF) type, whereas POFs occurred more commonly than COFs [3,19,20]. Until the year 2012, about 70 cases of COFs have been published in the literature [1,20]. Since then, more new cases of COFs have been reported [4-6,17,18,21-24]. Histopathologically, the World Health Organization (WHO) classified COFs into two variants, the epithelial-rich (complex) type (WHO type) [14,24] and the epithelial-poor (simple) type [6,17]. Clinically, the lesion of COFs usually grows slowly and leads to asymptomatic cortical expansion and bony swelling at the mandible or maxilla [1,4,5,24]. Radiologically, COFs present as a unilocular or multilocular radiolucent area with a well-defined margin [1,2,4,5]. Histopathologically, COF lesions are characterized by presence of odontogenic epithelial islands and a highly cellular fibro-collagenous connective tissue stroma [8,14,18,23,24]. It is noteworthy that the clinical and radiological features of COF are usually similar to other odontogenic and/or non-ondotogenic tumors of the jaw, such as unicystic ameloblastoma, keratocystic odontogenic tumor, dentigenous cyst, and hyperplastic dental follicle, etc., thus making the diagnosis more difficult [1,2,18,19]. Therefore, the combination of clinical, radiological and histopathological findings is necessary to confirm the diagnosis of COF [5,6,18,22,24]. In this study, we reported an epithelium-poor or the simple type of COF according to the clinical, radiological and histopathological findings.
Duo to the extreme rarity of COF, much less work has been done to elucidate the cellular and molecular mechanisms underlying its etiology and pathophysiology [5,14]. Recently, immunohistochemical examination of a COF showed that the odontogenic epithelia were positive for high-molecular-weight cytokeratins, vimentin, and CD99, and negative for CAM5.2, while the stroma contained some myofibroblasts and many fibroblast-like cells positive for CD99 [23]. In a collaborative retrospective study, Mosqueda-Taylor A, et al. reported that odontogenic epithelial islands in COF lesions were immunoreactive for cytokeratins (CK) AE1/AE3, CK5, CK14, CK19, and 34BE12, but negative for CK1 and CK18. They also found that COFs also contained a variable number of mast cells, Langerhans cells, and myofibroblasts [8]. However, the role of these cells in COF pathogenesis remains largely unknown. In the present study, we demonstrated, for the first time to our knowledge, that epithelial rests scattered within COF highly express epithelial stem cell-related genes, such as CD44, CD29, ALDH1, Bmi-1, and Oct4. In vitro primary culture of this COF lesion-derived cells yielded a unique subpopulation of epithelial stem-like cells, which express certain stem cell-related genes and exhibit self-renewal ability in vitro. These findings suggest that the epithelial rests of COF harbor a unique subpopulation of epithelial stem-like cells, which may become active in response to certain stimuli from their own microenvironment and thus contribute to the homeostasis of COF. However further studies are needed to identify the origins of these epithelial stem-like cells, their expression of other important stem cell-related markers and functional genes as well as their potential role in the development of odontogenic fibroma.
To date, accumulating evidence supports the notion that epithelial cells acquire remarkable plasticity or stem-like properties through bidirectional EMT and MET processes orchestrated by both intrinsic and extrinsic cues derived from the tumor microenvironment [15,16]. From this point of view, tumor stem cells may not be distinct entities but rather representative of a state of tumor cells that transiently acquire stem cell-like properties as consequences of bidirectional EMT and MET processes, thus contributing to uncontrollable invasion, metastasis and recurrence of tumors. In this case study, we demonstrated, for the first time, the expression of EMT related-genes in the epithelial islands or rests in COF tissue, suggesting the existence of EMT process actively undergoing in these so-called inactive epithelial islands or rests during COF progress. However, further studies are needed to verify this concept with more COF cases.
In conclusion, we have reported a case of an epithelium-poor or the simple type of COF with positive expressions of both stem cell- and EMT-related genes in the epithelial rests. Our findings support a novel concept that an active EMT process may facilitate the acquisition of epithelial stem-like properties for epithelial rests, and concomitantly contributes to the overgrowth of mesenchymal stromal tissues, and consequently, the aggressive progress of COF. Successful isolation and culture of these unique epithelial stem-like cells from COF tissues allows for the possibility of further studies on the pathogenesis of odontogenic fibroma and other types of benign tumors in the craniofacial and maxillofacial regions.
Acknowledgement
This work was supported by National Institute of Health Research Grant, R01DE 019932 (A. Le), Oral and Maxillofacial Surgery Foundation (OMSF) Research Grant (to Q. Xu and L. Carrasco), and the Schoenleber funding support (A. Le).
Ethical Statement
This study was approved by the Institutional Review Boards (IRB#817407) of the University of Pennsylvania.
Authors' Contributions
Conception and design: A. Le, L. Carrasco, Q. Xu; Acquisition of data: S. Wang, Q. Xu, B. Ford, Q. Zhang, P. Patel, F. Alawi; Analysis and interpretation of data: Q. Xu, S. Wang, F. Alawi; Writing, review, and/or revision of the manuscript: S. Wang, Q. Xu, B. Ford, P. Patel, Q. Zhang, L. Carrasco, A. Le; Study Supervision: L. Carrasco and A. Le.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
-
Hrichi R, Gargallo-Albiol J, Berini-Aytes L, Gay-Escoda C (2012) Central odontogenic fibroma: retrospective study of 8 clinical cases. Med Oral Patol Oral Cir Bucal 17: e50-55.
-
Hara M, Matsuzaki H, Katase N, Yanagi Y, Unetsubo T, et al. (2012) Central odontogenic fibroma of the jawbone: 2 case reports describing its imaging features and an analysis of its DCE-MRI findings. Oral Surg Oral Med Oral Pathol Oral Radiol 113: e51-e58.
-
Lin HP, Chen HM, Vu CH, Yang H, Kuo RC, et al. (2011) Odontogenic fibroma: a clinicopathological study of 15 cases. J Formos Med Assoc 110: 27-35.
-
Chhabra V, Chhabra A (2012) Central odontogenic fibroma of the mandible. Contemp Clin Dent 3: 230-233.
-
Venugopal S, Radhakrishna S, Raj A, Sawhney A (2014) Central odontogenic fibroma. Journal of Indian Society of Periodontology 18: 240-243.
-
Thankappan P, Chundru NSV, Amudala R, Yanadi P, Rahamthullah S, et al. (2014) Central odontogenic fibroma of simple type. Case Rep Dent 2014: 642905.
-
Dunlap CL (1999) Odontogenic fibroma. Seminars in Diagnostic Pathology 16: 293-296.
-
Mosqueda-Taylor A, Martinez-Mata G, Carlos-Bregni R, Vargas PA, Toral-Rizo V, et al. (2011) Central odontogenic fibroma: new findings and report of a multicentric collaborative study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 112: 349-358.
-
Crivelini MM, Felipini RC, Miyahara GI, de Sousa SC (2012) Expression of odontogenic ameloblast-associated protein, amelotin, ameloblastin, and amelogenin in odontogenic tumors: immunohistochemical analysis and pathogenetic considerations. J Oral Pathol Med 41: 272-280.
-
Alawi F, Quinn P (2004) Atypical central odontogenic fibroma recurring as ameloblastoma. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 98: 201.
-
Kinney LA, Bradford J, Cohen M, Glickman RS (1993) The aggressive odontogenic fibroma: report of a case. J Oral Maxillofac Surg 51: 321-324.
-
Slater LJ (1999) Odontogenic sarcoma and carcinosarcoma. Semin Diagn Pathol 16: 325-332.
-
Cercadillo-Ibarguren I, Berini-Aytes L, Marco-Molina V, Gay-Escoda C (2006) Locally aggressive central odontogenic fibroma associated to an inflammatory cyst: a clinical, histological and immunohistochemical study. J Oral Pathol Med 35: 513-516.
-
Ide F, Kikuchi K, Sakashita H, Muramatsu T, Kusama K (2015) Neurovascular involvement in central odontogenic fibroma: a potential source of confusion with invasive carcinoma. Histopathology 66: 1044-1046.
-
Kreso A, Dick JE (2014) Evolution of the cancer stem cell model. Cell Stem Cell 14: 275-291.
-
Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, et al. (2011) Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc Natl Acad Sci U S A 108: 7950-7955.
-
Puppala N, Madala JK, Mareddy AR, Dumpala RK (2016) Central Odontogenic Fibroma of the Mandible. Journal of Dentistry for Children 83: 94-97.
-
Santoro A, Pannone G, Ramaglia L, Bufo P, Muzio LL, et al. (2016) Central odontogenic fibroma of the mandible: A case report with diagnostic considerations. Ann Med Surg (Lond) 5: 14-18.
-
Chrcanovic B, Freire-Maia B, Gomez R (2014) Small central odontogenic fibroma mimicking hyperplastic dental follicle and dentigerous cyst. J Maxillofac Oral Surg 13: 332-336.
-
De Matos FR, de Moraes M, Neto AC, da Costa Miguel MC, da Silveira ÉJD (2011) Central odontogenic fibroma. Annals of diagnostic pathology 15: 481-484.
-
Pippi R, Santoro M, Patini R (2016) The central odontogenic fibroma: How difficult can be making a preliminary diagnosis. J Clin Exp Dent 8: e223-225.
-
Soolari A, Khan A (2015) Central odontogenic fibroma of the gingiva: a case report. Open Dent J 8: 280-288.
-
Iordanidis S, Poulopoulos A, Epivatianos A, Zouloumis L (2013) Central odontogenic fibroma: Report of case with immunohistochemical study. Indian J Dent Res 24: 753-755.
-
Veeravarmal V, Madhavan RN, Nassar MM, Amsaveni R (2013) Central odontogenic fibroma of the maxilla. J Oral Maxillofac Pathol 17: 319.





