International Journal of Stem cell Research and Therapy
Kartogenin Induced Chondrogenesis of Stem Cells and Cartilage Repair
Fazal-Ur-Rehman Bhatti1, Karen A Hasty1,2,3 and Hongsik Cho1,2,3*
1Department of Orthopaedic Surgery and Biomedical Engineering, University of Tennessee Health Science Center, Memphis, TN, USA
2Campbell Clinic, Memphis, TN, USA
3Veterans Affairs Medical Center, Memphis, TN, USA
*Corresponding author: Hongsik Cho, PhD, Department of Orthopaedic Surgery and Biomedical Engineering, University of Tennessee Health Science Center and Campbell Clinic Research 151 VA Medical Center, 1030 Jefferson Ave, Memphis, TN 38138, USA, E-mail: hcho4@uthsc.edu
Int J Stem Cell Res Ther, IJSCRT-3-036, (Volume 3, Issue 2), Short Review; ISSN: 2469-570X
Received: April 25, 2016 | Accepted: June 26, 2016 | Published: July 01, 2016
Citation: Fazal-Ur-Rehman B, Hasty KA, Cho H (2016) Kartogenin Induced Chondrogenesis of Stem Cells and Cartilage Repair. Int J Stem Cell Res Ther 3:036. 10.23937/2469-570X/1410036
Copyright: © 2016 Fazal-Ur-Rehman B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Osteoarthritis (OA) is predicted to be the fourth leading cause of disability in the world by the year 2020. OA results in damage to cartilage tissue and underlying subchondral bone. Current therapeutic options for osteoarthritis (OA) are limited due to the unique nature of cartilage tissue. Alternatively, mesenchymal stem cells (MSCs) have been utilized for cartilage repair, but the formation of an intact neocartilage similar to hyaline cartilage is still a challenge. Kartogenin (KGN), a small heterocyclic, drug-like compound was discovered in the year 2012. KGN was found to induce chondrogenesis of MSCs and protect chondrocytes against OA. At the molecular level, KGN dissociates Filamin A from CBFβ that translocates into the nucleus and interacts with RUNX1 to transcribe genes involved in the chondrogenesis of MSCs. KGN maintains the balance between anabolism and catabolism of cartilage by providing protection against cytokine-induced cartilage damage. KGN also prevents apoptosis of MSCs and chondrocytes during stress conditions that mimic OA in vitro. Moreover, KGN repaired the damaged cartilage in chemically- and surgically-induced animal models of OA. KGN treatment in mouse and rabbit models of OA improved as assessed by the gross morphology, histological features, MRI, micro-CT and serum markers of OA. Here we present a brief review of KGN in terms of cartilage repair both in vitro and in vivo. To the best of our knowledge, this is a first review that discusses the potential of KGN for cartilage repair.
Keywords
Mesenchymal stem cells, Chondrogenesis, Kartogenin, Cartilage repair
Introduction
Osteoarthritis (OA) is the chronic joint disease that is responsible for functional disability, particularly in the elderly population [1]. The rate of OA is growing rapidly around the globe. In the year 2010, OA was reported to be the eleventh leading cause of years lived with disability among 289 diseases [2]. Further, OA is likely to be the fourth leading cause of physical disability by the year 2020 [3]. Therefore, OA has been known as the major concern during the bone and joint decade [4]. The mediators of OA include degradation of articular cartilage, changes in subchondral bone, the formation of osteocytes, inflammation of synovium and hypertrophy of chondrocytes [5]. Owing to its aneural, a vascular and a lymphatic nature, cartilage tissue fails to heal itself naturally [6]. The therapeutic options available so far for OA can be broadly classified as pain management and surgical intervention [7].
Mesenchymal stem cells (MSCs) have been looked forward as a reliable source to treat defects of articular cartilage and hence OA [8-10]. MSCs can be isolated from various adult tissues. Furthermore, MSCs possess the capability of self-renewal and differentiation into various cell lineages, including chondrocytes, osteoblasts, and adipocytes [8]. The ultimate goal of this approach is to overcome the limited number of chondrocytes available to repair the damaged cartilage. In this aspect, many compounds have been studied that bring about the chondrogenesis of MSCs. This review discusses one such compound known as 'Kartogenin (KGN)'. The interesting feature of KGN is that due to its small size it does not initiate the immune response and also it has been shown to have no toxic effect on different cell types even at a concentration of 100 mM [11]. We present here a brief review about how KGN has been exploited in the field of cartilage repair since its discovery.
Discovery of Kartogenin
In the year 2012, Kristen Johnson and his colleagues published the discovery of KGN after screening 22,000 drug-like molecules [12]. These compounds were similar to the natural ligands that are involved in cell signaling and differentiation. KGN is a small heterocyclic compound that induced chondrogenesis of human bone marrow MSCs by forming cartilage nodules in vitro. Interestingly, these nodules expressed collagen II and aggrecan that are specific for hyaline cartilage. In addition to this, no hypertrophic markers were expressed in either chondrocytes or bone cells. Furthermore, long-term culture of differentiated chondrocytes showed no production of matrix metalloproteinases (MMPs), enzymes known to cause degradation of cartilage matrix. In fact, levels of inhibitors of MMPs were increased. It was also found that KGN protected chondrocytes from damage induced by cytokines as the release of proinflammatory NO and by-products of glycosaminoglycan of tissue degradation were suppressed in cultured chondrocytes and cartilage explants.
Later, KGN was administered via the intra-articular route in chemically- and surgically-induced mouse models of OA (Figure 1A and Figure 1B). Obvious reasons for selection of these models were that collagenase-induced OA mimics chronic injury while surgical ligament transection mimics acute injury. Cartilage was regenerated in both mouse models along with a reduction in serum levels of cartilage breakdown products and improved weight bearing outcomes. Moreover, the repair was observed at early stages of OA. These results indicated that KGN has both regenerative and protective effects. Furthermore, only 0.1% of the intraarticular dose of KGN was found in the serum. This percentage was found to be negligible to induce any toxic effects.
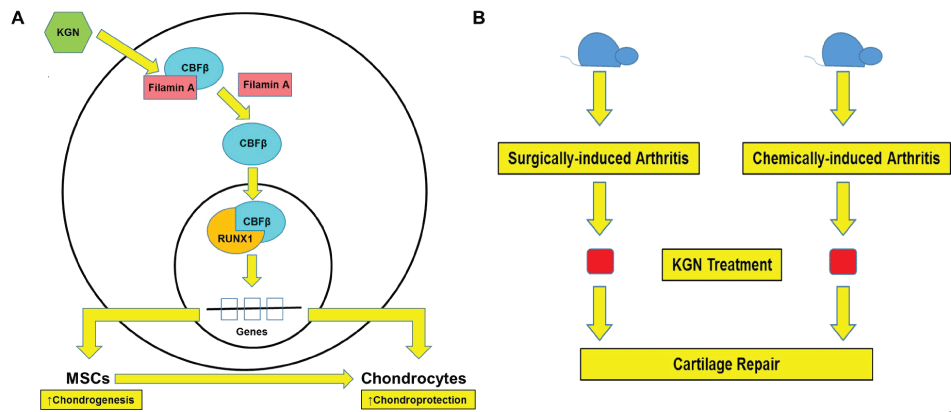
.
Figure 1: Discovery of KGN. (A) Molecular mechanism of action of KGN. In the absence of KGN, CBFβ is bound to Filamin A in the cytoplasm. KGN dissociates this interaction, CBFβ dislocates into the nucleus and interacts with RUNX1. This interaction results in the transcription of genes responsible for chondrogenesis and other mechanisms that are known for chondroprotection; (B) Surgically- and alchemically-induced mouse model of OA were developed and treated with KGN. Cartilage repair was observed in both mouse models.
View Figure 1
Molecular Mechanisms of Kartogenin (KGN)
It was previously known that MSCs undergo chondrogenesis via Runx1/CBFα 2 [13]. Johnson et al. while studying the mechanism of KGN action found that KGN disrupts the interaction between CBFβ and Filamin A. As a result, CBFβ translocates into the nucleus where it binds to RUNX1. The CBFβ-RUNX1 activates the transcription of genes that mediate chondrogenesis (Figure 1A). These results were supported by the experimental work on MSCs in which KGN enhanced the localization of CBFβ into the nucleus. In addition, knockdown of CBFβ expression blocked chondrogenesis of MSCs, whereas overexpression of CBFβ increased chondrogenesis. The role of RUNX1 was confirmed by microarray analysis [12]. In addition, recent studies showed KGN enhanced chondrogenesis by increasing the expression of chondrogenic markers such as Sox-9, collagen II and aggrecan [14,15].
Furthermore, KGN has been reported to play its role in the skeletal growth and development of mouse [16]. Researchers treated committed preskeletal MSCs that were isolated from mouse embryo limb buds and whole limb explants with KGN. It was found that KGN stimulated cartilage nodule formation, digit cartilaginous anlage elongation, the formation of synovial joint, interzone compaction, tendon maturation, and interdigit invagination. Authors also found that expression of lubricin/prg4 was enhanced by KGN that is vital for completion of cavitation by the end of mouse embryogenesis [17]. Deficiency or mutation of lubricin has been linked to joint disease [18]. Furthermore, it was found that KGN regulates canonical TGFβ and BMP signaling by regulating the phospho-Smads as compared to TGFβ [16]. It is known that TGFβ1 is responsible for phosphorylation of both TGFβ Smads (Smad 2/3) and BMP Smads (Smad 1/5/8) [19,20]. Smad 1/5/8 act upstream of RUNX2 that in turn causes hypertrophy and terminal differentiation of chondrocytes. On the other hand, KGN only increased the expression of Smad 2/3 while suppressed the expression of Smad 1/5/8. This is an indication of the chondroprotective effect of KGN. Moreover, this regulatory control by KGN seems to be specific for cartilage as KGN caused type-I collagen synthesis in human dermal fibroblasts by the activation of the Smad 4/5 pathway [21].
Kartogenin (KGN) in Cartilage Repair
In vitro studies
One of the ways by which OA can be characterized is the loss of aggrecan from the cartilage tissue, particularly in the pericellular matrix. This loss of aggrecan is also accompanied by the loss of glycosaminoglycan hyaluronan (HA). HA is known to retain aggrecan at the cell surface by linking with CD44 receptor [22]. A study examined the effect of KGN on chondrocytes, bioengineered neocartilages and cartilage explants obtained from human and steer cartilage samples and treated with IL-1β [23]. KGN prevented IL-1β induced damage to these coats, neocartilages and cartilage explants. Authors assumed the chondroprotective effect of KGN rather than anabolic effect by observing no significant changes in the anabolic markers of cartilage tissue such as collagen II, aggrecan, and Sox9.The chondroprotective nature of KGN was confirmed by examining the degradation of aggrecan by IL-1β that increases the terminating ITEGE-containing fragment of aggrecan G1 domain. The release of ITEGE-containing fragment reduced after KGN treatment in human cartilage explants. This decrease was caused by inhibition of an aggrecanase, ADAMTS5. The overall conclusion of this study was that KGN exerts its effects by retention of aggrecan and CD44 and not by synthesizing any anabolic marker of the cartilage tissue.
Deficiency of lubricin has been associated with reduced articular joint friction that increases mechanical stress on cartilage surface and ultimately results in cartilage destruction [24]. A study conducted on bone marrow MSCs and chondrocytes from Sprague-Dawley (SD) rats examined the role of transforming growth factor-β1 (TGF-β1) and bone morphogenetic protein-7 (BMP-7) in combination with KGN on the production of lubricin [25]. It was found that combination of all these three components not only triggered chondrogenesis of MSCs but also enhanced the production of lubricin from chondrocytes. Gene expression analysis showed that production of lubricin is increased by prg4 and aggrecan genes. On the other hand degradation of lubricin is decreased by probable regulation of ADAMTS5 and c-Myc that are known to cause aggrecan degradation and apoptosis respectively. Future studies on protein expression analysis of ADAMTS5 and c-Myc needs to be done to strengthen this observation. Nonetheless, this study sheds light on the role of KGN that it may be involved in different pathways to regulate cartilage homeostasis.
In vivo studies
Animal models of OA are used to study the pathological changes in the joints during the progression of OA. Details on animal models of OA have been covered elsewhere [26,27]. In general, the defect is created in the joint to cause OA. The injury could be either acute or chronic depending on the type of animal model used. The later agent of choice is used to repair the damaged cartilage and outcomes are measured.
Studies in mouse model of OA
Microparticles and nanoparticles, such as targeted nanosomes, are gaining considerable attention to be used as a delivery system to repair damaged cartilage in mouse knee [28]. A study was published in which, nanoparticles and microparticles were synthesized that contain KGN conjugated with low and medium molecular weight chitosan (CHI) [29]. Later, hMSCs derived from bone marrow were induced with both types of KGN-conjugated particles to undergo chondrogenesis. Although, both particles resulted in chondrogenesis of hMSCs, however, results were more significant with KGN-CHI nanoparticles. In vivo analysis was done on asurgically-induced mouse model of OA developed through transection of anterior cruciate ligament (ACLT) via intraarticular injection of KGN-CHI (KGN ~ 25 μM) particles. It was observed that KGN-CHI microparticles were retained longer in the knee joint probably owing to their much bigger size. Healing of the cartilage was observed for both types of particles. Also, KGN-CHI particles were retained in OA joints for 3 weeks. In brief, this study presented an effective approach to delivering KGN into the affected joint to yield cartilage repair.
The progression of OA ultimately affects the underlying subchondral bone [5]. Therefore, a study was conducted not only to study the effects of KGN on cartilage but also on the subchondral bone in ACLT mouse model of OA [30]. Weekly, 125 μM KGN was injected intraarticularly in mice one week after surgery until 12 weeks. On the basis of magnetic resonance imaging (MRI), it was found that KGN retained the integrity of cartilage by preventing proteoglycan loss and maintenance of water content. Micro-CT was done to analyze the subchondral bone that showed that KGN significantly prevented the OA-related changes in the architecture of subchondral bone. Furthermore, serum levels of cartilage oligomeric matrix protein (COMP) and C-terminal telopeptide of collagen type I (CTX-I) were examined. COMP and CTX-1 are markers of cartilage and bone turnover respectively that increase during OA. It was found that KGN prevented the OA-related increase of these markers. In short, this study showed that KGN reduced degradation of cartilage, subchondral bone changes and turnover of both cartilage and bone. However, this study was limited due to the sample size in each group (n = 6 per group). Apart from this, the addition of gene expression analysis and protein analysis may further strengthen the findings of this study.
Studies in rabbit model of OA
One of the techniques used to repair full-thickness cartilage defects is microfracture. However, studies have shown that it fails in the long run [31]. To address this issue a study was conducted in female New Zealand White rabbits by creating full-thickness cartilage defect that measured about 3.5 mm in diameter and 3 mm in depth and was created in the patellar grooves of right femur [32]. Animals were injected intraarticularly with 10 μM KGN as a treatment. Follow-up was done at 4 and 12 weeks after KGN administration. Gross morphological features showed filling of cartilage defects at 4 (50% healing) and 12 (100% healing) weeks as compared to an untreated control. The histological score also indicated a similar pattern by showing theformation of hyaline cartilage, intact repair tissue and reconstruction of the subchondral bone. Furthermore, histology revealed the formation of fibrotic tissue in the untreated group whereas no fibrosis was observed in KGN treated group. Immunohistochemistry further showed increased expression of collagen II in KGN treated group with decreased expression of collagen I. The condition was vice versa in an untreated control. This is an important finding as most of the healing approaches suffered due to the formation of fibrotic tissue along with the newly formed cartilage. Although, no gene expression analysis was carried out in this study but still the results of this clearly showed that KGN may prove a useful agent to treat full-thickness cartilage defects in rabbits.
Another study by the same group on rabbit model of microfracture studied the effect of KGN incorporated into poly (lactic-co-glycolic acid) (PLGA) scaffold and stabilized with photo-cross-linkable acrylated hyaluronic acid (m-HA) [11]. Follow-up in this study was also done at 4 and 12 weeks. Surprising results of this study were similar to their previous study done earlier. Gross morphological features showed initial signs of healing at 4 weeks and formation of hyaline-like cartilage at 12 weeks. Proteoglycan staining also showed integrated new tissue formation at 4 and 12 weeks. Immunohistochemistry also showed increased collagen II and decreased collagen I in the treated group. We suggest that these studies need to be further strengthened by molecular evidence.
The most recent study on full-thickness cartilage defects in rabbits was carried out by utilizing KGN-incorporated thermogel to support bone marrow MSCs [33]. Thermogel was constituted from poly(l-lactide-co-glycolide)-poly(ethylene glycol)-poly(l-lactide-co-glycolide) (PLGA-PEG-PLGA) for the reason that both PEG and PLGA are approved by U.S. Food and Drug Administration (FDA) [34]. Gross morphological, histology and biochemical evidence all showed that KGN-loaded thermogel yielded better results than the untreated control. The authors concluded that their developed system is effective in maintain MSCs under stress conditions of OA and adding KGN is an added advantage. However, like previous studies their data lacked the molecular analysis. Furthermore, authors need to provide more strong evidence for the enhanced survival of MSCs using this system.
Conclusion
The discovery of KGN certainly has paved a new dimension in the field of cartilage repair. KGN has emerged as a promising small molecule that can be used to develop stem cell-based cartilage repair. Its efficacy to induce chondrogenesis, chondroprotective effect and biosafety have already been established. Although promising but results of the studies conducted so far on KGN are limited. This may be due to the short-term stability of KGN in anaqueous medium. We recommend strictly following the manufacturer's guidelines when using KGN for long-term studies. In addition, some studies indicated that KGN might have a slight effect on hypertrophy and calcification [12,35].
Further studies are needed to exploit the molecular mechanisms by which KGN exert its effects in vivo. For instance, it is still not clear that how many pathways involve KGN. Moreover, more studies are required to develop effective delivery systems in order to deliver an effective dosage of KGN to the target tissue.
Acknowledgement
H.C. was supported by grants from William and Ella Owens Medical Research Foundation. K.A.H was supported by grants from Department of Veterans Affairs (VA Merit Review award), NIH (R21: AR060408) and the UTHSC (CTSI award).
Conflict of Interest
The authors declare no conflict of interest.
References
-
Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman BN, et al. (1995) The incidence and natural history of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum 38: 1500-1505.
-
Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, et al. (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2163-2196.
-
Woolf AD, Pfleger B (2003) Burden of major musculoskeletal conditions. Bull World Health Organ 81: 646-656.
-
Woolf A (2000) The bone and joint decade 2000-2010. Ann Rheum Dis 59: 81-82.
-
Goldring MB, Goldring SR (2010) Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci 1192: 230-237.
-
Buckwalter JA, Mankin HJ (1998) Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect 47: 477-486.
-
Hunter DJ (2011) Pharmacologic therapy for osteoarthritis--the era of disease modification. Nat Rev Rheumatol 7: 13-22.
-
Filardo G, Perdisa F, Roffi A, Marcacci M, Kon E (2016) Stem cells in articular cartilage regeneration. J Orthop Surg Res 11: 42.
-
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143-147.
-
Muraglia A, Cancedda R, Quarto R (2000) Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci 113: 1161-1166.
-
Shi D, Xu X, Ye Y, Song K, Cheng Y, et al. (2016) Photo-Cross-Linked Scaffold with Kartogenin-Encapsulated Nanoparticles for Cartilage Regeneration. ACS Nano 10: 1292-1299.
-
Johnson K, Zhu S, Tremblay MS, Payette JN, Wang J, et al. (2012) A stem cell-based approach to cartilage repair. Science 336: 717-721.
-
Wang Y, Belflower RM, Dong YF, Schwarz EM, O'Keefe RJ, et al. (2005) Runx1/AML1/Cbfa2 mediates onset of mesenchymal cell differentiation toward chondrogenesis. J Bone Miner Res 20: 1624-1636.
-
Zhang J, Wang JH (2014) Kartogenin induces cartilage-like tissue formation in tendon-bone junction. Bone Res 2.
-
Yuan T, Zhang J, Zhao G, Zhou Y, Zhang CQ, et al. (2016) Creating an Animal Model of Tendinopathy by Inducing Chondrogenic Differentiation with Kartogenin. PLoS One 11.
-
Decker RS, Koyama E, Enomoto-Iwamoto M, Maye P, Rowe D, et al. (2014) Mouse limb skeletal growth and synovial joint development are coordinately enhanced by Kartogenin. Dev Biol 395: 255-267.
-
Koyama E, Shibukawa Y, Nagayama M, Sugito H, Young B, et al. (2008) A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev Biol 31: 62-73.
-
Marcelino J, Carpten JD, Suwairi WM, Gutierrez OM, Schwartz S, et al. (1999) CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet 23: 319-322.
-
Drissi MH, Li X, Sheu TJ, Zuscik MJ, Schwarz EM, et al. (2003) Runx2/Cbfa1 stimulation by retinoic acid is potentiated by BMP2 signaling through interaction with Smad1 on the collagen X promoter in chondrocytes. J Cell Biochem 90: 1287-1298.
-
Kempf H, Ionescu A, Udager AM, Lassar AB (2007) Prochondrogenic signals induce a competence for Runx2 to activate hypertrophic chondrocyte gene expression. Dev Dyn 236: 1954-1962.
-
Wang J, Zhou J, Zhang N, Zhang X, Li Q (2014) A heterocyclic molecule kartogenin induces collagen synthesis of human dermal fibroblasts by activating the smad4/smad5 pathway. Biochem Biophys Res Commun 450: 568-574.
-
Ariyoshi W, Knudson CB, Luo N, Fosang AJ, Knudson W (2010) Internalization of aggrecan G1 domain neoepitope ITEGE in chondrocytes requires CD44. J Biol Chem 285: 36216-36224.
-
Ono Y, Ishizuka S, Knudson CB, Knudson W (2014) Chondroprotective Effect of Kartogenin on CD44-Mediated Functions in Articular Cartilage and Chondrocytes. Cartilage 5: 172-180.
-
Waller KA, Zhang LX, Elsaid KA, Fleming BC, Warman ML, et al. (2013) Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc Natl Acad Sci U S A 110: 5852-5857.
-
Liu C, Ma X, Li T, Zhang Q (2015) Kartogenin, transforming growth factor-beta1 and bone morphogenetic protein-7 coordinately enhance lubricin accumulation in bone-derived mesenchymal stem cells. Cell Biol Int 39: 1026-1035.
-
Kuyinu EL, Narayanan G, Nair LS, Laurencin CT (2016) Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res 11: 19.
-
Bendele AM (2001) Animal models of osteoarthritis. J Musculoskelet Neuronal Interact 1: 363-376.
-
Cho H, Pinkhassik E, David V, Stuart JM, Hasty KA (2015) Detection of early cartilage damage using targeted nanosomes in a post-traumatic osteoarthritis mouse model. Nanomedicine 11: 939-946.
-
Kang ML, Ko JY, Kim JE, Im GI (2014) Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials 35: 9984-9994.
-
Mohan G, Magnitsky S, Melkus G, Subburaj K, Kazakia G, et al. (2016) Kartogenin treatment prevented joint degeneration in a rodent model of osteoarthritis: A pilot study. J Orthop Res.
-
Goyal D, Keyhani S, Lee EH, Hui JH (2013) Evidence-based status of microfracture technique: a systematic review of level I and II studies. Arthroscopy 29: 1579-1588.
-
Xu X, Shi D, Shen Y, Xu Z, Dai J, et al. (2015) Full-thickness cartilage defects are repaired via a microfracture technique and intraarticular injection of the small-molecule compound kartogenin. Arthritis Res Ther 17: 20.
-
Li X, Ding J, Zhang Z, Yang M, Yu J, et al. (2016) Kartogenin-Incorporated Thermogel Supports Stem Cells for Significant Cartilage Regeneration. ACS Appl Mater Interfaces 8: 5148-5159.
-
Archana Swami, Michaela R. Reagan, Pamela Basto, Yuji Mishima, Nazila Kamaly, et al. (2014) Engineered nanomedicine for myeloma and bone microenvironment targeting. Proc Natl Acad Sci U S A 111: 10287-10292.
-
Blanco FJ, Ruiz-Romero C (2013) New targets for disease modifying osteoarthritis drugs: chondrogenesis and Runx1. Ann Rheum Dis 72: 631-634.





