International Journal of Stem cell Research and Therapy
Advancing Research in Regeneration and Repair of the Motor Circuitry: Non-Human Primate Models and Imaging Scales as the Missing Links for Successfully Translating Injectable Therapeutics to the Clinic
Magdalini Tsintou1,2#, Kyriakos Dalamagkas1# and Nikos Makris1,2*
1Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital, Harvard Medical School, USA
2Center for Neural Systems Investigations, Massachusetts General Hospital, Harvard Medical School, USA
#These authors contributed equally to this work
*Corresponding author: Nikos Makris, Associate Professor of Psychiatry & Neurology, Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital, Harvard Medical School, USA, Tel: +1617-726-5743, E-mail: nikos@cma.mgh.harvard.edu
Int J Stem Cell Res Ther, IJSCRT-3-042, (Volume 3, Issue 2), Review Article; ISSN: 2469-570X
Received: July 04, 2016 | Accepted: October 25, 2016 | Published: October 28, 2016
Citation: Tsintou M, Dalamagkas K, Makris N (2016) Advancing Research in Regeneration and Repair of the Motor Circuitry: Non-Human Primate Models and Imaging Scales as the Missing Links for Successfully Translating Injectable Therapeutics to the Clinic. Int J Stem Cell Res Ther 3:042. 10.23937/2469-570X/1410042
Copyright: © 2016 Tsintou M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Regeneration and repair is the ultimate goal of therapeutics in trauma of the central nervous system (CNS). Stroke and spinal cord injury (SCI) are two highly prevalent CNS disorders that remain incurable, despite numerous research studies and the clinical need for effective treatments. Neural engineering is a diverse biomedical field that addresses these diseases using new approaches. Research in the field involves principally rodent models and biologically active, biodegradable hydrogels. Promising results have been reported in preclinical studies of CNS repair, demonstrating the great potential for the development of new treatments for the brain, spinal cord and peripheral nerve injury.
Several obstacles stand in the way of clinical translation of neuroregeneration research. There seems to be a key gap in the translation of research from rodent models to human applications, namely non-human primate models, which constitute a critical bridging step. Applying injectable therapeutics and multimodal neuroimaging in stroke lesions using experimental rhesus monkey models is an avenue that a few research groups have begun to embark on. Understanding and assessing the changes that the injured brain or spinal cord undergo after an intervention with biodegradable hydrogels in non-human primates seem to represent critical preclinical research steps.
Existing innovative models in non-human primates allow us to evaluate the potential of neural engineering and injectable hydrogels. The results of these preliminary studies will pave the way for translating this research into much needed clinical therapeutic approaches. Cutting edge imaging technology using connectome scanners represents a tremendous advancement, enabling the in vivo, detailed, high-resolution evaluation of these therapeutic interventions in experimental animals. Most importantly, they also allow quantifiable and clinically meaningful correlations with humans, increasing the translatability of these innovations to the bedside.
Keywords
Neuroregeneration, Repair, Motor circuitry, Central nervous system, Non-human primate models, Neural tissue engineering
Introduction
The limited regenerative capacity of the CNS makes neurological conditions devastating, offering limited therapeutic options to patients. As shown in figure 1, it is not only the mechanical gaps that disrupt neuronal function in brain or spinal injury, but also the triggered cascade of events that leads to secondary neuronal degeneration and death. Therefore, there is a pressing clinical need for the development of therapeutic strategies for currently untreatable disorders of the CNS. Neural tissue engineering is a highly diverse biomedical field that combines experimental and computational neuroscience, clinical neurology, biomaterial science, nanotechnology and many other fields to address neurological diseases from a new perspective. This field seems to hold the promise of effective treatments, but there remain strategic steps that need to be followed to reach the full potential that this technology can offer.
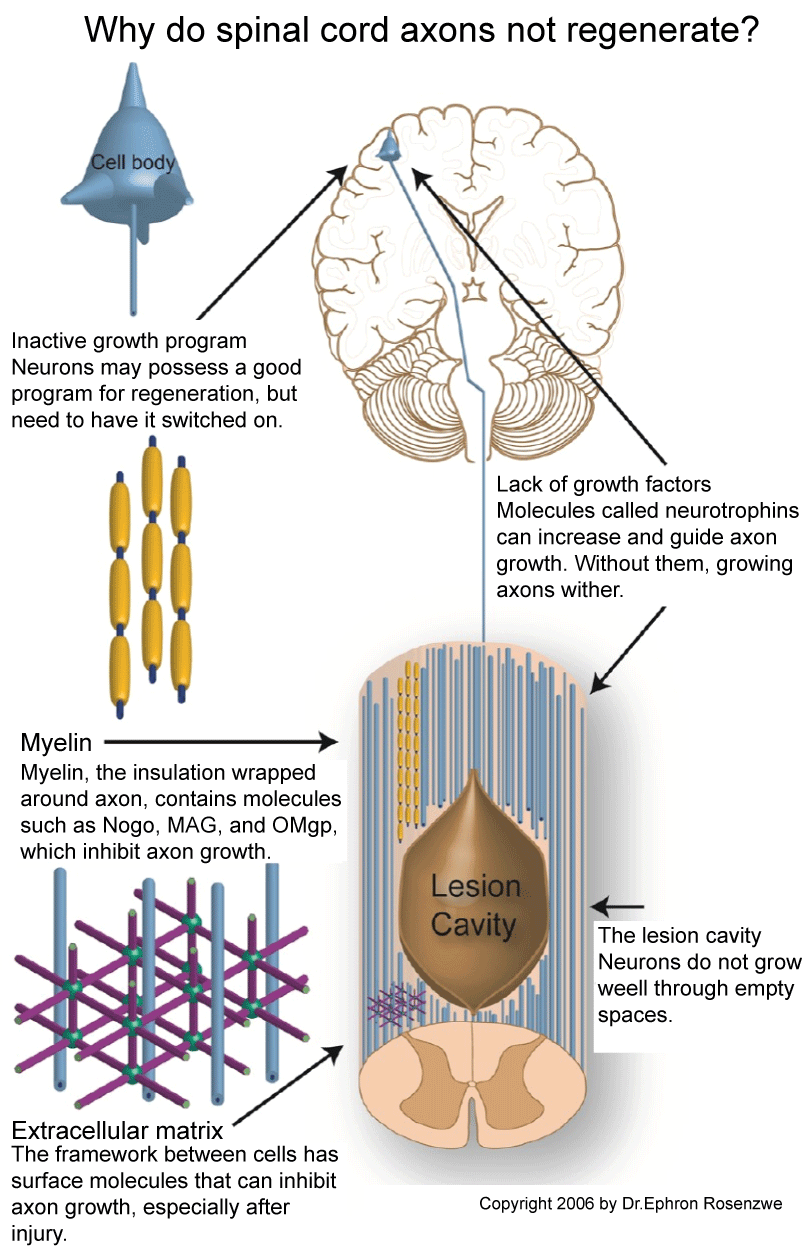
.
Figure 1: The figure illustrates the various inhibitory factors blocking axonal regeneration in the central nervous system (CNS). The CNS is unable to regenerate despite its regenerative potential due to the imbalance of inhibitory and promoting factors for regeneration [34].
View Figure 1
In this review we aim to provide a succinct outline of the most clinically translatable current animal models, focusing on next steps that would allow advances in neural repair to progress from bench to bedside. We focus principally on spinal cord injury (SCI) and ischemic stroke, because these are the clearest and most reproducible models for developing initial CNS therapies and also because they are based on known etiology and widely studied recovery patterns. Importantly, the results of these studies can be easily quantified to assess and monitor the progress of cavitary lesions using neuroimaging tools. These results could then be applied to other clinical conditions of the CNS involving neurodegeneration (i.e., stroke, traumatic brain injury, amyotrophic lateral sclerosis, multiple sclerosis).
Despite the complexity of the CNS and the challenges of developing effective neuroregeneration methods, there is a considerable amount of data regarding disease models focusing on repair of the nervous system [1-12]. One widely explored approach involves the utilization of structural support by biodegradable, biocompatible, injectable hydrogels in stroke and SCI applications, along with sustained and targeted release of trophic factors and/or stem cells to trigger an endogenous neuroregenerative response [7-12]. In the following section we will mention some of the significant achievements in preclinical research work based on small animal models (i.e., rats, mice) that have allowed us to move closer from bench to bedside treatment protocols.
Regeneration and repair: Selected preclinical achievements that open the path to animal models closer to humans
The majority of preclinical data for CNS repair is based on rodent animal models [13]. Rodents are widely used for preclinical investigations given their low cost, small size, ease of handling and rapid growth and reproduction rate. Their use can be highly valuable in terms of understanding the neurobiology of disorders in relatively complex in vivo settings compared to in vitro. The field is advancing so rapidly that there are already significant accomplishments in terms of nerve repair within the CNS of animal models.
The pioneering work of Tuszynski, Lu and colleagues is one of the clearest recent examples of the potential of CNS utilizing tools offered by neural engineering [14,15]. Using fibrin matrices loaded with a cocktail of growth factors and green fluorescent protein (GFP)-expressing neural stem cells (NSCs) in a complete spinal transection and hemisection rodent SCI model, Tuszynski, et al. have accomplished, to the best of our knowledge, the longest-distance axonal sprouting after SCI. Axons emerged by post-operative day 2 and extended at a rapid rate of 1-2 mm/day, sprouting tens of thousands of axons from the lesion site over virtually the entire length of the rat CNS. Despite Tuszynski, et al.'s success in rat models, in a collaborative attempt with Friedli and colleagues they emphasized the importance of non-human primate models for the development of clinically relevant treatments [16].
Carmichael and colleagues have focused their studies on stroke repair strategies [17]. To this end, they have combined rodent models, non-human primate models and imaging for further assessment and monitoring of intervention-related outcomes. To achieve sustained release of brain-derived neurotrophic factor (BDNF) within the CNS after ischemic stroke, they used a hyaluronan (HA) hydrogel-BDNF combination. BDNF was detected over 3 weeks post-operatively, promoting behavioral recovery and axonal sprouting within the motor system. Importantly, Carmichael, et al. found that magnetic resonance imaging (MRI) allowed detection of the hydrogel-BDNF combination and tracked changes over time in living mice and in non-human primates, which is not easily achievable with all brain-biocompatible biomaterials. They also observed that in both animal models, BDNF achieved significant diffusional distances in the brain. Specifically BDNF was seen up to 2 cm from the infract in the non-human primate models, an important fact that suggests translatable clinical potential in humans.
Admittedly these are only two examples of several promising results reported in the literature, but an exhaustive consideration of regenerative studies is beyond the scope of this review. Among the several experimental rodent disease models and the different ways researchers have tried to mimic human pathology in order to obtain clinically-relevant results, there are a few that have paved the way in neuregeneration and neurorepair research, as discussed below [18].
Main experimental rodent models used for SCI
The gold standard for closely mimicking the most common SCI injuries is the contusion model. This is the most clinically relevant model, demonstrating high reproducibility. In addition to this model, there are compression (prolonged spinal cord compression), distraction (stretches the cord), dislocation (replicates human vertebral displacement) and transection or hemisection (complete or partial cuts of the spinal cord) models. The reproducibility of these methods varies considerably. Contusion along with compression models are the most clinically relevant, resulting in the most reproducible lesions. Nevertheless, it is common practice for researchers to utilize transection models, because they permit studying SCI regeneration in complete lesions [18].
Main experimental rodent models used for ischemic stroke
In an attempt to mimic the ischemic insults in the human brain, many different animal stroke models have been explored. Among these, the four predominant models for investigating NSC responses are the middle cerebral artery occlusion (MCAO), common carotid artery occlusion (CCAO), devascularization via pial vessel disruption (PVD) and photothrombosis models. MCAO closely resembles the results of human strokes by causing a striatal infarct that can extend to the cortex after an adequate amount of occlusion time. CCAO, which is also known as transient global ischemia, given the "global" deprivation of blood supply in the entire brain, is a simpler model in terms of surgery but less clinically relevant. Devascularization by PVD is an excellent model for assessing functional recovery due to disruption of specific motor or sensory brain areas by local cortical ischemic damage. Finally, photothrombosis is an important method obviating the need for craniotomy, because it uses an argon-ion laser and photosensitive dye to cause focal cortical damage, enabling an accurate stroke lesion induction non-invasively [18].
Despite the abundance of preclinical rodent models, there are differences between rodents and humans in neuroanatomical and functional complexity that cannot be overlooked. These differences significantly limit the clinical utility of the preclinical therapies based on rodent models.
Why is there a gap between successful rodent preclinical models and their clinical potential?
The rapid pace of advances in neuroregenerative therapies and the impressive achievements in small animal models have motivated researchers to try translating treatments directly from rodents to humans, albeit with discouraging outcomes. Rodents represent readily available models that can be used for a deeper understanding of basic biological mechanisms and for proof of concept for preclinical research hypotheses. However, attempts at direct clinical translation to humans have proven problematic or even impossible to date, principally due to issues of scaling and complexity.
One obvious key problem for the direct clinical translation from rodents to humans is the significantly smaller size or the rodents' nervous system compared to humans, limiting generalizability regarding the repair of critical gap defects in humans. Moreover, the rat species-specific neurobiological regenerative profile differs significantly from that in humans [19]. After SCI, for example, inflammation is less pronounced in humans, even though cytokine expression is similar, demyelination is probably less, Wallerian degeneration is much more prolonged, glial scar with chondroitin sulfate production is less extensive, Schwannosis is extensive, the inhibitory protein Nogo-A is rarely found in the periaxonal myelin sheath, and the myelin-associated glycoprotein (MAG) persists longer, disappearing between 14 days and 4 months post-injury. A major difference, for example, that makes mice an inappropriate model for the study of human SCI is the lack of cyst formation that occurs in humans. Such differences could significantly affect the translation of results from rodents to humans in a variety of ways [20].
When considering rodent preclinical models, it is important to note that the brain of a mouse is 1/1000th that of the human and that in the humans axonal sprouting of a few millimeters is rarely adequate to repair CNS damage that normally extends over a few centimeters [21]. Furthermore, the significantly less complex rodent brain, with many fewer synapses and connections within its neuronal networks, may respond to certain regenerative interventions in ways that would not reach the threshold for clinical improvement in humans. Thus, it is perhaps not surprising that many Phase II and Phase III human clinical trials based on preclinical rodent data have failed, raising questions regarding the feasibility of such direct clinical translation [21].
In an attempt to clarify biological and functional divergence among species, Friedli and colleagues examined the reorganization and function of the corticospinal tract (CST) after SCI in rats, monkeys and humans [16]. CST is known to heavily affect human voluntary movement ability even though its contribution to locomotion is debated. In lower mammals, CST is not necessary for non-complex movement execution. Analysis of SCI lesions in all models indicated that monkeys and humans have the potential for synaptic reorganization of the spared CST fibers above and below the lesion, bridging the area across the hemisection and improving fine movement control capabilities. By contrast, such reorganization was not seen in rats. Based on this fact, the rodent models do not appear adequate for evaluating the restoration of fine motor skills and voluntary movements after an injury.
Thus, it is evident that there is a substantial need for non-human primate models before proceeding to clinical trials, to ensure minimal risk and maximum success based on the similar underlying pathophysiological mechanisms in humans and non-human primates. Due to the higher cost and complexity of experiments with non-human primates, there have been suggestions for the incorporation of other relatively larger animals (compared to rodent model) in neuroregeneration research (e.g., dogs, cats, pigs), in order to acquire adequate supportive data for clinical translation to humans.
Larger mammals as means to increase translatability
Larger domesticated mammals such as dogs, pigs, sheep, and goats have been suggested as effective alternatives to increase the clinical translation potential of novel theranostics. Indeed, such animal models do offer significant advantages compared to rodents due to their larger gyrencephalic brains with gray/white matter proportions that are closer to humans. These animals have a longer life span, which facilitates longitudinal studies critical for most neural engineering and stem cell applications, and they have greater physiological and anatomical resemblance to humans [22].
Larger mammals, mainly cats or dogs, have been popular models in neuroscience due to their ability to withstand extensive surgeries, their lower cost, and their larger size that allows for more complex interventions. However, after the mid-1980's, new regulations and growing public opinion against the use of companion animals for basic research purposes led to a reduction in the number of studies with feline models. Similarly, in recent years there has been a notable shift away from using dogs and toward the use of swine disease models, especially mini-pigs [23].
Swine models have been used increasingly in research due to similarities of their nervous system (especially the brain) to that of humans. The size and anatomic characteristics of pig brains have made this model attractive for CNS research [24]. There are certain limitations, however, due to differences in vascular and vertebral structure in comparison with humans. Although pig spinal vasculature is remarkably similar to humans, there are some significant differences. For example, the pig brain is supplied mainly by the internal carotid arteries, contrary to humans. In pigs, there is a larger plexus of vessels in the lower neck, larger bilateral vertebral arteries contributing to the circle of Willis, notably extensive branching, collaterals and shunting around larger arteries, and other vascular differences [25]. In addition, the vertebral formula of pigs differs from humans (C7, T14-15, L6-7, S4, Cy20-23) with the cauda equine originating at the S2-3 level. Structural difference (e.g., narrow intervertebral spaces and more prominent vertebral processes) may influence CNS related protocols, such as those in SCI applications [26].
There is also a recent trend toward miniature swine models (mini-pigs), due to ease of handling and closer approximation to average human size at full maturity. Conventional swine breeds typically reach 100 kg or 220 lb by the 4th month of age and can triple this weight by full maturity, posing practical challenges. In addition, the mini-pig's slower growth curve make this model of particular importance for capturing the late-stage outcomes of interventions, i.e., outcomes related to the chronic SCI stage [27]. Husbandry and handling of mini-swine is also easier due to their favorable temperament [28].
No matter how advantageous these animal models are for ensuring better quality and more clinically meaningful data, there remain limitations due to the lack of standardized procedures, and limited availability of species-specific antibodies, protocols and reagents. Moreover, certain species-specific pathophysiological or anatomical differences may influence the regenerative results regarding more hierarchically complicated structures such as the brain [29]. After proof of concept with rodents or with larger non-primate mammals, the next essential step before proceeding to clinical trials is testing the neuroregenerative method on non-human primates. Non-human primates provide a model highly relevant to human pathology and behavior that may permit the development of nerve repair therapeutics more likely to be successful in clinical trials.
The missing link: Non-human primate models for motor circuitry repair
Although non-human primates are expensive to maintain and require an experienced team with specific skills for handling the animals and evaluating their progress, non-human primate disease models have provided preclinical results that have helped pave the way for human CNS repair [30-32]. Such studies remain at a preliminary stage due to the limited number of animals tested and the limited number of researchers having access to such models. However, there are already indications that nerve repair can occur in those models with significant functional restoration.
Current developments indicate that the monkey model has great potential, especially when testing for the recovery of skilled movement. The under-representation of monkey models in translational research is a significant gap that impedes progress towards clinical translation. This is supported by recent lesional and behavioral studies implementing injectable therapeutics in rhesus monkeys after the induction of stroke in motor cortex, in the dedicated area for fine finger movements [33]. There are studies of injectable substances in the form of a gel material, which can act as scaffold loaded with growth factors, stem cells and cytokines. These substances can also be introduced in the form of stem cells directly injected into the cavity or exosomes from mesenchymal stem cells (Figure 2). The rationale for using these materials is that they will produce neuronal bridging, i.e., filling-in, and thus will generate connectivity across the lesion [10,34-41].
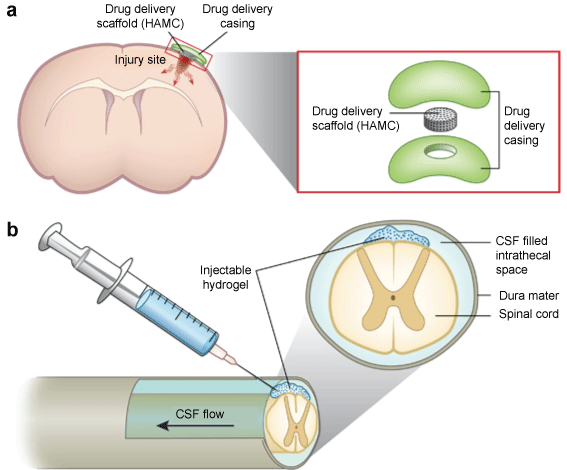
.
Figure 2: This is an illustrative demonstration of an injectable hydrogel applied to an ischemic stroke and a spinal cord injury (SCI) model: a) Stroke model: The drug-containing hydrogel (HAMC) is placed on top of the cortex, permitting diffusion into the brain. Additionally, other neurotrophic factors and/or stem cells can be delivered instead according to the focus and hypothesis of the experiment. The arrows in the horizontal cross-section indicate diffusion in all directions; b) SCI model: The rational is the same as in stroke lesions. The hydrogel is injected into the space between the spinal cord and dura matter called intrathecal space in order to structurally bridge the lesion site and provide the needed biomolecules through diffusion of the embedded substance [67].
View Figure 2
To this end, a translational pipeline that would maximize the safety and the clinical success of any prospective human treatment strategies has been suggested, as shown in figure 3. A neuroregenerative therapy could first be tested in small animal models (rodents), in order to gain an understanding of the repair mechanisms and the factors contributing to this process. The therapeutic vehicle would subsequently be tested in non-human primate models that more accurately represent human pathology, thereby achieving high-level, complex, clinically significant correlations. With the help of high-resolution imaging, such correlations can be quantified to establish objective scales for assessing the nerve repair potential. This "rodent-monkey-human" translational pipeline seems to be a solid basis for future translational research on nerve repair.
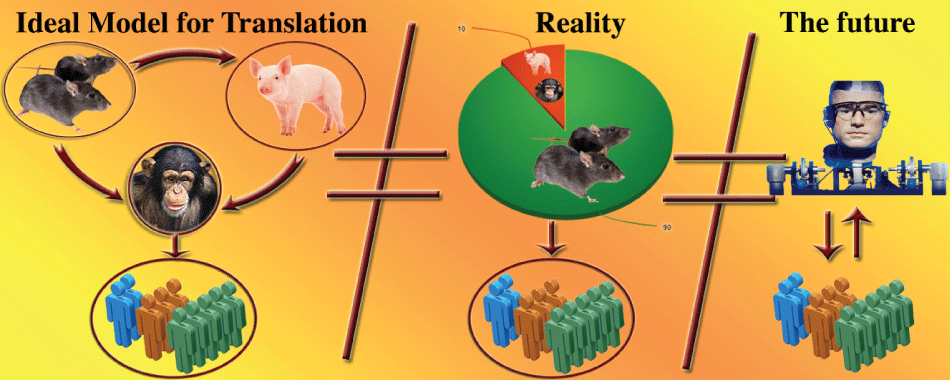
.
Figure 3: Illustrative demonstration of the suggested pipeline (on the left) for taking a therapy more safely and effectively from bench to bedside. An injectable hydrogel loaded with growth factors and/or stem cells can be initially tested in rodent models in order to study the pathophysiological mechanisms involved and establish an initial proof of concept for the proposed treatment strategy. The hydrogel can also be tested in larger mammals (e.g., mini-pigs) after the initial proof of concept to allow more generalizability to humans. After initial success in rodents and/or larger animals, the hydrogel should be tested in non-human primate models in order to address more complex questions of relevance to humans that cannot be addressed in other species due to differing neurobiological regenerative profiles. Finally, if the treatment is safe and successful for these animal models, it can proceed to human clinical trials. At each step imaging data can help to quantify the results and establish standards that can be clinically meaningful for assessing the progress of the patients and the regenerative potential of the treatment. In the middle figure, the contrast of such pipelines to current research reality is illustrated. In particular, over 90% of the animals used for research purposes are rodents based on current estimations. In many cases promising results in rodents have led to clinical trials without further testing in other animal models, leading to discouraging failures of trials and significant increase in the cost (financial as well as quality of life). The right side of the image is an illustrative representation of the future. Animals testing may become unnecessary and inappropriate due to advances in the field of tissue engineering. Human-based artificial tissue development would allow direct testing of novel theranostics within appropriate platforms directly related to human pathophysiology and anatomy. This could become an ideal setting for maximizing the translational potential of new therapeutic approaches.
View Figure 3
In a series of experiments, using devascularization via PVD in non-human primate ischemic stroke models, Rosene, Moore and colleagues, demonstrated the recovery pattern for hand motor function [33,42,43]. They also measured the delay for recovery based on age (130-150 days of recovery for middle-aged animals compared to the 65-80 days of recovery for younger animals) and the statistically significant motor functional recovery in a treatment group with human umbilical tissue-derived cells (hUTCs) compared to the control group. The hand motor area and, the control of the digits in particular, is a vital area of brain research for nerve repair and neurorehabilitation for improving the quality of life of patients with CNS disorders and restoring their fine motor skills.
Quantifying the recovery pattern in the aging brain with the use of imaging is also of high importance given that many CNS disorders target different age groups of patients [44,45]. This is needed to fully understand human brain degenerative changes and, ultimately, for developing repair and neuroprotection methods. Such understanding could subsequently inform other areas of aging research and the application of anti-aging drugs or nutrients that could, in turn, indirectly help in neural repair and neuroprotection processes. One interesting example in this regard is the neuroprotective nutrient curcumin, which appears to be beneficial for many CNS conditions (e.g., SCI, intracerebral hemorrhage, brain ischemia, and various encephalopathies) [46,47]. Nevertheless, its beneficial effects need to be evaluated in non-human primates en route to clinical translation. An important step in this direction is the use of neuroimaging technology, in particular for the delineation of nervous system white matter architecture. In fact, the assessment of all changes in and around a lesion can be done by using current Connectome scanners (http://www.humanconnectome.org/data/) and multimodal neuroimaging.
Neuroimaging prepares the ground for clinical translation
The advent of neuroimaging and its tremendous development over the last thirty years have opened up a huge opportunity in medical diagnostics and translational research, making in vivo, non-invasive interventions and treatment assessment an everyday reality [48]. Development of a highly reproducible and reliable non-human primate model for neuroregeneration is challenging. Neuroimaging and quantifiable high-resolution image analysis will be critical for assessing regenerative outcomes in order to translate novel therapies to the clinical domain.
Current structural imaging allows the characterization of a vast variety of parameters of brain tissue, in terms of biophysical properties, size and brain structure based on architectural and connectional factors [49-51]. Furthermore, using functional imaging and functional connectivity analyses, motor circuitry can be functionally evaluated [52-54]. Thus, the structural, functional and behavioral effects of a therapeutic intervention on an injured brain's plasticity, regeneration and repair can be measured in vivo in a non-invasive and objective way, assessing the gray and white matter [50,55].
There are hydrogels that have been visualized and studied with MRI, along with some basic CNS lesion characteristics (i.e., volume of lesion) [17]. In ischemic stroke models, it has been observed that hydrogels can lead to expansion of the cavitary lesion in the 2-week post-injection period. Therefore, we need to be able to visualize the hydrogel and track its degradation rate, as well as to quantify lesion size, correlate it with functional outcomes and, possibly, with in vivo visualized cells. Recently, Nicholls, et al. have demonstrated a reliable in vivo imaging method for tracking transplanted cells without the use of transfected vector, using DNA-gadolinium-gold nanoparticles [56]. However, in this study the false negatives were 30%, indicating a significant underestimation of the transplanted cells population that needs to be reduced in future work.
A critical notion in comparative neuroimaging is the harmonization of structure across species, which involves the utilization of well-informed brain atlases in neuroanatomy [57,58]. Brain atlases have been developed for several species, so that anatomical-clinical correlations may be possible, along with quantification of the therapeutic outcomes [59].
Monkey brain atlases
Several brain MRI atlases have been developed for different primate species such as chimpanzees, macaques and marmosets [60-62]. These atlases can be used to assess the structural as well as functional integrity of the nervous system of monkeys using MRI scanners. Considering the great homology of non-human primate DNA to humans, up to 98% in chimpanzees, we can guardedly optimistic that an intervention found to be successful in a monkey model may be appropriate for testing in humans.
Several projects have been launched worldwide, e.g., in the United States, Europe, China and Japan for creating human brain maps of both functional and structural connectivity using non-invasive imaging technology. The Human Connectome Project (HCP) represents one such initiative in the United States (http://www.humanconnectome.org/data/). The HCP is aimed at setting a normal baseline for the human brain, and establishing key clinical correlations with pathological human disease models. Once the human neural architecture has been investigated at high resolution both structurally and functionally, it would seem feasible to establish associations with non-human primate models. This creates a unique opportunity to develop a translational pipeline between humans and monkeys that can be used to accelerate the development of new therapies and interventions for the human CNS.
Ethical considerations- could we develop cures without the use of animals?
Although regulations exist to protect the well-being of animals and their ethical use for research purposes (e.g., the Animal Welfare Act (AWA) in United States), the animal research setting is far from ideal. AWA does not address animals such as rats, mice and birds bred for research use, therefore excluding approximately 90-95% of animals used in research laboratories. This does not mean that the excluded animals are not protected by other federal laws, however the regulations are less strict. Given that national annual statistical reports are based only on animals covered by AWA, there is no way to have an accurate overall enumeration of animals used for research purposes in United States. For the 10% of larger animals covered by AWA (dogs, cats, non-human primates, guinea pigs, hamsters, rabbits and other warm-blooded animals) the law sets the minimal standards for housing, feeding, handling, veterinary care or psychological care, where applicable.
AWA standards have made it more difficult for such animals to be used for research purposes without appropriate justification (Figure 3 and Figure 4), thereby gradually decreasing the number of these animals used for research. Nevertheless, the U.S. Department of Agriculture (USDA) states that the overall use of animals has not changed, but has shifted towards rodents. This could possibly increase poorly justified use of rodent models as well as methodological research errors due to the choice of such models as inappropriate for translational purposes. Therefore, there is a need for further development of regulatory requirements to ensure the continuous appropriate and justified use of animals for research purposes, with proper husbandry techniques. Only highly trained and specialized personnel should be allowed to handle animals in order to minimize suffering and stress during the research process. Additional research aimed at exploring the distress mechanisms of animals during therapeutic interventions should be considered in order to optimize animal welfare regulations.
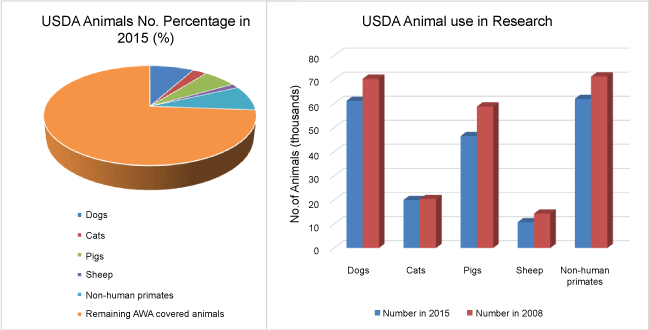
.
Figure 4: The left side of the figure illustrates the most useful models for human neuroscience (i.e., pigs, non-human primates) that are the rarely used in research settings, due to factors such as increased administrative costs, the necessity for highly trained staff members for animal handling, and the stricter regulations of AWA. It should be noted that, despite the fact that it is estimated that rodent models represent 90-95% of the animals used in research settings, they are not included in the graph. Because these animals are not covered by the AWA, accurate national statistics related to their use in research settings are not available. It is estimated by the USDA however, that their use has increased due to less strict regulations, lower costs, and the development of genetically modified species.
On the right side of the figure the gradual decrease in the number of larger animals used in research settings indicates that the USDA regulations have become stricter and that researchers are possibly discouraged from using larger animal models in the presence of alternatives such as i.e., rodent models.
Abbreviations used: USDA: United States Department of Agriculture; AWA: Animal Welfare Act.
View Figure 4
Larger animals, such as non-primates and companion domesticated animals raise an even higher level of concern for the society, given their psychosocial presence and privileged position among humans. Obviously, the use of each animal model should be carefully considered, and preliminary testing should be conducted in smaller animals in order to get a better understanding of the potential mechanisms and limitations. The use of larger animals, especially non-human primate models, remain a necessity for fully understanding disorders of the human central nervous system that affect complicated neuronal networks and are life-threatening or highly debilitating.
Apart from ethical issues that one needs to consider before designing an animal-based experiment, there is also the need to find a solution for the increasing number of failing clinical trials. Such trials significantly increase not only financial costs, but also the cost in human and animal lives. Technology seems to be close to offering a solution, offering the possibility of the development of cures without the use of animals. Biomedical therapeutics ultimately may lead to the development and use of artificial organs in research so that, in the future, not only will animals be unnecessary for research applications, but the models themselves will be based on human's pathophysiology and human-based tissues, resulting in more efficient design of clinical trials [63-66].
Conclusion
The field of neural engineering and neuroregeneration is undoubtedly a highly promising research area that integrates medical rationale and clinical need with the development of clinical treatments. The rapid advances of the field have yielded a large amount of data from small animal models (mainly rodents), setting the basis for a deeper understanding of neurobiology that can address significant clinical issues in humans. Nevertheless, there markable accomplishments in CNS repair using such animal models are at variance with the efficacy of clinical translation. Rodents and other small animals have different nervous system anatomy and pathology, and respond to neural injury, differently from humans, therefore clinical trials cannot be based solely on small animal models. As neural engineering and neuroregeneration research have progressed, there is an escalating need for the development of a more complex, structured pipeline for clinical translation. This pipeline should entail a series of steps, including in vitro and in vivo small animal models, as well as in vivo non-human primate models and imaging in all in vivo steps, to assess potential clinical human applications. Although non-human primate models may appear to be less cost-effective for research use, there is a significant long-term gain to be realized. This is due to the significant health-related and high financial risk involved in the direct translation from small animal models to humans. Until research without animal use is made possible with artificial human-based organs, the establishment of a "rodent-monkey-human" pipeline would accelerate the field of CNS regeneration and repair research, overcoming significant barriers in this much needed and important endeavor of biomedical research.
Acknowledgements
We would like to thank Dr. Douglas Rosene, Dr. Tara Moore and the rest of their team in Boston University for sharing their expertise on non-human primate models, focusing mainly on ischemic stroke. We would like to thank Dr. Edward Yeterian, and the anonymous reviewers for providing useful comments on the manuscript.
Nikos Makris is supported by the NIH National Center for Complementary and Integrative Health (NCCIH) of the National Institutes of Health (NIH) under award number R21AT008865.
Conflicts of Interest
Nothing to declare.
Author Contribution
Authors contributed equally to this work.
References
-
Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, et al. (1995) Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature 378: 498-501.
-
Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME (1994) Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature 367: 170-173.
-
So K-F, Aguayo AJ (1985) Lengthy regrowth of cut axons from ganglion cells after peripheral nerve transplantation into the retina of adult rats. Brain Res 328: 349-354.
-
Schwab ME, Thoenen H (1985) Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J Neurosci 5: 2415-2423.
-
Aguayo AJ, Rasminsky M, Bray GM, Carbonetto S, McKerracher L, et al. (1991) Degenerative and Regenerative Responses of Injured Neurons in the Central Nervous System of Adult Mammals. Philos Trans Biol Sci 331: 337-343.
-
Aguayo AJ (1985) Axonal regeneration from injured neurons in the adult mammalian central nervous system. Synaptic Plast 457-484.
-
Winter CC, Katiyar KS, Hernandez NS, Song YJ, Struzyna LA, et al. (2016) Transplantable living scaffolds comprised of micro-tissue engineered aligned astrocyte networks to facilitate central nervous system regeneration. Acta Biomater 38: 44-58.
-
Hsieh FY, Tseng TC, Hsu SH (2015) Self-healing hydrogel for tissue repair in the central nervous system. Neural Regen Res 10: 1922-1923.
-
Elliott Donaghue I, Tator CH, Shoichet MS (2016) Local Delivery of Neurotrophin-3 and Anti-NogoA Promotes Repair After Spinal Cord Injury. Tissue Eng Part A 22: 733-741.
-
Elliott Donaghue I, Shoichet MS (2015) Controlled release of bioactive PDGF-AA from a hydrogel/nanoparticle composite. Acta Biomater 25: 35-42.
-
Rivet CJ, Zhou K, Gilbert RJ, Finkelstein DI, Forsythe JS (2015) Cell infiltration into a 3D electrospun fiber and hydrogel hybrid scaffold implanted in the brain. Biomatter 5: e1005527.
-
Li H, Ham TR, Neill N, Farrag M, Mohrman AE, et al. (2016) A Hydrogel Bridge Incorporating Immobilized Growth Factors and Neural Stem/Progenitor Cells to Treat Spinal Cord Injury. Adv Healthc Mater 5: 802-812.
-
Pankevich DE, Wizemann TM, Mazza A-M, Altevogt BM (2012) International Animal Research Regulations: Impact on Neuroscience Research: Workshop Summary. National Academies Press.
-
Lu P, Wang Y, Graham L, McHale K, Gao M, et al. (2012) Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150: 1264-1273.
-
Lu P, Woodruff G, Wang Y, Graham L, Hunt M, et al. (2014) Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 83: 789-796.
-
Friedli L, Rosenzweig ES, Barraud Q, Schubert M, Dominici N, et al. (2015) Pronounced species divergence in corticospinal tract reorganization and functional recovery after lateralized spinal cord injury favors primates. Sci Transl Med 7: 302ra134.
-
Cook DJ, Nguyen C, Chun HN, Llorente IL, Chiu AS, et al. (2016) Hydrogel-delivered brain-derived neurotrophic factor promotes tissue repair and recovery after stroke. J Cereb Blood Flow Metab 12.
-
Grégoire CA, Goldenstein BL, Floriddia EM, Barnabé-Heider F, Fernandes KJ (2015) Endogenous neural stem cell responses to stroke and spinal cord injury. Glia 63: 1469-1482.
-
Kaplan HM, Mishra P, Kohn J (2015) The overwhelming use of rat models in nerve regeneration research may compromise designs of nerve guidance conduits for humans. J Mater Sci Mater Med 26: 226.
-
Hagg T, Oudega M (2006) Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma 23: 264-280.
-
Dobkin BH (2007) Curiosity and cure: translational research strategies for neural repair-mediated rehabilitation. Dev Neurobiol 67: 1133-1147.
-
Sørensen JC, Nielsen MS, Rosendal F, Deding D, Ettrup KS, et al. (2011) Development of neuromodulation treatments in a large animal model-Do neurosurgeons dream of electric pigs? Progress in Brain Research 194: 97-103.
-
(2009) National Research Council (US) Committee on Scientific and Humane Issues in the Use of Random Source Dogs and Cats in Research. Scientific and Humane Issues in the Use of Random Source Dogs and Cats in Research. Washington (DC): National Academies Press (US).
-
Lind NM, Moustgaard A, Jelsing J, Vajta G, Cumming P, et al. (2007) The use of pigs in neuroscience: modeling brain disorders. Neurosci Biobehav Rev 31: 728-751.
-
Strauch JT, Lauten A, Zhang N, Wahlers T, Griepp RB (2007) Anatomy of spinal cord blood supply in the pig. Ann Thorac Surg 83: 2130-2134.
-
Swindle MM, Smith AC (2007) Swine in the laboratory: surgery, anesthesia, imaging, and experimental techniques. CRC Press.
-
Swindle MM, Smith AC, Laber-Laird K, Dungan L (1994) Swine in Biomedical Research: Management and Models. ILAR J 36: 1-5.
-
Simmons GH, Padilla J, Jenkins NT, Laughlin MH (2014) Exercise training and vascular cell phenotype in a swine model of familial hypercholesterolaemia: conduit arteries and veins. Exp Physiol 99: 454-465.
-
Moran CJ, Ramesh A (2016) The benefits and limitations of animal models for translational research in cartilage repair. J Exp Orthop 3: 1.
-
Joers VL, Emborg ME (2009) Preclinical assessment of stem cell therapies for neurological diseases. ILAR J 51: 24-41.
-
Freund P, Schmidlin E, Wannier T, Bloch J, Mir A, et al. (2006) Nogo-A-specific antibody treatment enhances sprouting and functional recovery after cervical lesion in adult primates. Nat Med 12: 790-792.
-
Zörner B, Schwab ME (2010) Anti-Nogo on the go: from animal models to a clinical trial. Ann N Y Acad Sci 1: E22-34.
-
Moore TL, Pessina MA, Finklestein SP, Kramer BC, Killiany RJ, et al. (2013) Recovery of fine motor performance after ischemic damage to motor cortex is facilitated by cell therapy in the rhesus monkey. Somatosens Mot Res 30: 185-196.
-
Tsintou M, Dalamagkas K, Seifalian AM (2015) Advances in regenerative therapies for spinal cord injury: a biomaterials approach. Neural Regen Res 10: 726-742.
-
Kumar P, Choonara YE, Modi G, Naidoo D, Pillay V (2015) Multifunctional therapeutic delivery strategies for effective neuro-regeneration following traumatic spinal cord injury. Curr Pharm Des 21: 1517-1528.
-
Führmann T, Obermeyer J, Tator CH, Shoichet MS (2015) Click-crosslinked injectable hyaluronic acid hydrogel is safe and biocompatible in the intrathecal space for ultimate use in regenerative strategies of the injured spinal cord. Methods San Diego Calif 84: 60-69.
-
Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig A-K, et al. (2015) Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl Med 4: 1131-1143.
-
Pakulska MM, Ballios BG, Shoichet MS (2012) Injectable hydrogels for central nervous system therapy. Biomed Mater 7: 024101.
-
Wei JJ, Chen YF, Xue CL, Ma BT, Shen YM, et al. (2016) Protection of Nerve Injury with Exosome Extracted from Mesenchymal Stem Cell. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 38: 33-36.
-
Elliott Donaghue I, Tator CH, Shoichet MS (2015) Sustained delivery of bioactive neurotrophin-3 to the injured spinal cord. Biomater Sci 3: 65-72.
-
Tukmachev D, Forostyak S, Koci Z, Zaviskova K, Vackova, et al. (2016) Injectable Extracellular Matrix Hydrogels as Scaffolds for Spinal Cord Injury Repair. Tissue Eng Part A 22: 306-317.
-
Moore TL, Killiany RJ, Pessina MA, Moss MB, Rosene DL (2010) Assessment of motor function of the hand in aged rhesus monkeys. Somatosens Mot Res 27: 121-130.
-
Moore TL, Killiany RJ, Pessina MA, Moss MB, Finklestein SP, et al. (2012) Recovery from ischemia in the middle-aged brain: a nonhuman primate model. Neurobiol Aging 33: 619.
-
Moss MB, Moore TL, Schettler SP, Killiany R, Rosene D (2007) Successful vs. Unsuccessful Aging in the Rhesus Monkey. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL): CRC Press/Taylor & Francis.
-
Salat DH (2011) The declining infrastructure of the aging brain. Brain Connect 1: 279-293.
-
Aggarwal BB, Gupta SC, Sung B (2013) Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br J Pharmacol 169: 1672-1692.
-
Gupta SC, Patchva S, Koh W, Aggarwal BB (2012) Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin Exp Pharmacol Physiol 39: 283-299.
-
Kopp N (2009) How Technologies of Imaging are Shaping Clinical Research and Practice in Neurology. Med Stud 1: 315-328.
-
Wen X, Wang Y, Zhang F, Zhang X, Lu L, et al. (2014) In vivo monitoring of neural stem cells after transplantation in acute cerebral infarction with dual-modal MR imaging and optical imaging. Biomaterials 35: 4627-4635.
-
Schmierer K, Parkes HG, So P-W, An SF, Brandner S, et al. (2010) High field (9.4 Tesla) magnetic resonance imaging of cortical grey matter lesions in multiple sclerosis. Brain J Neurol 133: 858-867.
-
Kim JH, Loy DN, Wang Q, Budde MD, Schmidt RE, et al. (2010) Diffusion tensor imaging at 3 hours after traumatic spinal cord injury predicts long-term locomotor recovery. J Neurotrauma 27: 587-598.
-
Stephan KE, Iglesias S, Heinzle J, Diaconescu AO (2015) Translational Perspectives for Computational Neuroimaging. Neuron 87: 716-732.
-
Li S, Ma Z, Tu S, Zhou M, Chen S, et al. (2014) Altered resting-state functional and white matter tract connectivity in stroke patients with dysphagia. Neurorehabil Neural Repair 28: 260-272.
-
Li R, Hettinger PC, Liu X, Machol J, Yan J-G, et al. (2014) Early evaluation of nerve regeneration after nerve injury and repair using functional connectivity MRI. Neurorehabil Neural Repair 28: 707-715.
-
Tajiri N, Dailey T, Metcalf C, Mosley YI, Lau T, et al. (2013) In vivo animal stroke models: a rationale for rodent and non-human primate models. Transl Stroke Res 4: 308-321.
-
Nicholls FJ, Rotz MW, Ghuman H, MacRenaris KW, Meade TJ, et al. (2016) DNA-gadolinium-gold nanoparticles for in vivo T1 MR imaging of transplanted human neural stem cells. Biomaterials 77: 291-306.
-
Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, et al. (1999) MRI-Based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. NeuroImage 9: 18-45.
-
Makris N, Kennedy DN, Boriel DL, Rosene DL (2010) Methods of MRI-based structural imaging in the aging monkey. Methods 50: 166-177.
-
Bohland JW, Wu C, Barbas H, Bokil H, Bota M, et al. (2009) A Proposal for a Coordinated Effort for the Determination of Brainwide Neuroanatomical Connectivity in Model Organisms at a Mesoscopic Scale. PLOS Comput Biol 5: e1000334.
-
Li L, Preuss TM, Rilling JK, Hopkins WD, Glasser MF, et al. (2010) Chimpanzee (Pan troglodytes) Precentral Corticospinal System Asymmetry and Handedness: A Diffusion Magnetic Resonance Imaging Study. PLOS ONE 5: e12886.
-
Okano H, Miyawaki A, Kasai K (2015) Brain/MINDS: brain-mapping project in Japan. Philos Trans R Soc Lond B Biol Sci 370.
-
Frey S, Pandya DN, Chakravarty MM, Bailey L, Petrides M, et al. (2011) An MRI based average macaque monkey stereotaxic atlas and space (MNI monkey space). NeuroImage 55: 1435-1442.
-
The Incredible Bionic Man. Smithsonian Channel.
-
Teoh GZ, Klanrit P, Kasimatis M, Seifalian AM (2015) Role of nanotechnology in development of artificial organs. Minerva Med 106: 17-33.
-
Huh D, Kim HJ, Fraser JP, Shea DE, Khan M, et al. (2013) Microfabrication of human organs-on-chips. Nat Protoc 8: 2135-2157.
-
Huh D, Leslie DC, Matthews BD, Fraser JP, Jurek S, et al. (2012) A Human Disease Model of Drug Toxicity-Induced Pulmonary Edema in a Lung-on-a-Chip Microdevice. Sci Transl Med 4: 159ra147-159ra147.
-
Tam RY, Fuehrmann T, Mitrousis N, Shoichet MS (2014) Regenerative therapies for central nervous system diseases: a biomaterials approach. Neuropsychopharmacology 39: 169-188.





