International Journal of Radiology and Imaging Technology
Transarterial Chemoembolization (TACE) as A Palliative Treatment Option for Liver Metastases from Non-Small Cell Lung Cancer (NSCLC)
Tatjana Gruber-Rouh1, Nagy N N Naguib1,2, Martin Beeres1, Benjamin Kaltenbach1, Thomas J Vogl1 and Nour-Eldin A Nour-Eldin1,3*
1Institute for Diagnostic and Interventional Radiology, Johann Wolfgang Goethe-University Frankfurt, Germany
2Department of Diagnostic and Interventional Radiology, Alexandria University Hospital, Egypt
3Department of Diagnostic and Interventional Radiology, Cairo University Hospital, Egypt
*Corresponding author:
Nour-Eldin A Nour-Eldin, MD, MSc, Institute for Diagnostic and Interventional Radiology, Johann Wolfgang Goethe-University Frankfurt, Theodor-Stern-Kai 7, 60590 Frankfurt am Main, Germany, Tel: +49-69-6301-87200, Fax: +49-69-6301-7258, E-mail: nour410@hotmail.com
Int J Radiol Imaging Technol, IJRIT-2-019, (Volume 2, Issue 2), Original Research
Received: October 13, 2016; Accepted: November 23, 2016; Published: November 26, 2016
Citation: Gruber-Rouh T, Naguib NNN, Beeres M, Kaltenbach B, Vogl TJ, et al. (2016) Transarterial Chemoembolization (TACE) as A Palliative Treatment Option for Liver Metastases from Non-Small Cell Lung Cancer (NSCLC). Int J Radiol Imaging Technol 2:019.
Copyright: © 2016 Gruber-Rouh T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: The study aimed at evaluating the local tumor control, survival data and prognostic factors following treatment with transarterial chemoembolization (TACE) in the palliative therapy of patients with liver metastases from non-small-lung-cancer (NSCLC).
Material and methods: The study was retrospectively performed following approval of the ethical committee. 56 patients (mean age, 53.4 years) with liver metastases of NSCLC (Stage IV) undergone repeated TACE. Overall, 214 TACE procedures were administered (mean, 3.8 sessions/patient). The administered chemotherapeutic agents included mitomycin, gemcitabine and cisplatin. For embolization lipiodol and degradable starch microspheres (DSM) were used.
Results: The local response of the tumors was assessed by MRI using RECIST 1.1. Patient survival was evaluated using Kaplan-Meier method and compared with the log-rank-test. The local tumor control was: partial response in 14.3%, stable disease in 51.8% and progressive disease in 33.9% of patients. The patients’ survival rate was 59%, 9% and 2% after 1, 2 and 3 years. The median survival time was 11 months. Initial tumor response (p = 0.04) and high tumor vascularity (p = 0.03) were statistically significant factors for patient`s survival.
Conclusion: TACE could be used as a therapeutic option for the palliative management of selected patients with hepatic metastases of NSCLC origin with satisfactory local tumor control capability.
Keywords
Liver metastases, NSCLC, TACE, Survival data, Prognostic factors
Introduction and Background
Non-small cell lung cancer (NSCLC) represents an important cause of mortality worldwide due to its aggressiveness and metastatic data after initial curative treatment [1].
Approximately 40-50% of NSCLC patients presents with metastatic stage IV, and are not candidates for resection. Those patients are treated with systemic chemotherapy [2-4]. Systemic therapy is the only treatment proven to increase survival by up to 8-12 months, decrease symptoms and improve quality of life [5,6]. Resistance to systemic therapy is main obstacle in the treatment metastatic patients [2].
For patients with hepatocellular carcinoma and liver metastases from colorectal carcinoma, transarterial chemoembolization is an effective treatment option, [7-9].
To our knowledge, we present the first report of case series; in which patients with inoperable and chemotherapy refractory liver metastases of NSCLC, were treated with TACE. The single-centre study included a relatively large number of patients with failed systemic chemotherapy or progression under systemic chemotherapy. The aim of the study was to present the possible clinical impact of chemoembolization of liver metastases on survival and local tumor control in patients with NSCLC. The prognostic factors for patient`s survival were evaluated.'
Materials and Methods
Patients
The study demonstrates a single-center 15-year retrospective analysis of TACE of liver metastases in patients from Germany, Denmark, Holland, Canada and the United States with NSCLC.
Between December 2000 and August 2015, 56 patients with systematic chemotherapy refractory liver metastasis of NSCLC were repeatedly treated with transarterial chemoembolization (TACE). Overall 214 TACE treatments were performed every 4 weeks with a mean of 3.8 sessions /patient (range, 2-9). The mean age of the patients was 53.4 (range, 45-76 years). An ethical committee approval was obtained before the study and all patients signed consent prior to treatment.
All of the clinical data were obtained either by contacting the patients themselves or by contacting their treating physicians. In addition we reviewed our database of TACE and all patients’ electronic medical records. Analysis of the MRI and CT studies was performed by three senior radiologists. All included patients had systematic chemotherapy refractory liver metastases without extrahepatic disease.
All patients received TACE treatment using mitomycin, gemcitabine and cisplatin (n = 56).
Inclusion criteria
The treatment decision for patients was performed in a multidisciplinary tumor board. TACE indications were hepatic metastasis with no response, or systemic toxicity to systemic chemotherapy and isolated metachronous liver metastasis after resection of primary tumor. Patients were inoperable either due to co-medical morbidities or due to refusal of surgery. All alternatives were discussed with the patients and all patients provided approval of treatment as well as the approval of the use of their data anonymously for research purposes. The main aim of TACE treatment was to achieve sufficient volume reduction of the hepatic lesions with preservation of the hepatic function. The aim of palliation was to relieve pain and abdominal discomfort due pressure on nearby organs, with subsequent improvement of the quality of life. In all patients histopathological confirmation of liver metastases was carried out.
For all patients, we calculated the total volume amount of all hepatic lesions per patient in addition to the depending liver volume in order to estimate the hepatic tumor load. Only those patients with < 70% hepatic tumor-involvement were treated. To be eligible for TACE, patients had to fulfil certain laboratory and clinical criteria including adequate hematic, hepatic and renal functions in addition to an ECOG performance score of 0 or 1.
Exclusion criteria
We excluded patients with > 70% hepatic involvement by the tumor since treating such patients might impair the remaining liver function and lead to liver failure.
In addition we excluded patients with total thrombosis of the main stem of the portal vein, patients with extrahepatic metastases and those with renal (creatinine level > 2 mg/dl in serum), hepatic, respiratory or cardiovascular failure. Inadequate performance status as judged by an (ECOG > 1), nutritional impairment, high serum total bilirubin level (> 3mg/dL) and poor hepatic synthesis (albumin level < 2.0 mg/dL in serum) were further exclusion criteria.
TACE- therapy
The interventional procedure was performed according to the standard technique of Chemoembolization. The femoral artery was punctured using the Salinger’s technique. This was followed by introduction of a femoral sheath. A 5F Pig-Tail catheter (Boston Scientific) was used to perform an angiographic view of the abdominal aorta and its major branches, The Pig-Tail catheter was exchanged over the guide wire for a 5F Side-Winder Catheter (Terumo, Tokyo, Japan) and this was used for selective catheterization and angiographic demonstration of the superior mesenteric artery and the celiac trunk. In addition an indirect photography was performed to ensure patency of the portal vein. This was followed by selective catheterization of the hepatic artery. Further selective catheterization of segmental and subsegmental branches of the hepatic artery was performed depending on the location, size and arterial feeding vessel of the target tumor. In case of involvement of both hepatic lobes, we treated the lobe with the higher tumor burden first. The other lobe was being treated in another session of chemoembolization.
The chemotherapeutic drugs used were mitomycin (8 mg/m2 , Medac®, Hamburg, Germany), gemcitabine (1000 mg/m² , Gemzar®, Eli Lilly and Company, Indianapolis, IN), and cisplatin (35 mg/m2 , Cisplatin Teva®, Radebeul, Germany). Vascular occlusion (Embolization) was performed after injection of the chemotherapeutic drugs using lipiodol (maximal dose of 6 ml, Guerbet®, Sulzbach, Germany), followed by an injection of 200-450 mg of degredable starch microspheres (200 µm) (EmboCept®, PharmaCept GmbH, Berlin, Germany).
The embolizing material was injected under fluoroscopic guidance until the end point of embolization was reached (stasis of flow). To be eligible for the study patients should have at least 3 sessions of chemoembolization performed with 4 week interval between sessions.
Following treatment patients were transferred for clinical observation at least for 8 hours and were discharged (in absence of complications) on the same day of the procedure. The treatment sessions were repeated until the end point of treatment was reached, this was defined as a state of stable disease for two successive sessions or in case of disease progression. After treatment end patients were followed by MRI until patient death. In case of new lesions or disease progression during follow-up (after initial stabilization), patients were retreated using the previous protocol as long as they meet the inclusion criteria for treatment.
MRI follow-up
Follow-up MRI was performed to evaluate the tumor response. For the purpose of planning the intervention, unenhanced and contrast-enhanced MR imaging, with 0.1 mmol/kg body weight of gadopentetate dimeglumine (Magnevist; Schering, Berlin, Germany), was performed in all patients. A 1.5-T MRI-system (Magnetom Espree; Magnetom Avanto-fit; Siemens, Erlangen, Germany) was used.
Enhanced MR imaging was performed before first and 4 weeks after third TACE. Unenhanced MR imaging was performed with a 1.5-T system before every TACE treatment.
Following treatment end patients were followed up using MRI performed every month for a period of three months this was followed by MRI examination every three months for the rest of patients’ life. None of the included patients was lost to follow-up.
4-6 hours after embolization, retention of iodized oil in the liver metastases was confirmed with findings by unenhanced computed tomography (CT).
Quantitative and statistical evaluation
Datasets of all patients were evaluated retrospectively. Each clinical data was obtained either by contacting the patients themselves or by contacting their treating physicians. In addition, we reviewed our database of TACE and the depending patients’ files. Event occurrences were reported.
All MRI and CT evaluations were performed by three radiologists (with more than 3, 10 and 18 years of experience in abdominal imaging) in consensus. The local response of the tumor was assessed by MRI, using Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) [10]. Statistical analysis was performed using BiAs 10.12 software. Survival times, starting at point of first chemoembolization, were calculated to obtain the median and mean survival times by using the Kaplan-Meier method and compared with the log-rank test. Survival rates were calculated in terms of 1-, 2- and 3-year survival, also dating from the start of TACE treatment. Subgroup analysis and differences in survival between groups were assessed by log-rank test. P ≤ 0.05 was considered significant.
Results
Local and clinical results
Location of the tumor was in 67.9% (38/56) bilobar and in 32.1% (18/56) in the right liver lobe. The numbers of liver lesions were as follows: We had 56 patients, 60.7% (34/56) with multiple lesions (≥ 5), 12.5% (7/56) with only one lesion, 16.1% (9/56) had two liver lesions, and 10.7% (6/56) had three or four liver tumors.
Using angiography and post-interventional CT scans we documented in 39.3% (22/56) hypervascular metastatic liver lesions and in 60.7% (34/56) hypovascular liver lesions.
The post-interventional evaluation was based on the RECIST 1.1 und all patients were revealed using this criteria: partial response in 14.3% (8/56) (Figure 1), stable disease in 51.8% (29/56) and progressive disease in 33.9% (19/56) (Table 1).
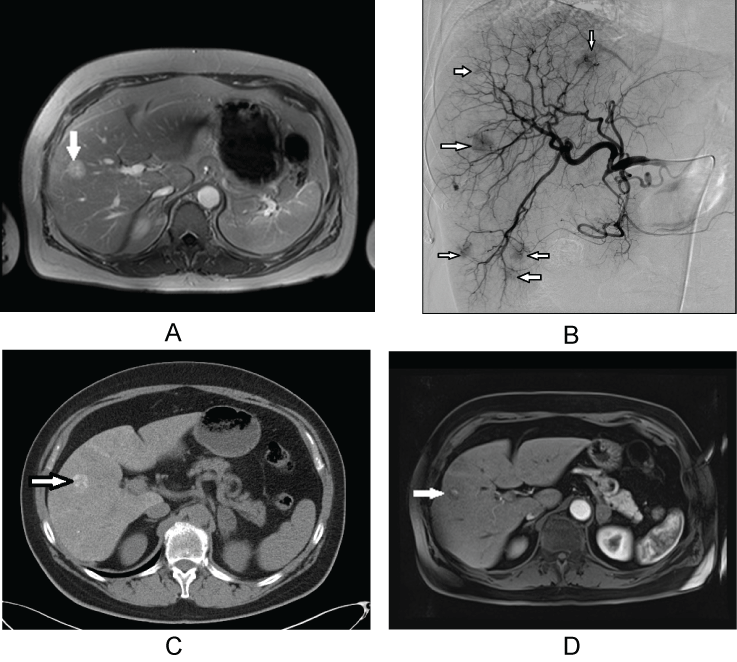
.
Figure 1: : 62-year-old women with metachronous liver metastases of NSCLC and morphologic features of complete response after treatment with transarterial chemoembolization. The patient is currently in follow up.
a) Pretreatment contrast-enhanced axial MRI scan shows metastatic liver lesion (arrow) in segment 8/5; b) Selective digital subtraction angiogram obtained during TACE reveals the hypervascularity of the liver metastases (arrows); c) CT scan after transarterial embolization shows lipiodol retention in metastatic lesion in segment 8/5 (arrow); d) Contrast-enhanced T1-weighted MR image after image 5 years. No newly developed lesions were detected.
View Figure 1
![]()
Table 1: Characteristics of patients with NSCLC.
View Table 1
Survival analysis
The median and mean survival times from the beginning of interventional treatment with TACE were 11 and 15.3 months. Survival rate from the start of TACE was 59% after 1-year, 9% after 2-years, and 2% after 3-years due to Kaplan Meier evaluation with BiAs 10.12 (Figure 2).
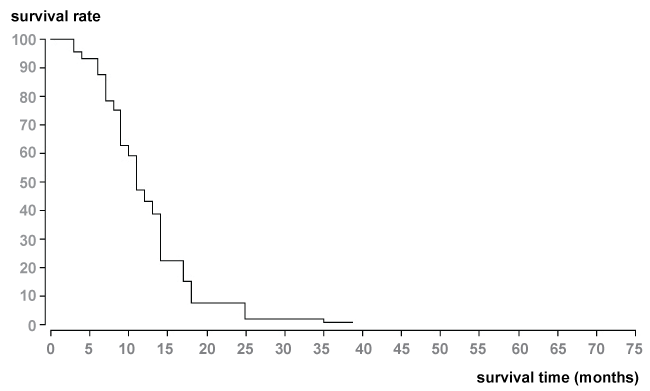
.
Figure 2: Survival data (Kaplan-Meier method) of patients with liver metastases of NSCLC (n = 56). Median survival time was 11 months from the start of chemoembolization therapy.
View Figure 2
Median and mean survival of patients with partial response (PR) after first chemoembolization therapy was 14.0 months and 32.2 months. Median and mean survival of patients with stable disease (SD) was 12.0 months and 12.3 months, and median and mean survival of patients with progression disease (PD) was 8.3 months and 9 months (Figure 3). The survival time analyses showed statistical differences among the groups using the log-rank test (ρ = 0.04).
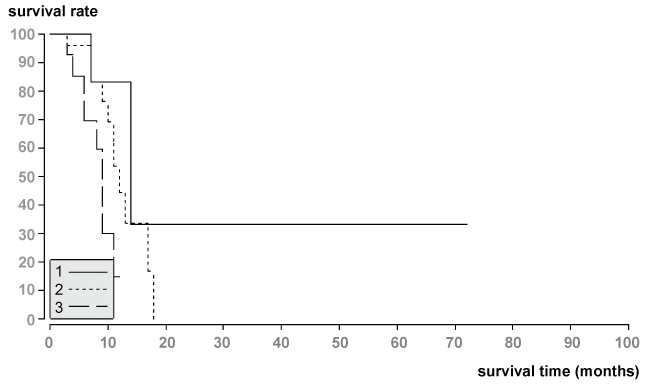
.
Figure 3: Survival data of patients with tumor response of liver metastases according to the RECIST 1.1.-Kriteria.
1.The median survival time of patients with PR (n = 8) was 14.0 months.
2.The median survival time of patients with SD (n = 29) was 12.0 months.
3.The median survival time of patients with PD (n = 19) was 8.3 months.
View Figure 3
Prognostic factors analysis
Using log-rank test initial local tumor response (p = 0.04; Figure 3) and tumor vascularity (p = 0.03; Figure 4) were significant factors for patient’s survival. There is low probability of survival in patients with progressive disease (median survival time = 8.3 months) and tumor hypovascularity (median survival time = 9 months). However, initial tumor response (median survival time = 14 months) and tumor hypervascularity (median survial time = 14 months) were positive prognostic factors for patient’s survival.
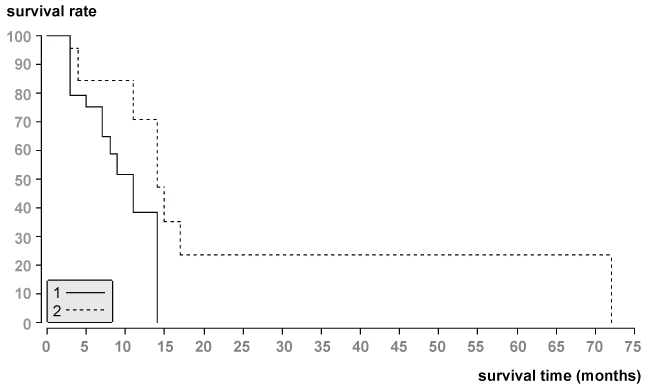
.
Figure 4: Survival data of patients with hypervascularity and hypovascularity of liver metastases from NSCLC according to the Kaplan-Meier method. Survival times from the start of TACE showed statistical difference between the two groups (p = 0.03).
1.The median survival time of patients with hypervasvularity of NSCLC was 14 months.
2.The median survival time of patients with hypovascularity of NSCLC was 9 months.
View Figure 4
None statistically significant factors for survival were number of tumor lesions (p = 0.94), and tumor allocation within the liver (p = 0.82). Table 2 summarizes the survival data and the different prognostic factors.
![]()
Table 2: Survival data and prognostic factors.
View Table 2
Complications after chemoembolization
All our treated patients were monitored for “postembolization syndrome” and potential complications. Generally, the majority of patients tolerated chemoembolization well and all patients were discharged from hospital on the day of treatment. However, 17 patients (30%) had symptoms in the form of abdominal pain, nausea and vomiting for 2 to 7 days. No major complications or allergic reactions were reported in our patient group.
Discussion
NSCLC is the most lethal cancer due to its high rate of metastasis. Metastatic disease including liver metastasis (Stage IV) is a predictor of poor prognosis [1]. Median survival of patients with NSCLC in Stagy IV is 8-12 months and 5-years survival rate is 1%. In Stage IV of NSCLC, systemic treatment is based on the combination of carboplatin or cisplatin with drugs such as paclitaxel, gemcitabine, docetaxel, and vinorelbine which exhibit superior efficacy compared to single agent platinum therapy [5,6].
Because the liver does not have only one site of metastases from NSCLC, the experience with interventional loco-regional therapies of liver metastases is limited.
For patients with malignant liver lesions, chemoembolization with chemotherapeutic agents and embolizing agents, is a therapeutic option, that may help to prolong survival, relieve symptoms, and improve the quality of life [11].
The concept of chemoembolization has been used in the management of patients with unresectable hepatocellular carcinoma and with liver metastases from colorectal cancer, breast cancer, neuroendocrine malignant tumors, ocular melanoma and sarcoma [12-17].
As with any type of cancer treatment, transarterial chemoembolization is adjusted to fit each person's individual needs and depends on the size, location, and extent of the tumor and general health.
However, to our knowledge there have been no previous studies describing evaluation of chemoembolization for liver metastases from NSCLC.
The current single-centre study was administered on a large number of patients (n = 56) in order to examine the response and survival data of patients who undergo chemoembolization of liver metastases from NSCLC. In our study, 56 patients with chemotherapy refractory hepatic metastases were treated with TACE. We achieved median and mean survival time of 11 and 15.3 months from the beginning of chemoembolization therapy, and the survival rate was 59% at 1 year, 9% at 2 years, and 2% at 3 years. However, positive prognostic factors for patient’s survival were initial tumor response, and tumor hypervascularity.
Our results indicate that TACE is a minimally invasive but non-standard therapy option for palliative treatment of liver metastases in patients with NSCLC. A major advantage of transarterial chemoembolization is that it can be easily performed with local anesthesia in an outpatient setting and has a low complication rate.
The current study has several limitations. First, the study design was retrospective. Secondary, the lack of control group of patients receiving only systemic chemotherapy represents a further limitation of the current study. Thirdly, more patients should have been recruited and a prospective randomized study would be more accurate to assess treatment safety and efficacy of TACE in patients with liver metastases from NSCLC. However, given the small number of patients who require this therapy, it may be difficult to perform a prospective study with including a randomized protocol with control group.
In conclusion, TACE of liver metastases could be applied as a therapeutic option for palliative treatment in achieving local tumor control in selected patients with NSCLC. Chemoembolization can be considered as a palliative therapeutic tool with low incidence of complications and reasonable level of tolerability.
References
-
Cancer. World Health Organization.
-
Janet Wangari-Talbot, Elizabeth Hopper-Borge (2013) Drug Resistance Mechanisms in Non-Small Cell Lung Carcinoma. J Can Res Updates 2: 265-282.
-
Aisner DL, Marshall CB (2012) Molecular pathology of non-small cell lung cancer: a practical guide. Am J Clin Pathol 138: 332-346.
-
Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger KR, et al. (2011) International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 6: 244-285.
-
Azzoli CG, Baker S Jr, Temin S, Pao W, Aliff T, et al. (2009) American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Clin Oncol 27: 6251-6266.
-
Goffin J, Lacchetti C, Ellis PM, Ung YC, Evans WK (2010) First-line systemic chemotherapy in the treatment of advanced non-small cell lung cancer: a systematic review. J Thorac Oncol 5: 260-274.
-
Yi-Xiang J Wáng, Thierry De Baere, Jean-Marc Idée, Sébastien Ballet (2015) Transcatheter embolization therapy in liver cancer: an update of clinical evidences. Chin J Cancer Res 27: 96-121.
-
Llovet JM, Sala M, Castells L, Suarez Y, Vilana R, et al. (2000) Randomized controlled trial of interferon treatment for advanced hepatocellular carcinoma. Hepatology 31: 54-58.
-
Tellez C, Benson AB, Lyster MT, Talamonti M, Shaw J, et al. (1998) Phase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer 82: 1250-1259.
-
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, et al. (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228-247.
-
Stuart K (2003) Chemoembolization in the management of liver tumors. Oncologist 8: 425-437.
-
Ho AS, Picus J, Darcy MD, Tan B, Gould JE, et al. (2007) Long-term outcome after chemoembolization and embolization of hepatic metastatic lesions from neuroendocrine tumors. Am J Roentgenol 188: 1201-1207.
-
Brown DB, Geschwind J-F H, Soulen MC, Millward SF, Sacks D (2006) Society of Interventional Radiology position statement on chemoembolization of hepatic malignancies. J Vasc Interv Radiol 17: 217-223.
-
Vogl TJ, Zangos S, Eichler K, Yakoub D, Nabil M (2007) Colorectaal liver metastases:regional chemotherapy via transarterial chemoembolization (TACE) and hepatic chemoperfusion: an update. Eur Radiol 17: 1025-1034.
-
Vogl TJ, Trapp M, Schroeder H, Mack M, Schuster A, et al. (2000) Transarterial chemoembolization for hepatocellular carcinoma: volumetric and morphologic CT criteria for assessment of prognosis and therapeutic success- results from a liver transplantation center. Radiology 214: 349-357.
-
Llovet JM, Real MI, Montaña X, Planas R, Coll S, et al. (2002) Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 359: 1734-1739.
-
Takayasu K, Arii S, Ikai I, Omata M, Okita K, et al. (2006) Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology 131: 461-469.





