International Journal of Stem cell Research and Therapy
Mechanical Loading Promoted Discogenic Differentiation of Human Mesenchymal Stem Cells Incorporated in 3D-PEG Scaffolds with rhGDF5 and RGD
S. Guggisberg1, L. M. Benneker2, M. J. Keel2 and B. Gantenbein1*
1Tissue & Organ Mechano Biology, Institute for Surgical Technology and Biomechanics, University of Bern, Switzerland
2Department for Orthopedic Surgery and Traumatology, Inselspital, Switzerland
*Corresponding author: Benjamin Gantenbein, University of Bern, Medical Faculty, Institute for Surgical Technology and Biomechanics, Tissue & Organ Mechanobiology, Stauffacherstrasse, Bern, Switzerland, Tel: 4131-631-5951, Fax: 4131-631-5960, E-mail: benjamin.gantenbein@istb.unibe.ch
Int J Stem Cell Res Ther, IJSCRT-2-006, (Volume 2, Issue 1), Research Article; ISSN: 2469-570X
Received: March 10, 2015 | Accepted: March 26, 2015 | Published: March 28, 2015
Citation: Guggisberg S, Benneker LM, Keel MJ, Gantenbein B (2015) Mechanical Loading Promoted Discogenic Differentiation of Human Mesenchymal Stem Cells Incorporated in 3D-PEG Scaffolds with rhGDF5 and RGD. Int J Stem Cell Res Ther 2:006. 10.23937/2469-570X/1410006
Copyright: © 2015 Guggisberg S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Hydrogels have been described as ideal scaffolds for cells of 3D tissue constructs and hold strong promises with respect to in vitro 3D-cell-culture, where cells are isolated from native extracellular matrix (ECM). Synthesized polyethyleneglycol (PEG) hydrogels are appealing with regard to potential for cell therapy or as vehicles for drug delivery or even to regenerate tissue with similar hydrogel-like properties such as the nucleus pulposus of the intervertebral disc (IVD). Here, we tested whether incorporation of RGD motive would hinder discogenic differentiation of primary bone marrow-derived human mesenchymal stem cells (hMSCs) but favor proliferation of undifferentiated hMSCs. HMSCs were embedded in +RGD containing or without RGD PEG hydrogel and pre-conditioned with or without growth and differentiation factor-5 (rhGDF-5) for 13 days. Afterwards, all hMSCs-PEG gels were subsequently cyclically loaded (15% strain, 1Hz) for 5 consecutive days in a bioreactor to generate an IVD-like phenotype. Higher metabolic activity (resazurin assay) was found in groups with rhGDF5 in both gel types with and without RGD. Cell viability and morphology measured by confocal laser microscopy and DNA content showed decreased values (~60%) after 18 days of culture. Real-time RT-PCR of an array of 15 key genes suspected to be distinctive for IVD cells revealed moderate response to rhGDF5 and mechanical loading as also shown by histology staining. Preconditioning and mechanical loading showed relatively moderate responses revealed from both RT-PCR and histology although hMSCs were demonstrated to be potent to differentiate into chondrocyte-progenitor cells in micro-mass and 3D alginate bead culture.
Keywords
3D cell culture, Biodegradable hydrogel, Human mesenchymal stem cells, RGD, Gene expression, Intervertebral disc
Introduction
It seems evident that 3-dimensional (3D) cell culture is providing a much more authentic environment for primary cells, which are usually isolated from their native 3D tissue complex for cell culture [1]. Thus, two-dimensional cell culture is very limited especially for applications in tissue engineering where cells are often isolated from their native matrix and then re-imbedded into a different type of a 3D matrix. These matrices are most often in literature derived from plant or animals. It is a sort of "gold standard" in cartilage or intervertebral disc (IVD) engineering to use either micromass culture or embedding in alginate or collagen-derived hydrogels also for mechanobiological studies [2]. Hydrogels have been pushed back into focus lately in biomaterial sciences due to the advantage of drug delivery and possibility to use these carriers as a scaffold for tissue regeneration [3,4]. There is a clinical demand to use a more defined extracellular environment than plant or animal derived hydrogel [1,5,6]. Although, to our knowledge there is scientific evidence lacking that artificial scaffolds would be superior to natural polymers. In cartilage and intervertebral disc research the gold standard for culturing cells in 3D is 1.2% alginate [7]. However, the usage of serum in culture media has been applied extensively and seems to be a pre-requisite for expanding primary cells such as human mesenchymal stem cells (hMSCs). However, culturing hMSCs under serum free defined conditions has been noticed to be very difficult in alginate bead, fibrin gel and 1-2% agarose conditions [8]. It is very likely that the lack of migration availability in alginate and fibrin carriers is causing increased cell death in combination to serum starvation. It also seems that reduced pH as it is the case in the IVD environment is a detrimental factor for the survival of hMSCs and matrix production [9]. Furthermore, it has been investigated how the adhesion of cells in 3D hydrogels could be improved by the incorporation of the amino acid sequence arginine-glycine-aspartic acid (RGD) motive via a5β1 integrin signaling [10-12]. It has been speculated that hMSC can thrive especially well in the undifferentiated state in presence of RGD [10]. Recently, it has been investigated whether hMSCs can be pushed towards the discogenic pathway by the addition of RGD motive [13,14]. The recent study found a weaker effect of transforming and growth factor β3 (TGFβ3) [10] if RGD motive was added to an alginate culture system. However, Villanueva et al. (2009) [11] report opposite results on chondrocytes using a PEG-hydrogel system and RGD motive. Thus, there is a general confusion whether RGD motive is a requirement to improve discogenic differentiation or not. On the other hand, the presence of human recombinant growth and differentiation factor 5 (rhGDF5=CDMP1=BMP14) [15-17] and dynamic loading [18,19] have been described as one of the potent factors that could direct discogenic differentiation of hMSCs. Recently, a consortium was formed to define the discogenic phenotype [20] more closely. There were a number of markers pointed out that could possibly define the nucleus pulposus cell (NPC) phenotype, such as expression of glypican 3 (GPC3), cytokeratins (KRT8,19), junctophilin (JPH3), SOX-2, carboanhydrase 12, paxillin (PAX1) based on transcriptome studies [20-22]. Therefore, we aim to investigate the combined influence of RGD, rhGDF5 and mechanical loading on the fate of hMSCs.
We hypothesized that primary hMSCs cultured in artificial PEG hydrogels can be driven towards the intervertebral-disc like, more precisely to the nucleus pulposus cell (NPC)-like cell phenotype by pre-conditioning with rhGDF5 [15,16] and dynamic compression. Furthermore, we hypothesized that 3D cell culture in a PEG environment along with mechanical loading and stimulation with external application of rhGDF5 would push the hMSCs towards incorporation of RGD causes spreading of cell phenotype, which could enhance mechano-transduction and thus mechano-sensitivity of undifferentiated hMSCs. However, discogenic differentiation into NPC-like cells (here called "discogenesis") might be blocked or hindered in presence of RGD.
Materials and Methods
Experimental design
This experiment aims to analyze the discogenic differentiation of hMSCs in hydrogel with the RGD motive, rhGDF5 and dynamic axial compression. Human bone marrow derived hMSCs were embedded in commercially available PEG hydrogel with +RGD or without -RGD motive (Q-Gel®, QGelbioinc., Lausanne, Switzerland). Lausanne, Switzerland) and molded in silicon rings forming silicon-PEG 2-part constructs to facilitate dynamic loading. The concentration of the RGD was 50 μM as described in [23,24]. The two types of silicon-PEG constructs were cultured in 2 different culture media; one control media without -GDF5 and one contain +GDF5 and dexamethasone (dex) (Biovision, San Francisco, CA). The use of two types of PEG gels (-RGD, + RGD) and two culture mediums (-GDF5, +GDF5+dex) resulting in 4 experimental groups: 1) +RGD+GDF5, 2) +RGD-GDF5, 3)-RGD+GDF5, 4)-RGD-GDF5 (Figure 1). The concentration of dexamethasone (Sigma, Buchs, Switzerland) was 10-7 M. After 13 days of culture, all silicon-PEG constructs were mechanically loaded for 5 days. Data were collected at day 0 (after 2D expansion and right after seeded into the PEG gel), day 13 (after cultured with or without rhGDF5 and before loading is applied) and day 18 (after 5 days of loading) for subsequent analysis (Figure 1).
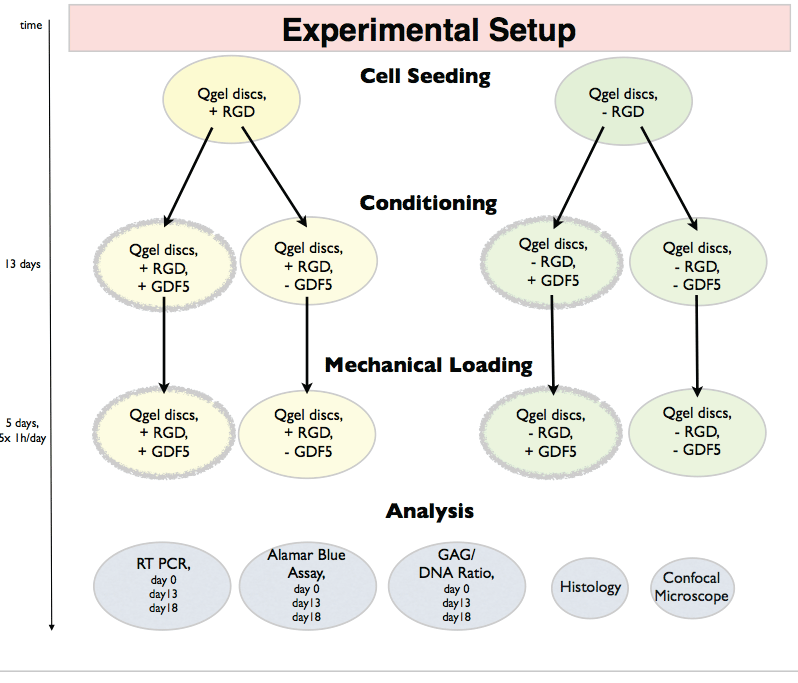
.
Figure 1: Experimental design and outcome parameters of pre-conditioning of human mesenchymal stem cells (hMSCs) in PEG hydrogels in presence or absence
of RGD and culturing in serum free control media or media contain GDF5 and dexamethasone.
View Figure 1
Cell source and expansion
Human bone marrow was obtained from patients undergoing spine surgery with written consent. The procedure was ethically approved by the committee of the Canton of Bern, Switzerland (KEK # 187/10). Bone marrow aspirations were collected from 6 patients (aged 41-79 yrs, mean age was 58.5 ± 15.42 yrs) (Table 1) from the lumbar or thoracic vertebrae. Human mesenchymal stem cells (hMSCs) were amplified from the mononuclear cell fraction after density gradient centrifugation (Histopaque-1077, Sigma-Aldrich, Buchs, Switzerland) by selection for plastic adherence for 2-3 passages. Additionally, two representative donors were characterized by staining for specific markers, i.e. CD34, CD90, CD105, by FACS and differentiated for adipo- and osteogenesis for as previously described [17] (data not shown). The hMSCs were expanded and kept mitotic using a-Minimal Essential Medium (α-MEM) with 10% fetal calf serum (FCS), 100 μg/ml penicillin, 100UI/ml streptomycin, and 5ng/ml bFGF-2.
![]()
Table 1: List of human donor material involved in this study
View Table 1
Carriers / Gel casting
PEG hydrogel containing MMP-degradable site with or without RGD was used to embed the hMSCs. Four million cells/mL hMSCs were seeded into PEG hydrogel discs according the manufacturers protocol. Lyophilised Qgel MT 3D matrix either including (50μM final concentration) or lacking covalently linked RGD (Arg-Gly-Asp) was resuspended with 400μl of Qgel buffer before 100μl of cell suspension (i.e. 2x106 cells) was added (Q-Gelbioinc., ref# 1001 and 1004, Lausanne, Switzerland). The cells mixed with Q-Gel hydrogel were distributed and molded into a 4 mm diameter and 3 mm height cylindrical shape inside a 8 mm diameter and 3mm height silicon ring forming a disc-like two-part construct, which resulted in ~1.2x105 cells per disc (30μl per disc from a cell density of 4x106 cells/mL PEG carrier) (Figure 2). The carriers were then incubated for 30-45 min in 37°C until the gel was completely jellified. Cells were maintained in serum-free high glucose (4.5g/L) Dulbecco's Modified Eagle Medium (HG-DMEM) containing 1% non-essential amino acids (Gibco), 50μg/mL ascorbic acid, ITS+, 100U/mL penicillin, 100mg/mL streptomycin, 2.5mg/mL amphotericin B, 100mg/mL sodium pyruvate (all from Sigma-Aldrich, Buchs, Switzerland), with or without 100ng/mL rhGDF5 (Biovision, San Franscisco, CA) and 10-7 M dexamethasone (Sigma-Aldrich, Buchs, Switzerland). To monitor the multipotency of the expanded hMSCs and to compare the 3D culture with previously described and established culture systems, hMSCs were additionally seeded in 1.2% alginate (Fluka, Sigma-Aldrich, Buchs, Switzerland) and micromass culture as a positive control. To encapsulate cells in alginate beads, cells were seeded in 1.2% alginate and dropped through a syringe with needle 22G in a 102mM CaCl2 solution [16]. Cells-seeded alginate beads were washed with 0.9% NaCl within 5 min of gelation to prevent Ca-shock. Additionally, cells were cultured in "micromass" culture which were formed by spinning 250,000 cells at 500G for 5 min. Cells in either alginate beads and or micromass were cultured directly in control medium versus chondrogenic medium (i.e. +10-7 M dexamethasone + 10 ng/ml TGFβ1, Peprotechinc., London, UK) for 18 days.
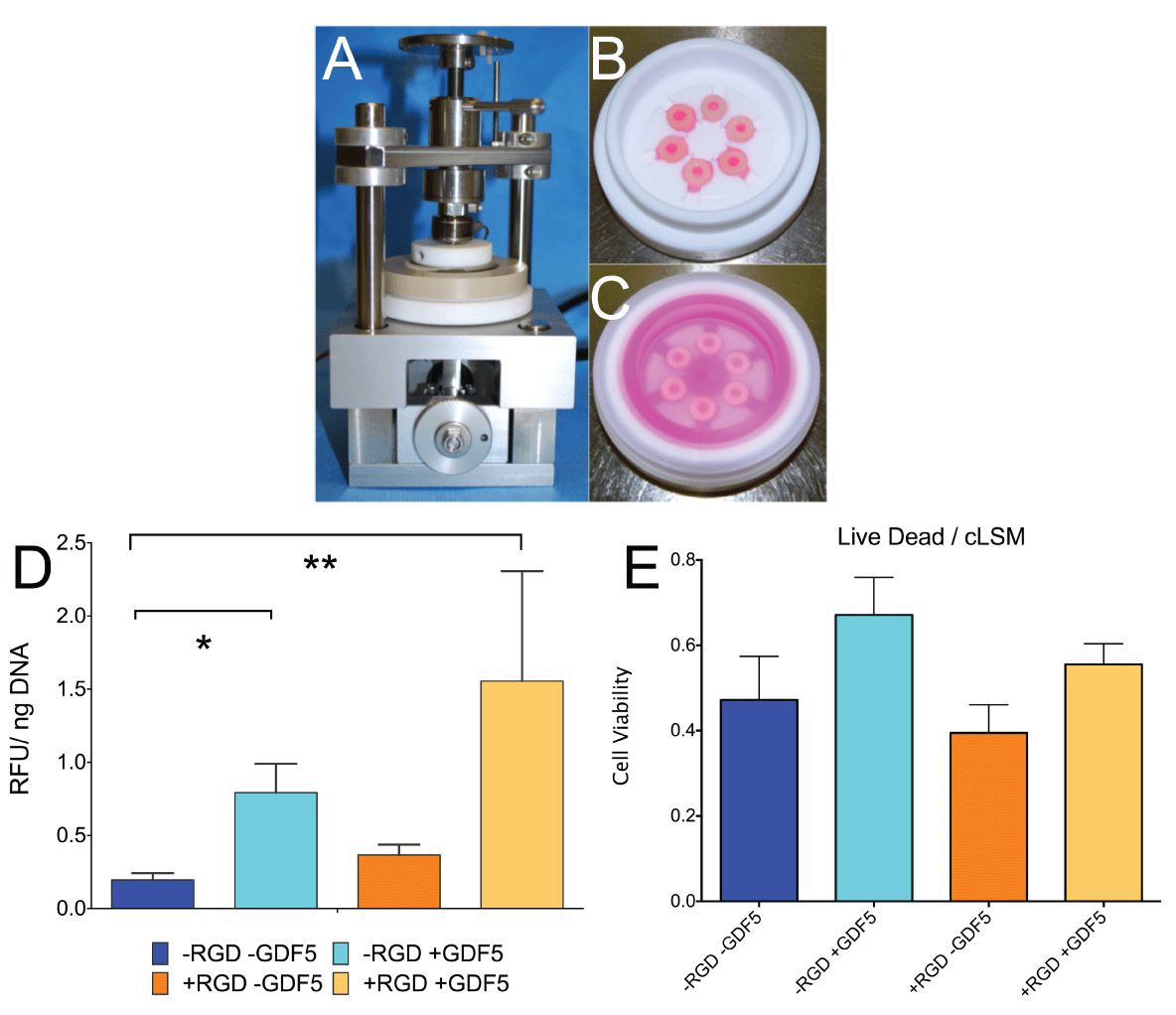
.
Figure 2: A. Experimental set-up of custom-made bioreactor for strain-controlled cyclic axial compression. B. PEEK loading chambers without cover showing 6 silicon-PEG constructs. C. loading chamber with 6 samples and serum free media covered by a transparent lid. D. Cell activity (resazurin assay) of hMSCs corrected per DNA content. Metabolism of hMSCs was significantly increased by addition of GDF5 and dexamethasone (10-7M) and was not dependent on RGD incorporation. * P< 0.05, ** P< 0.01. E. Cell viability as measured from LIVE/DEAD stain and confocal laser scanning microscopy according to the method of [27]. Bar plots represent means ± SEM (N=6 donor repeats).
View Figure 2
Mechanical loading
After 13 days of culture in serum free medium or in medium with GDF5 and 10-7M dexamethasone, dynamic axial compression loading of 15% strain at a frequency of 1Hz was applied to all silicon-PEG constructs. The loading was performed for 1 hour per day for 5 days using a custom-made loading bioreactor and chamber (Figure 2). Samples were collected for subsequent analysis after the final day of loading (day 18).
Quantification of total DNA content in specimens
Bisbenzimide, commonly known as Hoechst 33258 dye exhibits changes in fluorescence characteristics in the presence of DNA that allows accurate DNA quantification. DNA content was measured by fluorospectrometry (excitation at 350nm, emission at 450nm) using Hoechst 33258 dye (Sigma-Aldrich, Buchs, Switzerland) and purified calf thymus DNA as a standard. Three beads or two PEG carriers were digested in papain sodium acetate buffer pH 6.8 overnight at 60°C [16]. Fluorescence was measured with a Spectramax M5 microplate reader (Molecular Devices, Sunnyvale, California, United States).
Matrix content (dimethylmethylene blue dye assay)
For biochemical analysis of PEG gel carriers and alginate beads, 1 PEG carrier or 3 alginate beads were digested with papain digestion buffer and analyzed for sulphatedglycosaminoglycans (GAG). GAG content was determined by the dimethylmethylene blue (DMMB) dye method, using bovine chondroitin sulfate as standard [16]. Absorbance was read at 600 nm with a Spectramax M5 microplate reader (Molecular Devices).
Resazurin cell activity assay
Two alginate beads or 1 PEG carrier, about 200,000 cells were transferred to 500μl of DMEM containing 10% FCS and 50Mm resazurin (Sigma-Aldrich, Buchs, Switzerland) and let the live cells to metabolize glucose and nutrients at 37°C for a duration of 3h [25]. Fluorescence was measured at excitation wavelength 547nm and emission wavelength 582nm using a microplate reader (Molecular Devices).
LIVE/DEAD stain for 3D cell carriers
The cell viability was determined using our established protocols for the staining of 3D scaffold constructs using 2μmol/L calcein-AM and 1μmol/L ethidium-homodimer-1 (Sigma -Aldrich, Buchs, Switzerland) and using a confocal laser scanning microscope (cLSM510, Carl Zeiss, Jena, Germany). The stacks were analyzed using imageJ and our previous automated cell counting macro "cellcounter" available from imageJ (http://imagej.nih.gov/ij/macros/Cellcounter3D.txt) using the nucleus counter plug-in from the McMaster Biophotonics Facility [26,27].
Relative gene expression
For gene expression analysis, 3 alginate beads or 1 PEG carrier were flash-frozen in liquid N2 and pulverized. Total RNA was extracted from the constructs with TRI Reagent (Molecular Research Center, Cincinnati, USA) using a modified TRIspin method [28]. RNA was purified using GenElute™ Mammalian Total RNA Purification Kit with DNA digestion (Sigma-Aldrich, Buchs, Switzerland). RNA (100-200 ng) was used for reverse-transcription (RT) and subsequent PCR (RT-PCR) using gene-specific primers and probes [16]. Reverse transcription was performed using the iScript Kit and a my-cycler© Thermal Cycler (Bio-Rad, Basel, Switzerland). The resulting complementary DNA was prepared for RT-PCR using human-specific primers (Table 2) and Taq polymerase along with SYBR® Green I dye PCR was run on an IQ-5 RT-PCR system (all Bio-Rad, Reinach, Switzerland). The housekeeping gene 18S RNA was used as endogenous control. The relative gene expressions of day 13 and 18 samples relative to day 0 were calculated using the 2-ΔΔCt method [29].
![]()
Table 2: List of primers used for screen discogenic markers. Annealing temperature was 61°C and two-step protocol with 40s, 10s at 95°C and 30s expansion per cycle, 45 cycles in total
View Table 2
Histology
PEG carriers were embedded in OCT-compound (Tissue Tek, Alphen aan den Rijn, The Netherlands) for 20 min at room temperature and then snap-frozen in liquid N2 and stored at -80°C prior cutting. 12μm cryosections were made with a cryostat (Microm HM 560, Walldorf, Germany). Sections were stained with safranin-o & fast green and hematoxylin & eosin (H&E). High resolutionpictures were taken with a digital camera (Nikon 70D, Tokyo, Japan).
Statistics
The statistical analysis was performed by one-way ANOVA with Dunn's multiple comparison tests for all the analysis using PRISM softwarev6.0f (GraphPad, La Jolla, CA, USA). A significance value of p < 0.05 was specified for significance.
Results
Cell metabolism
Cell activity as measured with resazurin redox reaction was found significantly increased in both groups containing the growth factor GDF5 (79.3 ± 0.48 % P< 0.05 for -RGD and 155.5 ± 1.83 % for +RGD, P < 0.01) in the conditioning medium than without GDF5 (19.57 ± 11.26 % for -RGD and 36.54 ± 17.43 % for + RGD) after the 18 days of loading relative to the state of day 0 (= 100%) (Figure 2).
Cell viability and morphology
Confocal laser scanning microscopy revealed that there was a trend for more dead cells in gels cultured in the absence of GDF5 (-RGD 47.20 ± 25.09 % and +RGD 39.48 ± 16.17 %, respectively) compared to in the presence of GDF5 and dexamethasone (-RGD 67.12 ± 21.55 % and +RGD 55.53 ± 11.88 %, respectively). Under the confocal images, hMSCs appeared as wide-spread phenotype in the PEG gel with RGD and maintained as a round phenotype in the PEG gel without RGD (left column) (Figure 3). The presence of +GDF5 increased the spreading of hMSC in +RGD PEG gel (Figure 3, lower right) than -GDF5 (Figure 3,4).
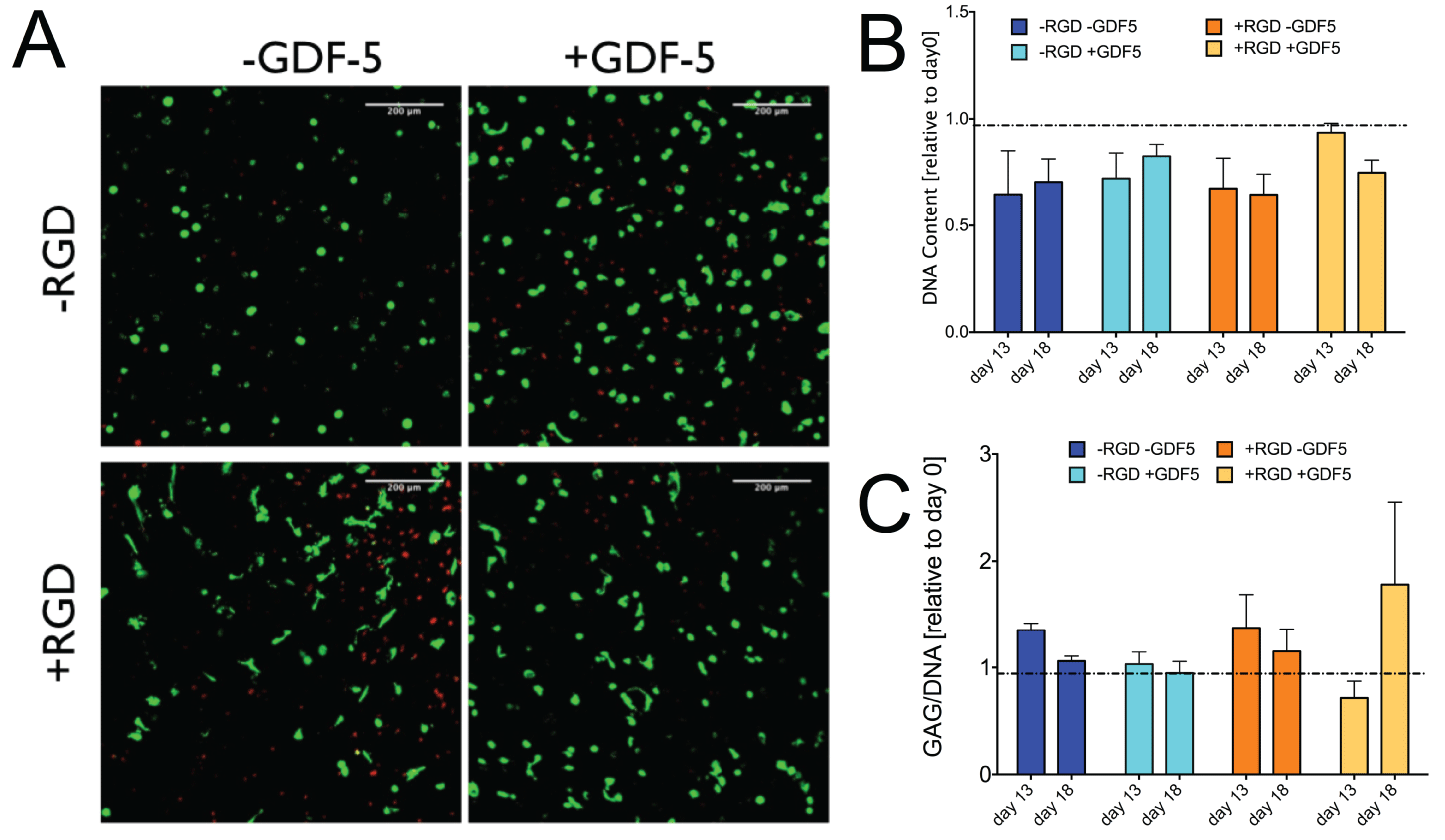
.
Figure 3: A. Representative confocal laser scanning microscope images of primary human mesenchymal stem cells cultured in MMP-degradable PEG hydrogel
with or without RGD; and with or without GDF5 for 18 days. Most hMSC were spread out in the presence of +RGD + GDF5. Green: live cells stained by calcain
AM, red: dead cell nucleus stained by ethidium homodimer-1. B. DNA content of the samples relative to day 0. There was a general decrease in DNA content
in all groups, independent of the presence of RGD or GDF5. C. Glycosaminoglycan (GAG)/DNA relative to day 0 control. GAG production per DNA was
maintained over the culture period in all groups. Barplots represent means ± SEM (N = 6 donor repeats).
View Figure 3
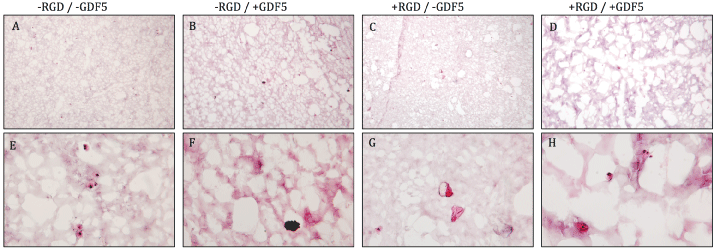
.
Figure 4: Hematoxylin & eosin stain of PEG hydrogels on cryosections after 18 days of preconditioning and mechanical loading. More void spaces were present in
the + GDF5 groups (column 2,4) compared to - GDF5 groups (column 1,3), indicating cells remodeling the surrounding matrix. Top row: low magnification, bottom
row: high magnification. Scale bar = 100 μm.
View Figure 4
Glycosaminoglycan / DNA content and histology
The cells did not produce significantly more proteoglycans but the DNA level showed a decrease in DNA content overall. Relative DNA content decreased by ~30% in groups without the growth factor but was kept more stable with decreases of 17.3% for the -RGD + GDF-5 group (Figure 3). Due to the decrease in DNA level, the GAG/DNA ratio showed values slightly higher than that of day 0 (Figure 3). Histology for safranin-O & fast green did not show strong production of glycosaminoglycans (data not shown). Figure 4 shows the hematoxylin & eosin staining of the PEG gel structure after 18 days of culture. More void spaces were present in the + GDF5 group than - GDF5 group indicating a higher rate of the PEG degradation in the presence of GDF5. Most significant loss of PEG matrix was the group with RGD and GDF5 (Figure 4, fourth column).
Gene expression
We found trends towards an increase of discogenic markers (i.e. aggrecan core protein (ACAN) and collagen type II (COL2A1)) (Figure 5). However, the expression of SRY-box containing gene 9 (SOX9) was found to be down-regulated for cells pre-conditioned in PEG hydrogel with GDF5 (day 13) and maintained at the same level after mechanical loading (day 18).
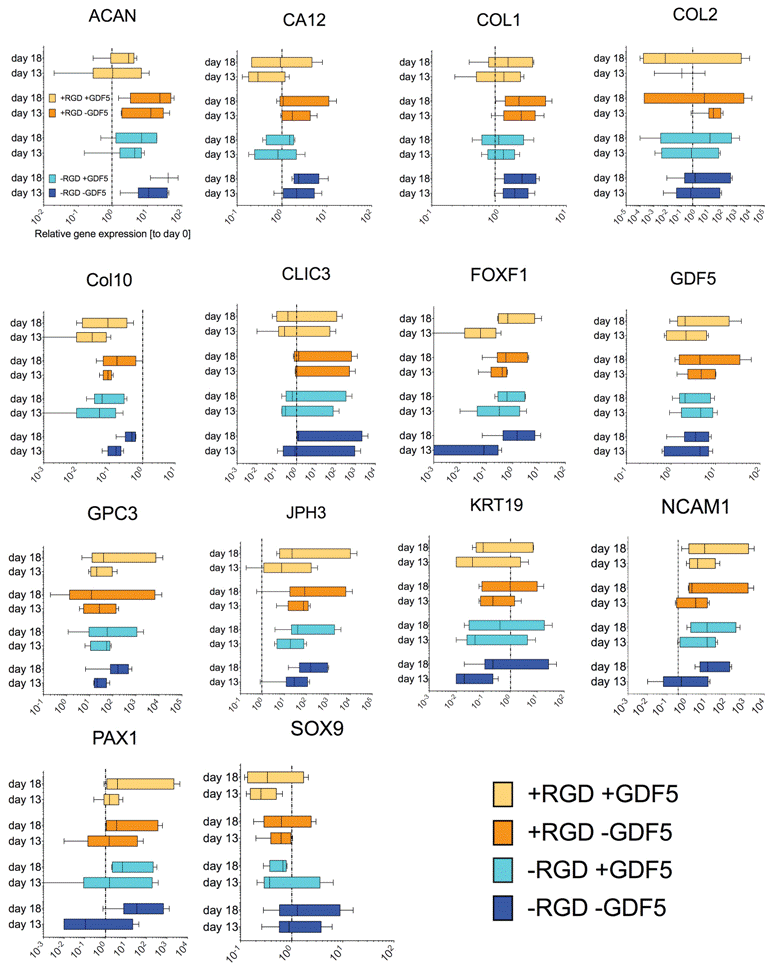
.
Figure 5: Relative gene expression of a screen of 15 genes of hMSCs seeded in PEG hydrogels after pre-conditioning with 100ng/mL rhGDF5 (day 13) and after mechanical loading at 1Hz (15% strain, 1h daily) (day 18). The genes have been described to be specific for intervertebral disc-like phenotype [20-22]. Box and whiskers represent medians and min. to max. (N=6 donor repeats).
View Figure 5
Application of mechanical loading did not seem to alter gene expression of all the genes that were tested in this study, as seen from the static gene expression level between day 13 and day 18. We found a relatively moderate response of hMSCs with respect to discogenic induction by GDF5 (Figure 5) as compared to the same donors under standard discogenic conditions using TGFβ (Figure 6). The expected up-regulation of discogenic markers was not observed although the donors possessed the discogenic potential as cultured in micromass culture or alginate bead culture (Figure 6).
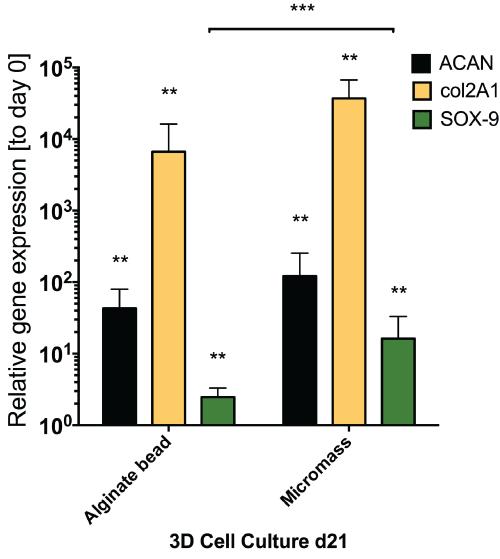
.
Figure 6: Relative gene expression for discogenic markers for analyzed donors (N = 4 out of 6) in standard 1.2% alginate beads and micromass culture (cell pellet) after 18 days of culture with TGFβ1. Major discogenic markers were all significantly increased after 18 days culture with TGFβ1 and dexamethasone confirming the discogenic potential of the cells used in this
study.** P < 0.05; *** P = < 0.01.
View Figure 6
Discussion
Cell viability of primary hMSCs in PEG hydrogels
Confocal laser scanning microscopy, DNA content and cellular activity demonstrated that under serum free conditions total living cell number decreased compared to the seeding day (day 0). However, a similar decreased cell viability was found for 1.2% alginate for MSCs [8,16] a material, which is used as a "gold standard" for chondrocytes and intervertebral disc cells and which seems to be ideal carrier for these cell types [30]. To our surprise, although the MMP-degradable modified PEG-hydrogel with RGD has been reported to be suitable for cell types such as endothelial cells [24] and mesenchymal stem cells [31], primary hMSCs seem to thrive with difficulties at cell densities and serum-free defined culture conditions, which have been used previously. The modification of the MMP-degradable PEG gel with RGD peptide did help the hMSC to attach and spread-out and to stay in an elongated cell shape. However, we were not able to demonstrate an improvement for chondrogenesis in the PEG environment lacking the RGD motive. Serum-free control condition seemed the most delicate condition for the hMSC to thrive for the 18 days culture period as has been demonstrated previously and GDF5 seemed more important than the RGD linker motif [8,16] (Figure 4).
RGD ligand for discogenic differentiation
The artificial MMP-degradable PEG hydrogels used in this study seem to improve cell-cell contacts and cell migration (time-lapse microscope movies, not shown) and that these primary cells seem to perform well in the cell carriers. HMSCs in PEG-hydrogels with covalently linked RGD motive showed a wide-spread cell phenotype from day 1 on and maintained until the end of the culture on day 18 (Figure 3). However, hMSC DNA content decreased over time and cells were difficult to differentiate. Our data showed a very weak effect of the growth factor GDF5 in contrast to previous studies that monitored gene expression at ACAN and COL2A1 [15,16], although our study clearly confirmed discogenic potential of hMSCs in 3D culture in alginate beads and in micromass culture. In our pilot experiment, the influence of the silicon on the hMSC tested to be non-toxic to cells, where hMSC embedded in PEG hydrogel with or without the silicon ring were cultured separately and result showed no difference in cell viability between the two treatments (data not shown).The silicon construct can be excluded to be a relevant factor to explain the non-responsiveness of the hMSCs. Connelly et al. (2007) [32] found that incorporation of RGD embedded into alginate has a blocking effect to differentiate. Salinas et al. (2009) [33] tested the RGD and KLER motives (a recognition site for decorin) in a PEG-peptide copolymer, it was found that cells produced 27-fold higher hydroxyl-proline in KLER-gels whereas the increase was only 2-fold in RGD-gels. Salinas et al.(2008) [34] found that hMSCs encapsulated in RGD-cleavable gels by day 21 of culture produce ten times as much glycosaminoglycan as cells with non-cleavable RGD functionalities,. They also found that 75% of the cells stain positive for collagen type II deposition when RGD is cleavable, as compared to 19% for cultures where RGD persists. In our case where RGD was persistently incorporated at a concentration of 50 μM and no increase of GAG/DNA production was noticed. It is not clear whether this is due to the non-degradable character RGD of the Qgel® PEG-hydrogel.
GDF5 and mechanical loading stimulations
Previous studies suggested that GDF5 is a more potent growth factor to direct discogenesis than TGFβ [15,16]. Gantenbein et al. (2010) [16] demonstrated a higher ratio of aggrecan/ col2a1 gene expression by MSC when stimulated by GDF5 than TGFβ1. Stoyanov et al. [15] compared the influence of TGFβ1 and GDF5 on the discogenic differentiation of hMSC in alginate beads under hypoxic and normoxic conditions. The results indicated that even under normoxic condition, the presence of GDF5 and dexamethasone increased the gene expression of aggrecan, Col2A1 and KRT-19 after 28 days of culture as comparable to TGFβ1. In our experiment, the preconditioning with GDF5 had a relatively neutral or even negative effect on the cells since RT-PCR revealed down-regulation of ACAN, COL2A1 and of SOX9. The presence of GDF-5 seems to increase cell activity (Figure 2) and the rate of PEG hydrogel degradation as shown by reduced amount of PEG hydrogel in histological evaluation (Figure 4). In recent findings by our group we could demonstrate that the IVD environment along with mechanical loading represents a major "discogenic" differentiation stimulus for hMSC; this stimulus being much stronger than the pre-conditioning in presence of 100ng/Ml GDF5 added to the media [35]. Several studies have shown that mechanical loading applied on stem cells might improve the outcome of chondrogenesis of stem cells [18,19,36]. Especially effective was the pre-conditioning of hMSCs embedded in PEG hydrogel the micro-environment of the IVD but also be non-viral transfection using GDF5 overexpression vector [17]. Previous studies demonstrated that loading parameters of 10 - 15% strain at frequency between 0.5- 1Hz should be favorable to hMSC differentiation under the stimulation of TGFβ in other cell carriers, e.g., fibrin [37,38], agarose [39] and polyurethane [19]. However, in contrast to these studies we applied cyclic strain at a magnitude of 15% strain at 1Hz for 5 consecutive days. The five consecutive days of loading had some minor recovery effect in the genes JPH3, NCAM1 and FOXF1 (Figure 5). However, most gene expression levels were found to be relatively even among groups (Figure 5) relative to day 0 controls. Some studies also showed that, even without the stimulation of TGFβ, mechanical loading alone could trigger discogenic differentiation of stem cells [40], possibly due to the fact that cyclic compression induces the production of TGFβ by stem cells [41]. Future work should also focus on a closely related cytokine, BMP13=GDF6. It could recently been demonstrated that GDF6 may be even more effective to produce a NPC-like phenotype than GDF5 but for sure both perform better than TGFβ1, which induces the osteogenic pathway [42]. We did not check for brachyury (T) and also not for CD24 or HIF-1α in our study, as these markers were recently proposed as stable expressed for the healthy NPC phenotype [20,43]. However, cyclic compression did not seem to enhance discogenic differentiation of hMSC in our model. It is likely that the silicon ring has a much higher compressive stiffness than the PEG hydrogel therefore the silicon ring carried most of the compressive force and hence forces applied on the cell-seeded PEG hydrogel became non-significant.
Conclusions
3D culturing of human mesenchymal stem cells in MMP-degradable PEG hydrogel incorporated with or without RGD and cyclic mechanical stimulation was tested using a daily 1h cyclic compression protocol. HMSC showed moderate response towards discogenic differentiation under these synthetic PEG 3D culture conditions, although the same hMSCs under "standard" chondrogenic micromass culture or alginate culture with TGFβ was successful to induce chondrogenesisas positive control.
Acknowledgements
We thank Samantha Chan and Elena Calandriello for assistance cell culture and in histology. Alexander Burki and for support on the uni-axial loading device. This work was financially supported by the Lindenhof Foundation Projects no. 13-02-F and 14-03-F. The imaging part of this study was performed with the facility of the Microscopy Imaging Center (MIC), University of Bern.
References
-
Rimann M, Graf-Hausner U (2012) Synthetic 3D multicellular systems for drug development. Curr Opin Biotechnol 23: 803-809
-
Huang AH, Farrell MJ, Kim M, Mauck RL (2010) Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogel. Eur Cell Mater 19: 72-85
-
Reitmaier S, Kreja L, Gruchenberg K, Kanter B, Silva-Correia J, et al. (2014) In vivo biofunctional evaluation of hydrogels for disc regeneration. Eur Spine J 23: 19-26
-
Tsai TL, Nelson BC, Anderson PA, Zdeblick TA, Li WJ (2014) Intervertebral Disc and Stem Cells Co-cultured in Biomimetic Extracellular Matrix Stimulated by Cyclic Compression in Perfusion Bioreactor. Spine J 14: 2127-2140
-
Pereira DR, Silva-Correia J, Oliveira JM, Reis RL (2013) Hydrogels in acellular and cellular strategies for intervertebral disc regeneration. J Tissue Eng Regen Med 7: 85-98
-
Lutolf MP, Gilbert PM, Blau HM (2009) Designing materials to direct stem-cell fate. Nature 462: 433-441
-
Wang H, Zhou Y, Huang B, Liu LT, Liu MH, et al. (2014) Utilization of stem cells in alginate for nucleus pulposus tissue engineering. Tissue Eng Part A 20: 908-920
-
Zeiter S, der Werf MV, Ito K (2009) The fate of bovine bone marrow stromal cells in hydrogels: a comparison to nucleus pulposus cells and articular chondrocytes. J Tissue Eng Regen Med 3: 310-320
-
Wuertz K, Godburn K, Iatridis JC (2009) MSC response to pH levels found in degenerating intervertebral discs. Biochem Biophys Res Commun 379: 824-829
-
Re'em T, Tsur-Gang O, Cohen S (2010) The effect of immobilized RGD peptide in macroporous alginate scaffolds on TGFbeta1-induced chondrogenesis of human mesenchymal stem cells. Biomaterials 31: 6746-6755
-
Villanueva I, Weigel CA, Bryant SJ (2009) Cell-matrix interactions and dynamic mechanical loading influence chondrocyte gene expression and bioactivity in PEG-RGD hydrogels. Acta Biomater 5: 2832 - 2846
-
Chang JC, Hsu SH, Chen DC (2009) The promotion of chondrogenesis in adipose-derived adult stem cells by an RGD-chimeric protein in 3D alginate culture. Biomaterials 30: 6265-6275
-
Ravindran S, Roam JL, Nguyen PK, Hering TM, Elbert DL, et al. (2011) Changes of chondrocyte expression profiles in human MSC aggregates in the presence of PEG microspheres and TGF-β3. Biomaterials 32: 8436-8445
-
You M, Peng G, Li J, Ma P, Wang Z, et al. (2011) Chondrogenic differentiation of human bone marrow mesenchymal stem cells on polyhydroxyalkanoate (PHA) scaffolds coated with PHA granule binding protein PhaP fused with RGD peptide. Biomaterials 32: 2305-2313
-
Stoyanov JV, Gantenbein-Ritter B, Bertolo A, Aebli N, Baur M, et al. (2011) Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur Cell Mater 21: 533-547
-
Gantenbein-Ritter B, Benneker LM, Alini M, Grad S (2011) Differential response of human bone marrow stromal cells to either TGF-β(1) or rhGDF-5. Eur Spine J 20: 962-971
-
Bucher C, Gazdhar A, Benneker LM, Geiser T, Gantenbein-Ritter B (2013) Nonviral Gene Delivery of Growth and Differentiation Factor 5 to Human Mesenchymal Stem Cells Injected into a 3D Bovine Intervertebral Disc Organ Culture System. Stem Cells Int 2013: 326828
-
Nagel T, Kelly DJ (2010) Mechano-regulation of mesenchymal stem cell differentiation and collagen organisation during skeletal tissue repair. Biomech Model Mechanobiol 9: 359-372
-
Li Z, Kupcsik L, Yao SJ, Alini M, Stoddart MJ (2010) Mechanical load modulates chondrogenesis of human mesenchymal stem cells through the TGF-beta pathway. J Cell Mol Med 14: 1338-1346
-
Risbud MV, Schoepflin ZR, Mwale F, Kandel RA, Grad S, et al. (2014) Defining the Phenotype of Young Healthy Nucleus Pulposus Cells: Recommendations of the Spine Research Interest Group at the 2014 Annual ORS Meeting. J Orthop Res 33: 283-293
-
Rutges J, Creemers LB, Dhert W, Milz S, Sakai D, et al. (2010) Variations in gene and protein expression in human nucleus pulposus in comparison with annulus fibrosus and cartilage cells: potential associations with aging and degeneration. Osteoarthritis Cartilage 18: 416 - 423
-
Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA (2010) Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther 12: R22
-
Ehrbar M, Rizzi SC, Schoenmakers RG, Miguel BS, Hubbell JA, et al. (2007) Biomolecular hydrogels formed and degraded via site-specific enzymatic reactions. Biomacromolecules 8: 3000-3007
-
Jo YS, Rizzi SC, Ehrbar M, Weber FE, Hubbell JA, et al. (2010) Biomimetic PEG hydrogels crosslinked with minimal plasmin-sensitive tri-amino acid peptides. J Biomed Mater Res A 93: 870-877
-
Xiao J, Zhang Y, Wang J, Yu W, Wang W, et al. (2010) Monitoring of Cell Viability and Proliferation in Hydrogel-Encapsulated System by Resazurin Assay. Appl Biochem Biotechnol 162: 1996-2007
-
Rasband WS (2007-2015) ImageJ, National Institutes of Health, Bethesda, Maryland, USA,
-
Gantenbein-Ritter B, Potier E, Zeiter S, van der Werf M, Sprecher CM, et al. (2008) Accuracy of three techniques to determine cell viability in 3D tissues or scaffolds. Tissue Eng Part C Methods 14: 353-358
-
Reno C, Marchuk L, Sciore P, Frank CB, Hart DA (1997) Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. Biotechniques 22: 1082-1086
-
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402-408
-
Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU (1998) In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 238: 265-272
-
Salinas CN, Anseth KS (2008) The influence of the RGD peptide motif and its contextual presentation in PEG gels on human mesenchymal stem cell viability. J Tissue Eng Regen Med 2: 296-304
-
Connelly JT, Garcia AJ, Levenston ME (2007) Inhibition of in vitro chondrogenesis in RGD-modified three-dimensional alginate gels. Biomaterials 28: 1071-1083
-
Salinas CN, Anseth KS (2009) Decorin moieties tethered into PEG networks induce chondrogenesis of human mesenchymal stem cells. J Biomed Mater Res A 90: 456-464
-
Salinas CN, Anseth KS (2008) The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials 29: 2370-2377
-
Malonzo C, Chan SCW, Kabiri A, Eglin D, Grad S, et al. (2013) A papain-induced disc degeneration model for the assessment of thermo-reversible hydrogel-cells therapeutic approach. J Tissue Eng Regen Med: 1667
-
Lee DA, Knight MM, Campbell JJ, Bader DL (2011) Stem cell mechanobiology. J Cell Biochem 112: 1-9
-
Mouw JK, Connelly JT, Wilson CG, Michael KE, Levenston ME (2007) Dynamic compression regulates the expression and synthesis of chondrocyte-specific matrix molecules in bone marrow stromal cells. Stem Cells 25: 655-663
-
Pelaez D, Huang CY, Cheung HS (2009) Cyclic compression maintains viability and induces chondrogenesis of human mesenchymal stem cells in fibrin gel scaffolds. Stem Cells Dev 18: 93-102
-
Elder SH, Kimura JH, Soslowsky LJ, Lavagnino M, Goldstein SA (2000) Effect of compressive loading on chondrocyte differentiation in agarose cultures of chick limb-bud cells. J Orthop Res 18: 78-86
-
Jung Y, Kim SH, Kim YH, Kim SH (2009) The effects of dynamic and three-dimensional environments on chondrogenic differentiation of bone marrow stromal cells. Biomed Mater 4: 055009
-
Huang CY, Hagar KL, Frost LE, Sun Y, Cheung HS (2004) Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells 22: 313-323
-
Clarke LE, McConnell JC, Sherratt MJ, Derby B, Richardson SM, et al. (2014) Growth differentiation factor 6 and transforming growth factor-beta differentially mediate mesenchymal stem cell differentiation, composition and micromechanical properties of nucleus pulposus constructs. Arthritis Res Ther 16: R67
-
Mwale F, Roughley P, Antoniou J (2004) Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: a requisite for tissue engineering of intervertebral disc. Eur Cell Mater 8: 58-63





