International Journal of Ophthalmology and Clinical Research
Flash Visual Evoked Potential versus Pattern Visual Evoked Potential in the Diagnosis of Strabismic Amblyopia
Amany Abd El-Fattah El-Shazly*, Walid Mohamed Abd E, Raouf El-Zawahry and Noha Ezzat Elsherbiny
Department of Ophthalmology, Ain Shams University, Cairo, Egypt
*Corresponding author: Dr. Amany Abd El-Fattah El-Shazly, Assitant professor of Ophthalmology, Department of Ophthalmology, Faculty of Medicine, Ain Shams University, Cairo, Egypt, Tel: 002 02 26429626, E-mail:
amany_elshazly@ymail.com
Int J Ophthalmol Clin Res, IJOCR-3-061, (Volume 3, Issue 3), Research Article; ISSN: 2378-346X
Received: May 25, 2016 | Accepted: July 29, 2016 | Published: August 01, 2016
Citation: El-Shazly AAEF, Walid MAE, El-Zawahry R, Elsherbiny NE (2016) Flash Visual Evoked Potential versus Pattern Visual Evoked Potential in the Diagnosis of Strabismic Amblyopia. Int J Ophthalmol Clin Res 3:061. 10.23937/2378-346X/1410061
Copyright: © 2016 El-Shazly AAEF, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: To compare the responses of flash visual evoked potential (FVEP) & pattern visual evoked potential (PVEP) in amblyopic eyes with the eyes of healthy children and to find out whether the flash VEP can help us in the diagnosis of amblyopia as it is the only test applicable to nonverbal and disabled children.
Methods: We included 60 children with unilateral amblyopia, their age ranging between 4 and 10 years. Thirty age and sex matched healthy children were included as a control group. The included children underwent complete ophthalmologic evaluation with focus on subjective monocular visual acuity, cycloplegic refractions, slit-lamp examination, fundus examination, orthoptic evaluation, stereoacuity, worth 4-dot test, FVEP & PVEP. All participants had stable fixation.
Results: The flash VEP P100 latency showed a statistical insignificant difference between the two studied groups (120.6 ± 13.45 & 116.0 ± 14.7 ms respectively) (p = 0.07). However, the pattern VEP P100 latency in amblyopic group (130.5 ± 6.8 ms) was statisticaly longer than that of control group (111.9 ± 4.2 ms) (p ≤ 0.0001). The amblyopic best corrected visual acuity (BCVA) showed significant correlation with the pattern VEP latency and amplitude (P < 0.001, < 0.001), however, it showed insignificant correlation with the flash VEP latency (P = 0.66).
Conclusion: Pattern VEP could be applied to diagnose amblyopia, but we cannot rely on the FVEP in this context as proved with multiregression analysis which showed that the pattern VEP latency and amplitude are the most important determinant factor for amblyopic best corrected visual acuity.
Keywords
Amblyopia, BCVA, Flash VEP, Pattern VEP
Introduction
Amblyopia (from the Greek, amblyos-blunt; ops-vision) is a developmental problem usually associated with physiological alterations in the visual cortex early on in life [1,2].
Amblyopia is an acquired unilateral or bilateral diminution of visual acuity with no obvious structural or pathological causes that can be detected by physical examination of the eye [3-5].
Amblyopia can be classified into three types: strabismic, anisometropic, and combined mechanism (strabismus and anisometropia both exist) amblyopia. The prevalence of these types gives the impression that they are influenced by the age of their occurrence; for children less than 3 years old, 50% present with strabismus and 18% present with anismetropia. However, reverse ratio appears in adults; Attebo et al. [6] found that in 50% of the adult patients, the reason of amblyopia was anisometropia while strabismus was accountable for only 19% of the cases. A possible explanation for this difference in frequency is that anisometropia may develop later or that it may need a longer duration to induce amblyopia [7].
It was suggested that there are two mechanisms for amblyopia, lack of satisfactory visual stimulation during infancy, causing visual deficiency and the abnormal binocular interaction [8].
The visual evoked potential (VEP), which is a method which shows the changes of the electrical potentials created by the brain which are recorded over the occipital cortex as a result of visual stimulations, is studied. This mostly diagnoses the affection of the optic nerve and visual pathways. The VEP is a practical, objective and efficient diagnostic method which displays the activity of the visual system at the level of the occipital visual cortex. The stimulation initiates from the massed activity of a huge number of cortical neurons [9].
The aim of electrophysiological studies in amblyopia is to understand the mechanism of diminution of visual acuity and reveal the position of major defects and their depth [3-5]. FVEPs are less sensitive than PVEPs to dysfunction of the visual projection pathways. Their use in clinical testing is generally limited to: (1) subjects with severe refractive errors or opacity of ocular media who cannot visually resolve a pattern stimulus and (2) subjects who are too young or too uncooperative to reliably fixate on a pattern stimulus.
Although the difference in visual acuity and refraction are important in diagnosis of amblyopia, there is still a need for a diagnostic tool to diagnose amblyopia in nonverbal infancy and early childhood, thus we will compare between flash visual evoked potential and pattern visual evoked potential for the diagnosis of amblyopia.
Aim of the work
To compare the responses of flash visual evoked potential (FVEP) & pattern visual evoked potential (PVEP) in amblyopic eyes with the eyes of healthy children and to find out whether the flash VEP can help us in the diagnosis of amblyopia as it is the only test applicable to nonverbal and disabled children.
Subjects and Methods
This cross-sectional study, from June 2013 to July 2014, included 60 patients (4-10 years old) with strabismic amblyopia which came to the outpatient clinic of Ain shams university hospital, they were selected via simple random sampling, as well as thirty age and sex matched healthy children included as a control group. The participating children were chosen in this age group to facilitate performing VEP without general anesthesia.
Amblyopia is diagnosed when the difference in visual acuity between the two eyes is greater than two lines as stated by Dr. Hahn's standard test chart (unilateral amblyopia) [10].
The participants were examined for the following inclusion criteria: unilateral amblyopia, at least a 2-line difference with the best optical correction in the visual acuity test between the eyes, stable fixation, clear media, normal fundus, age between 4 and 10 years and cooperative. All the children in the control group had full vision and were cooperative.
The exclusion criteria consisted of pathological complications as determined by ophthalmological evaluations, presence of a history of neurological diseases, previous eye surgery, abnormal retinal correspondence with the Bagolini test, and vertical deviations secondary to surgery. The participants' fixation was carefully evaluated by direct ophthalmoscopy.
All the procedures conducted in the studies which involved human participants were in accordance with the ethical standards of the Faculty of Medicine-Ain Shams University research ethics committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from the guardians of all the children before the study began.
Subjective visual acuity was measured monocularly by a standard Snellen distance chart. A cycloplegic refraction was performed at least 20 min after instillation of 1% cyclopentolate hydrochloride and 1% tropicamide (instilled 5 minutes apart) using autorefraction (Topcon KR-8800, Japan). Spherical equivelant (SE) was calculated as the amount of the spherical power and half of the cylinderical power [11]. Slit-lamp and fundus examination were preformed to exclude any abnormality of anterior and posterior segment. The orthoptic evaluation comprised of objective measurment of the angle of deviation and the assessment of fixation characteristics which was conducted by using visoscopy. Stereoacuity was conducted with the TNO Stereotest (Clement Clark International, UK) for all participatnts. We checked suppression with the worth 4-dot test. All participatnts had stable fixation. Amblyopia subjects were tested prior occlusion/penalization therapy.
Pattern visual evoked potentials (PVEP) recording
PVEP recording apparatus comprised of a Roland Reti (Model ISXEV 60, Germany), pupils were undilated, stimulation was monocular, eyes were refracted for the stimulus seeing distance (70 cm), parameters of the stimulation were as follows: 21″ cathode ray tube (CRT) monitor with a frame rate of 75 frames per second (fps), black/white reversing checkerboard was presented to the patient with a check size of 1°4′ and 0°16′, Michelson contrast was 97%, temporal frequency of 2 reversals per second (rev/s) (1 Hz) and luminance for the white elements was 120 candelas per square meter (cd/m2+) (mean luminance: 55 cd/m2).
According to the International 10/20 system, the scalp electrodes should be placed relative to bony landmarks, in proportion to the size of the head. The active-gold disk electrode is placed on the scalp over the visual cortex at Oz , the reference-gold disk electrode at Fz, while ground-gold disk electrode was placed on the forehead. Central fixation was preserved and properly monitored.
Recording system parameters were as follows: filters of frequency 1-100 Hz (notch filters were converted off), artifact reject threshold was 50 μV, sweep time of 300 milliseconds (ms), average of 100 sweeps. Two consecutive waveforms were recorded, then averaged off-line and analysed. The amplitude and latency of the P100-wave with manual correction to the automatic cursor placement were also analyzed. In order to estimate the bioelectrical function of macular versus the peripheral regions of the retina, stimulation with the small (0°16′) and large (1°4′) check stimuli were used. The PVEP test parameters were in accordance with the International Society for Clinical Electrophysiology of Vision (ISCEV) standard [12].
Flash visual evoked potentials (FVEP) recording
The FVEP recording equipment consisted of a Roland Reti (Model ISXEV 60, Germany), flash visual evoked potential produced by a brief luminance increment and a flash, which subtends a visual field of at least 20 deg, and the requirements were as follows; pupils were undilated and stimulation was monocular.
Electrodes: the active-gold disk electrode was placed on the scalp over the visual cortex (one centimetre above inion), the reference-gold disk electrode was placed at mid-point of a hypothetical line between inion and nose base), and the ground-gold disk electrode was placed on the forehead. The electrodes were positioned relative to bone landmarks according to the international 10/20 system. Central fixation was preserved and properly monitored.
The flash VEP was produced by a brief flash that subtends a visual field of at least 20 deg, existing in a dimly illuminated area. The strength (time-integrated luminance) of the flash stimulus was 3 (2.7-3.3) photopic candelas seconds per meter squared (cd•s•m-2). This can be attained by using an integrating bowl (ganzfeld).
The flash rate was 1 per second (1.0 Hz ± 20%). The VEP to flash stimulation was made up of a series of negative and positive waves. The earliest noticeable component had a peak time of about 30 ms post-stimulus and components were recordable and their highest latencies were up to 300 ms. Peaks are nominated as negative and positive in a numerical sequence. This reading is used to distinguish the flash VEP from the pattern-reversal VEP. The most characteristic components of the flash VEP are the N2 and P2 peaks. Calculations of P2 amplitude were made from the positive P2 peak at about 120 ms to the preceding N2 negative peak at around 90 ms. The FVEP test parameters were in agreement with the International Society for Clinical Electrophysiology of Vision (ISCEV) standard [12].
The VEP recording was repeated 3 times in cases where the participant's cooperation was poor. The fixation stability of the eyes was monitored closely by an experienced ophthalmologist.
Statistical analysis
We studied data using the Statistica software (version 8). Quantitative variables were stated as mean ± SD. Student's t-test was used to assess the statistical significance of differences between two groups. Descriptive statistics were done using chi-square analysis. Correlation analyses were achieved by calculating Pearson correlation coefficient (r). Regression analyses were done to assess the different factors that could affect visual evoked potential latency. P values were considered statistically significant if < 0.05.
Results
Participants were classified into two groups: amblyopic group (26 F/34 M) and control group (11 F /19 M). No gender differences were found between the two studied groups (χ2 = 0.37 and p = 0.54).
The general characteristics of patients and controls are shown in table 1. No significant differences in age was found between the two studied groups.
![]()
Table 1: The comparison between amblyopic and control group regarding basic characteristics.
View Table 1
Visual acuity (VA) and best corrected visual acuity (BCVA) of the amblyopic group were statistically lower than that of the control group. Both groups showed stable fixation, normal fundus, clear media, no abnormal retinal correspondence with the Bagolini test and no vertical deviations.
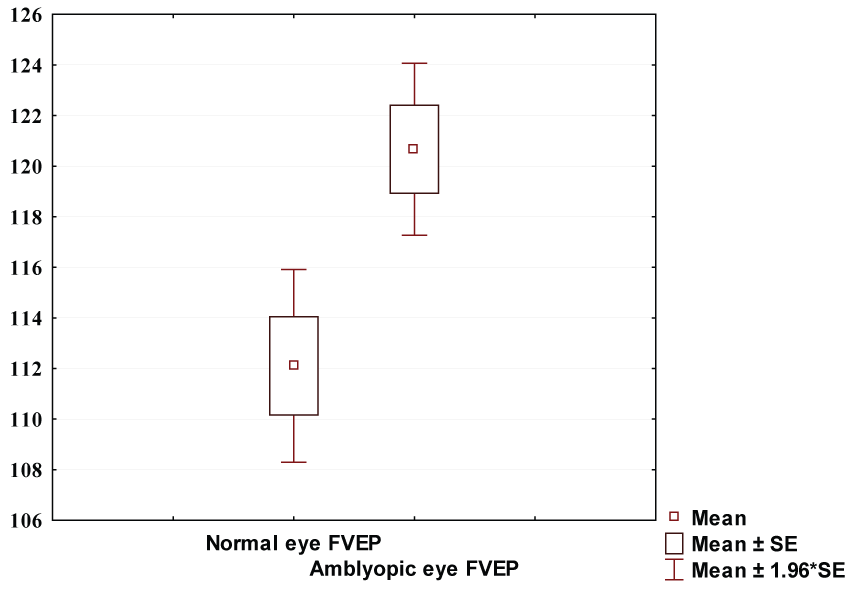
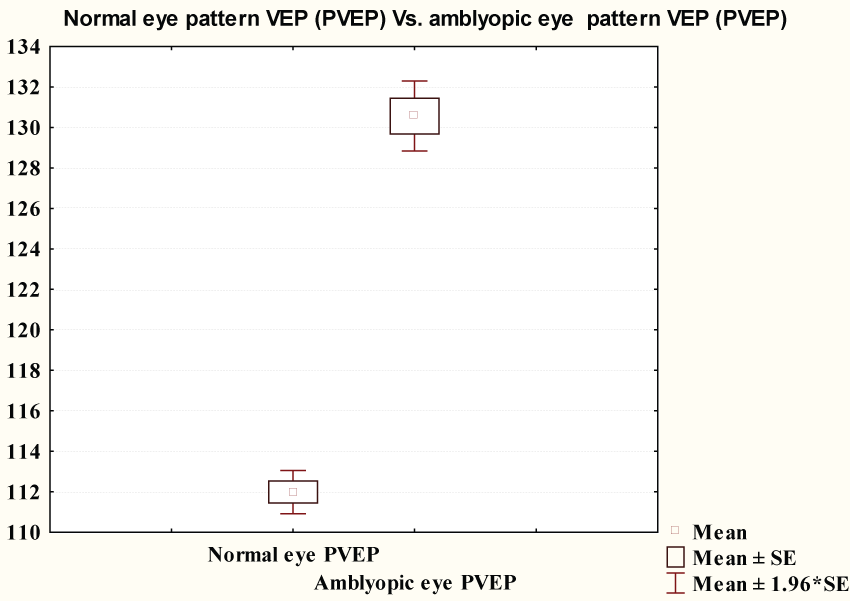
Flash VEP P100 latency showed no significant difference between the two studied groups (120.6 ± 13.45 & 116.0 ± 14.7 ms respectively) (p = 0.07). Figure 1 showed pattern VEP P100 latency in amblyopic group (130.5 ± 6.8 ms) was significantly longer than that of control group (111.9 ± 4.2 ms) (p ≤ 0.0001) (Figure 2).
.
Figure 2: Comparison between PVEP of controls and PVEP P100 latency of amblyopic group.
View Figure 2
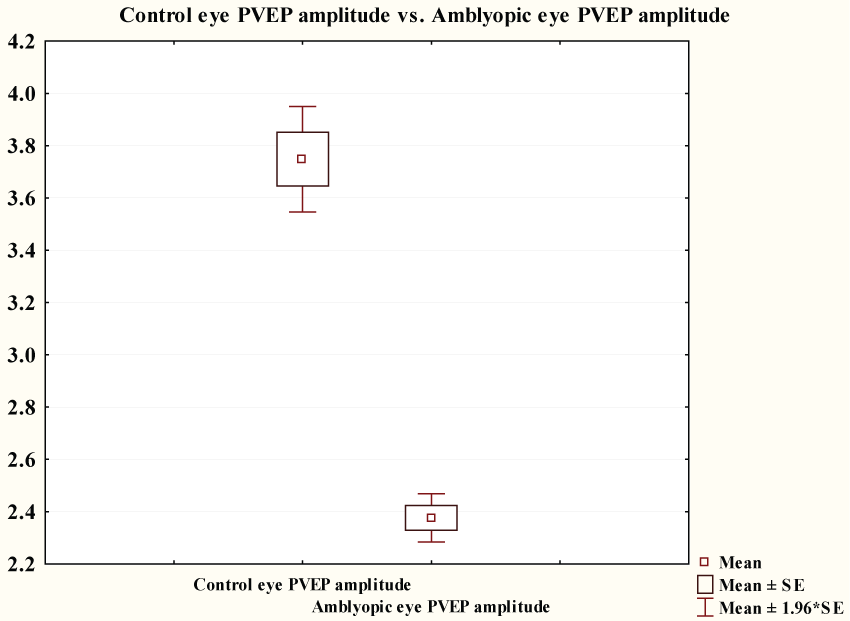
Figure 3 showed that pattern VEP P100 amplitude of amblyopic group (2.38 ± 0.36 μV) was significantly lower than that of control group (3.75 ± 0.79 μV) (p ≤ 0.0001).
.
Figure 3: Comparison between PVEP of controls and PVEP P100 amplitude of amblyopic group.
View Figure 3
Amblyopic eye FVEP (120.67 ± 13.45 ms) was significantly lower than that of amblyopic eye PVEP (132.73 ± 9.7 ms) with (p < 0.0001) .
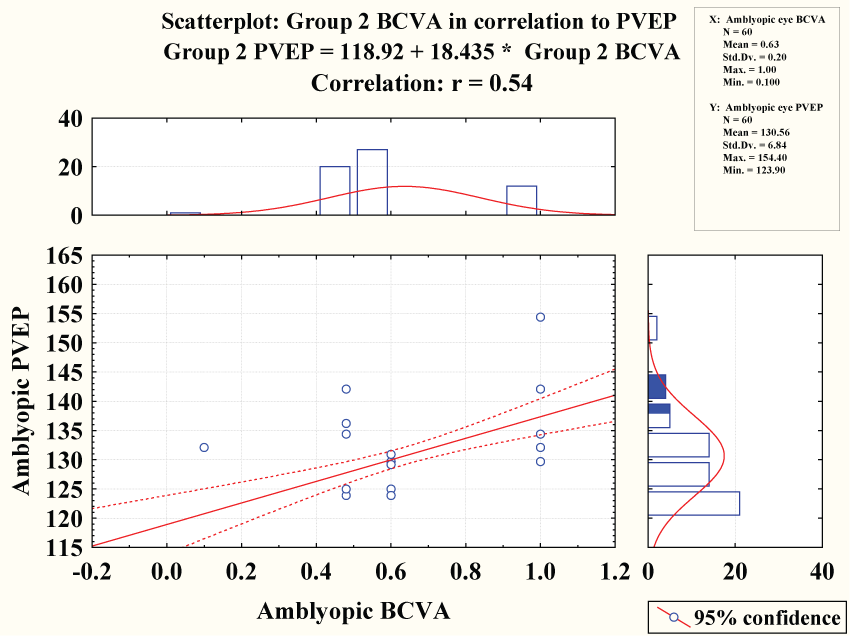
Amblyopic best corrected visual acuity showed negative correlation with pattern VEP latency (r = 0.54 & P < 0.0001) however it showed no significant correlation with flash VEP latency (r = 0.06 = & P = 0.66) figure 3.
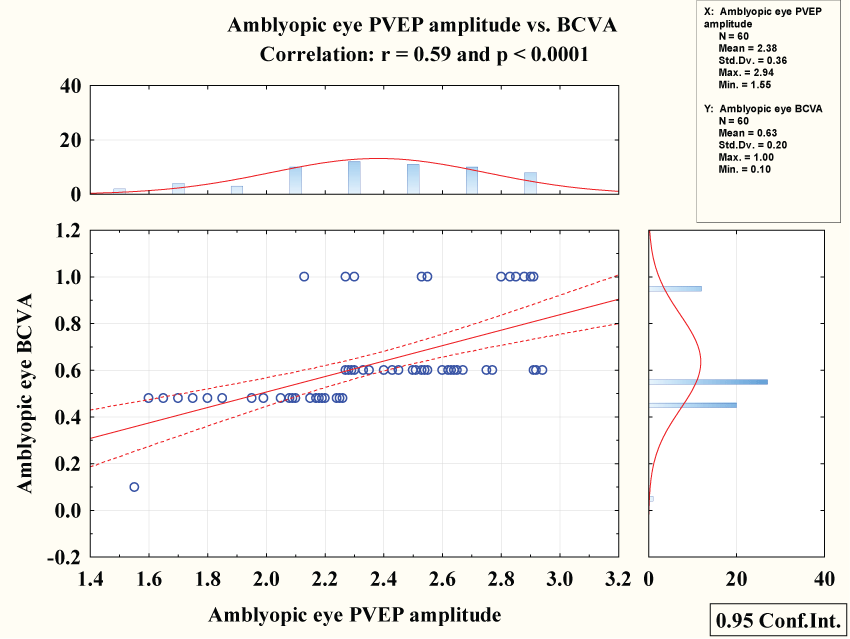
Amblyopic best corrected visual acuity showed negative correlation with pattern VEP latency (r = 0.54 & P < 0.0001) figure 4 while showed positive correlation with pattern VEP amplitude (r = 0.59 & P < 0.0001) figure 5.
We plotted multiple regression analyses for amblyopic eyes to show which factors in the study were the most important determinants of FVEP latency. We found that FVEP latency was not attributable to gender, VA and BCVA. Age was the most important determinant of FVEP latency (β = -0.38 & p = 0.04).
When we plotted multiple regression analyses for amblyopic eyes to show which factors in the study were the most important determinants of PVEP latency. We found that PVEP latency was not attributable to age and gender. VA and BCVA were the most important determinants of PVEP latency (β = 0.37 & 0.50 and & p = 0.02 & < 0.0001 respectively).
When we plotted multiple regression analyses for amblyopic eyes to show which factors in the study were the most important determinants of PVEP amplitude. We found that PVEP latency was not attributable to age, gender, VA and SE. BCVA were the most important determinants of PVEP amplitude (β = 0.58 & p < 0.0001).
Discussion
We tried to find another method other than visual acuity to diagnose and monitor the effect of treatment for amblyopia. Visual evoked potential has been proposed as another method to diagnose and detect the amount of expected vision improvement upon initiation of treatment [13-18].
In our study, we found that group 2's (ambylopic group) pattern VEP P100 latency (130.5 ± 6.8 ms) was statisticaly longer than group 1's (Control group) (111.9 ± 4.2 ms) [p ≤ 0.0001]. This is in agreement with Hosseinmenni et al. [19] who found P100 latency in amblyopic eyes was significantly increased compared to the normal group. Also Parisi et al. [20] stated that amblyopic eyes showed a significant increase in VEP P100 implicit time in comparison to the values observed in sound eyes and control eyes, may be attributable to a delay in postretinal neural conduction.
We also found negative significant correlation between pattern visual evoked potential latency with the best corrected visual acuity of patients with amblyopia (p ≤ 0.0001); while its correlation with flash visual evoked potential was insignificant (p = 0.43). Karlica et al. [21] found a statistically significant correlation between visual evoked potential amplitude values and visual acuity as well as between latency values and visual acuity. Also, Kubova et al. [22] reported that the amplitude of pattern-reversal VEPs was significantly smaller, the latency significantly longer and the magnitude of those changes correlated with the reduction of VA. Xiao et al. [23] found a significant prolongation of the latency and a decrease of amplitude of P100 waves was observed in the amblyopia group compared with the healthy control group. Heravian et al. [24] found that The P100 latency in PVEP was increased in strabismic amblyopia.
On the other hand, Halfeld Furtado de Mendonça et al. [25] found that there is no statistically significant difference between the amblyopic and sound eye of amblyopic children in the strabismic group for VEP P1 amplitude and latencies for any check sizes. Davis et al. [26] reported that strabismic amblyopes often showed supernormal flash visual evoked responses in the affected eye.
Regression analysis showed that the FVEP latency was not related to gender, VA and BCVA, while VA and BCVA were the most important determinant of the PVEP latency (β = 0.37 & 0.50 and & p = 0.02 & < 0.0001 respectively). Parisi et al. [27] confirmed that VEP P100 implicit time showed the highest sensitivity/specificity for the recognition of a visual dysfunction.
Conclusion
Pattern VEP could be applied to diagnose amblyopia, but we cannot rely on the FVEP in this context as proved with multiregression analysis which showed that the pattern VEP latency and amplitude are the most important determinant factor for amblyopic best corrected visual acuity.
Limitations
The small sample size and lack of multicentrality could be considered the prominent limitations in the our study. We didn't have child-friendly VEP systems so we had to repeat the test several times and exclude uncooperative children (limited facilities). We would suggest that further studies recruiting adequate numbers of subjects in multi-central locations be conducted to analyze the latency of VEP.
Competing Interests
The authors declare that they have no competing interests regarding the publication of this paper.
Conflict of Interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speaker's bureaus; membership, employment, consultancies, stock ownership or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Support
Costs were the responsibility of the authors and the instruments used in the study belong to Faculty of Medicine, Ain Shams University, a public government organization.
Acknowledgments/Disclosure
• The research was not funded All authors completed and submitted the ICMJE form for disclosure of potential conflict of interest. The authors indicate no relationships/conditions/circumstances that present a potential financial conflict of interest. Costs were the responsibility of the authors and instruments used in the study belong to Faculty of Medicine, a part of Ain Shams University, which is a public governmental organization. All authors attest that they meet the current ICMJE requirements to qualify as authors.
• No other financial disclosures.
Funding
No funding. Costs were the responsibility of the authors and instruments used in the study belong to Faculty of Medicine, a part of Ain Shams University, which is a public governmental organization.
References
-
Ciuffreda KJ, Levi DM, Selenow A (1991) Amblyopia: Basic and Clinical Aspects. Butterworth-Heinemann Press, Stoneham, MA.
-
Holmes JM, Clarke MP (2006) Amblyopia. Lancet 367: 1343-1351.
-
Heravian J, Ostadimoghadam H, Yekta AA, Hasanabadi H, Mahjoob M (2008) Simultaneous Pattern VEP in response to monocular and binocular stimulation in normal and amblyope subjects. Iran Red Crescent Med J 10: 69-74.
-
Wright KW, Eriksen KJ, Shors TJ (1987) Detection of amblyopia with P-VEP during chloral hydrate sedation. J Pediatr Ophthalmol Strabismus 24: 170-175.
-
Sokol S (1983) Abnormal evoked potential latencies in amblyopia. Br J Ophthalmol 67: 310-314.
-
Attebo K, Mitchell P, Cumming R, Smith W, Jolly N, et al. (1998) Prevalence and causes of amblyopia in an adult population. Ophthalmology 105: 154-159.
-
Birch EE (2012) Amblyopia and binocular vision. Prog Retin Eye Res 33: 67-84.
-
von Noorden GK (1985) Amblyopia: a multidisciplinary approach. Proctor lecture. Invest Ophthalmol Vis Sci 26: 1704-1716.
-
Ridder WH, Rouse MW (2007) Predicting potential acuities in amblyopes: predicting post-therapy acuity in amblyopes. Doc Ophthalmol 114: 135-145.
-
Baker DH, Simard M, Saint-Amour D, Hess RF (2015) Steady-state contrast response functions provide a sensitive and objective index of amblyopic deficits. Invest Ophthalmol Vis Sci 56: 1208-1216.
-
Gupta P, Cheung CY, Saw SM, Bhargava M, Tan CS, et al. (2015) Peripapillary choroidal thickness in young Asians with high myopia. Invest Ophthalmol Vis Sci. Feb 56: 1475-1481.
-
Odom JV, Bach M, Brigell M, Holder GE, McCulloch DL, et al. (2010) ISCEV standard for clinical visual evoked potentials standard (2009 update). Doc Ophthalmol 120: 111-119
-
Palmer EZ (1992) Amblyopia. Prepared by the American Academy of Ophthalmology, Quality of Care Committee. Pediatric Ophthalmology Panel 1-24.
-
Iliakis E, Moschos M, Hontos N, Tsalouki JK, Chimonidou E (1996) The prognostic value of visual evoked response latency in the treatment of amblyopia caused by strabismus. Doc Ophthalmol 92: 223-228.
-
Oner A, Coskun M, Evereklioglu C, Dogan H (2004) Pattern VEP is a useful technique in monitoring the effectiveness of occlusion therapy in amblyopic eyes under occlusion therapy. Doc Ophthalmol 109: 223-227.
-
Johansson B, Jakobsson P (2006) Fourier-analysed steady-state VEPs in pre-school children with and without normal binocularity. Doc Ophthalmol 112: 13-22.
-
Simon JW, Siegfried JB, Mills MD, Calhoun JH, Gurland JE (2004) A new visual evoked potential system for vision screening in infants and young children. J AAPOS 8: 549-554.
-
Ohn YH, Katsumi O, Matsui Y, Tetsuka H, Hirose T (1994) Snellen visual acuity versus pattern reversal visual-evoked response acuity in clinical applications. Ophthalmic Res 26: 240-252.
-
Hosseinmenni S, Talebnejad MR, Jafarzadehpur E, Mirzajani A, Osroosh E (2015) P100 Wave Latency in Anisometropic and Esotropic Amblyopia versus Normal Eyes. J Ophthalmic Vis Res 10: 268-273.
-
Parisi V, Scarale ME, Balducci N, Fresina M, Campos EC (2010) Electrophysiological detection of delayed postretinal neural conduction in human amblyopia. Invest Ophthalmol Vis Sci 51: 5041-5048
-
Karlica , Galetović D, Bućan K, Znaor L, Skelin S (2009) Changes in visual evoked potentials curve parameters in children with amblyopia. Acta Med Croatica 63: 321-324.
-
Kubová Z, Kuba M, Juran J, Blakemore C (1996) Is the motion system relatively spared in amblyopia? Evidence from cortical evoked responses. Vision Res 36: 181-90.
-
Xiao M, Wei X, Li Y, Xiong W, Xu S (2013) Pattern visual evoked potentials in normal-vision eyes of post-therapy amblyopia. Zhong Nan Da Xue Xue Bao Yi Xue Ban 38: 704-708.
-
Heravian J, Daneshvar R, Dashti F, Azimi A, Ostadi Moghaddam H, et al. (2011) Simultaneous pattern visual evoked potential and pattern electroretinogram in strabismic and anisometropic amblyopia. Iran Red Crescent Med J 13: 21-26.
-
Halfeld Furtado de Mendonça R, Abbruzzese S, Bagolini B, Nofroni I, Ferreira EL, et al. (2013) Visual evoked potential importance in the complex mechanism of amblyopia. Int Ophthalmol 33: 515-519.
-
Davis ET, Bass SJ, Sherman J (1995) Flash visual evoked potential (VEP) in amblyopia and optic nerve disease. Optom Vis Sci 72: 612-618.
-
Parisi V, Miglior S, Manni G, Centofanti M, Bucci MG (2006) Clinical ability of pattern electroretinograms and visual evoked potentials in detecting visual dysfunction in ocular hypertension and glaucoma. Ophthalmology 113: 216-28.