International Journal of Ophthalmology and Clinical Research
The Accuracy of Automated Angle Closure Screening Software
Anita Manassakorn1*, Chonlada Theeraworn2, Pished Bunnun2, Sirichai Nithi-Uthai2, Rangsarit Vanijjirattikhan2, Kittipong Ekkachai2, Waree Kongprawechnon3, Toshiaki Kondo3, Direk Patikulsila4 and Kanokvate Tungpimolrut2
1Department of Ophthalmology, Faculty of Medicine, Chulalongkorn University, Thailand
2National Electronics and Computer Technology Center, Klong Luang, Pathumthani, Thailand
3Sirindhorn International Institute of Technology, Thammasat University, Pathumthani, Thailand
4Department of Ophthalmology, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
*Corresponding author: Anita Manassakorn, MD, Department of Ophthalmology, Faculty of Medicine, Chulalongkorn University, 1873 Rama IV Road, Patumwan, Bangkok 10330, Thailand, E-mail: animanassa@gmail.com, animanassa@yahoo.com
Int J Ophthalmol Clin Res, IJOCR-3-060, (Volume 3, Issue 3), Research Article; ISSN: 2378-346X
Received: December 27, 2015 | Accepted: July 16, 2016 | Published: July 19, 2016
Citation: Manassakorn A, Theeraworn C, Bunnun P, Nithi-Uthai S, Vanijjirattikhan R, et al. (2016) The Accuracy of Automated Angle Closure Screening Software. Int J Ophthalmol Clin Res 3:060. 10.23937/2378-346X/1410060
Copyright: © 2016 Manassakorn A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: To evaluate the accuracy of automated angle closure screening software and van Herick grading (VH) compared to gonioscopy.
Methods: Peripheral anterior chamber angle images of 27 eyes were taken by slit lamp biomicroscope and analyzed by automated software. The software selected region of interest and applied a light reflection extraction to detect corneal thickness (CT) and peripheral anterior chamber depth (PACD). Three observers manually counted CT (CTm) and PACD (PACDm) pixels and compared with the CT and PACD pixel counts that were obtained by the software (CTa and PACDa). Inter-rater variability was evaluated using intraclass correlation coefficient (ICC). Bland and Altman plot was used to test agreement between manual and software pixel counts. The accuracy between software and VH vs. gonioscopy was analyzed using the area under receiver operating characteristic curve (AUC).
Results: Mean ± SD CTm counts were 64.6 ± 7.9, 66.2 ± 8.8, and 64.6 ± 7.5 pixels and PACDm counts were 65.8 ± 40.1, 62.7 ± 39.2, and 64.1 ± 39.7 pixels. The ICC (95% CI) for CTm and PACDm were 0.957 (0.918-0.979) and 0.997 (0.993-0.998). The limits of agreement in the Bland and Altman plot between manual and automated pixel counts for CT and PACD were -9.6 to 6.6 (mean ± SD difference: -1.5 ± 4.1) and -10.6 to 9.3 pixels (mean ± SD difference: -0.7 ± 5.1). With gonioscopy as a reference, AUCs of PACDa, PACDm, VH, CTa, and CTm were 0.818, 0.814, 0.809, 0.705, and 0.577, respectively.
Conclusion: The software exhibited excellent reliability and accuracy in the measurement of PACD for angle closure screening comparable to VH grading.
Keywords
Angle closure glaucoma, Glaucoma screening, Limbal anterior chamber depth, Peripheral anterior chamber depth, Van Herick classification
Abbreviations
ACA: Anterior Chamber Angle; AUC: Area under Receiver Operating Characteristic Curve; CT: Corneal Thickness; CTa: Corneal Thickness as Measured by Automated Software; CTm: Corneal Thickness as Measured by Manual Counting; ICC: Intraclass Correlation Coefficient; PACD: Peripheral Anterior Chamber Depth; PACDa: Peripheral Anterior Chamber Depth as Measured by Automated Software; PACDm: Peripheral Anterior Chamber Depth as Measured by Manual Counting; PACG: Primary Angle Closure Glaucoma; POAG: Primary Open Angle Glaucoma; ROI: Region of Interest; SPAC: Scanning Peripheral Anterior Chamber Depth Analyzer; VH: van Herick grading
Introduction
Glaucoma is the leading cause of irreversible blindness. The key challenge in detecting glaucoma is the asymptomatic characteristic of the disease. Patients may realize that they have peripheral visual field reduction only in the advanced and irreversible stages. Even in developed countries, about half of all glaucoma patients were not diagnosed [1]. Moreover, we still do not have a reliable and cost effective screening tool [2,3]. Primary open angle glaucoma (POAG) has a higher overall prevalence than primary angle closure glaucoma (PACG); however, PACG poses a greater threat to sight and is the type of glaucoma most often found in Asia [1,4].
Evaluation of the anterior chamber angle (ACA) is crucial. Although gonioscopy is currently the gold standard, it is a subjective qualitative method that is time consuming and requires a skilled observer. In addition, gonioscopy requires direct contact with the eye, which limits its use for screening purposes. Many ACA evaluation instruments, such as ultrasound biomicroscopy, anterior segment optical coherence tomography, and Scheimpflug imaging, were developed to provide quantitative results. However, major limitations of these instruments include high cost, lack of portability, invasiveness, and the need for experienced technicians. For angle closure screening, the flash light test is often used by non-medical healthcare personnel, however, agreement between observers has been low [5]. The van Herick (VH) grading system is another approach used to screen whether angle closure is likely to occur, with interobserver agreement of 0.50-0.73 [6,7], with 61.9% sensitivity, and 89.3% specificity, as compared with gonioscopy [6]. In addition, agreement among optometrists and between optometrists and ophthalmologist using VH system was moderate (κ = 0.54-0.65) [7,8].
A key advantage of angle closure screening is the prevention of acute angle closure attack; a disorder that can be treated by laser peripheral iridotomy with a success rate of 98% [9-11]. This diagnostic method can also benefit for new patients who have PACG, but still asymptomatic and have not yet received treatment. However, a shortage of ophthalmologists, especially in rural areas, has limited this diagnostic capability. To augment the ACA screening technique, we developed the automated software to measure temporal corneal thickness (CT) and peripheral anterior chamber depth (PACD). We evaluated the accuracy of the software application by comparing its results with van Herick grading and gonioscopy.
Methods
This pilot study composed of 2 component parts. The angle closure screening software was developed at the National Electronics and Computer Technology Center, Pathumthani, Thailand. Patient clinical examination was performed at the Department of Ophthalmology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand. This study protocol, including study protocol, patient information sheet and the consent form were approved by the Institutional Review Board of Chulalongkorn University, in accordance with the Declaration of Helsinki. All participants were informed about the study protocol by principle investigator (AM) and gave written informed consents before participate in the study. Inclusion criteria for this study were as follows: participants at least 40 years of age with no history of eye trauma or surgery, spherical refractive error < ± 6 diopters, and astigmatism < 3 diopters. Both open and angle closure are included. Angle closure was defined if the posterior trabecular meshwork was not seen at least 270° [12]. Informed consent was obtained from all subjects after an explanation of the nature and possible consequences of the study. Before being including in the study, all participants underwent complete eye examination with gonioscopy, using the Magna View Gonio Lens (Ocular Instruments Inc., Bellevue, WA, USA) by 1 observer (AM), within 1 week before the start of the study. On the examination day, slit lamp images were taken at the most temporal area, including cornea, peripheral anterior chamber, and iris, at 60 degrees using 1 mm width brightest light beam with 16x magnification (Topcon slit lamp, Model DC-3, Topcon Corporation, Tokyo, Japan) by 1 observer (AM). All images were then randomly rearranged by 1 observer (CT) and then classified by 3 ophthalmologists (AM, DP, LM), using the VH technique to determine either angle closure or open angle. According to VH classification, the angle is closed when CT is less than ¼ of PACD. Two out of three results were used to reach a final decision.
The development principle for the software was described previously [13,14] (Figure 1). In brief, a slit lamp image of 3.264 × 2.448 pixels was registered into the software. The image size was then reduced to 640 × 480 pixels to accelerate processing. To identify the region of interest (ROI), excluding eyelid, sclera and other unnecessary regional components, top-hat transform was applied and the image was split into red and blue channels for the purpose of performing channel subtraction. At this stage, the horizontal position of the light reflected onto the iris was recognized. The channel-subtracted image was then cropped at the left and right bounds and is performed with the roughly light reflection extraction to clearly identify the upper and lower end of the light reflected onto the iris. Subsequently, the identified ROI was scaled up to the original image size for purposes of cropping the ROI image from the original slit lamp image. Light reflection extraction was then applied to get the light reflected onto the cornea and iris components. There were 2 main processes to this step: extraction of the light reflected onto the iris and light reflected onto the cornea. These 2 processes were similar, except that the later process required 2 additional steps: image intensity clipping and power-law transformation. The process began by applying top-hat transform to an overexposed slit lamp image to remove excessive light reflections. Then, ROI images were classified into 2 types. The first type was adequately illuminated and the second type was over-exposed, with an extremely bright spot on the cornea.
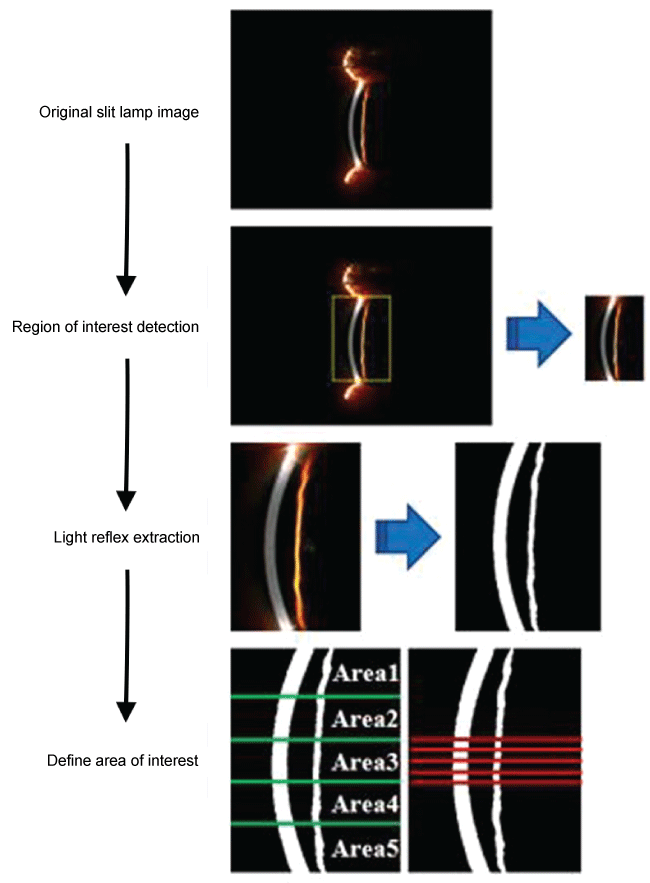
.
Figure 1: Process flowchart for angle closure screening software.
Process flowchart for angle closure screening software. From the original picture, region of interest was detected and cropped. After that, each light components were extracted to get each light bands and combined them together in one black and white image. Five horizontal lines in the middle area of the image were defined. Five pairs of peripheral anterior chamber depth (PACD) and corneal thickness (CT) along the lines were counted and used in further processes to give a classification result.
View Figure 1
Then, the image intensity clipping function was initiated to facilitate cornea reflected light extraction. Channel subtraction was then performed in order to make the reflection of interest the brightest component on the grayscale image. If the reflection of interest was the light reflected on the cornea, power-law transformation was used to enhance component intensity. Afterward, the global image threshold, morphological operation, connected-component labeling, and region properties operations were performed to make the reflection of interest more clear and to remove noises from the binary (black and white) image. When all processes in this step were finished, 2 binary images were generated; one image portrayed the light reflected on the iris and the other image, the light reflected on the cornea (Figure 2). These 2 images were then merged using OR operation and the resulting image was used for further calculation.

.
Figure 2:OR operation result.
OR operation was used to combine two extracted light components into one black and white image. This operation was needed for measuring peripheral anterior chamber depth (PACD) because PACD is a pixel gap between both light components.
View Figure 2
Because of limitations of eye exposure in most of the Asian population, the central part of the scanning slit may not be the thinnest part of the cornea. Therefore, CT and PACD are measured through the middle one-fifth of the image in vertical orientation that should cover the thinnest part (Figure 1). Measurement was performed in 5 locations that were equally divided from the top to the bottom of the middle one-fifth area. To avoid the extremely bright spot in some cases, the thinnest CT in that area, instead of mean CT, was used to represent CT in the calculation of PACD (Figure 3). To test the accuracy of pixel detection, all images were shuffled and manually counted (CTm and PACDm) by 3 observers (PB, CT, and SN) who did not know the results of gonioscopy. Intraclass correlation coefficient (ICC) was used to identify any correlation. Then, agreement between the automated CT and PACD pixel counts (CTa and PACDa) from the software and the manual pixel counts using Bland and Altman plot. The diagnostic accuracy of the software was evaluated by comparing the results from manual and automated pixel counting and VH grading to gonioscopy, using the area under receiver operating characteristic curve (AUC). All statistical analyses were performed using SPSS Statistics version 17.0 (SPSS, Chicago, IL, USA).
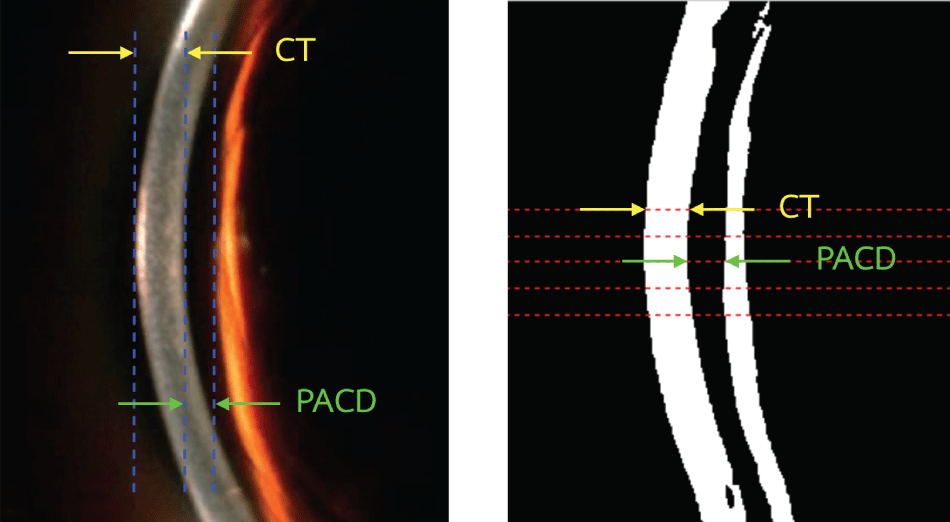
.
Figure 3: Region of interest and landmark of peripheral anterior chamber depth (PACD) and corneal thickness (CT).
Left picture is a region of interest (ROI) taken from a full resolution picture. It was used in the manual counting method. Three vertical dash lines were manually chosen. Counted pixels between these lines were assumed to be PACD and CT, respectively. Right picture was a pre-processed ROI resulted from feeding the left picture into the program. The program automatically chose 5 horizontal dash lines for measuring PACD and CT. It counted pixels in each lines from black to black for PACD (green arrow) and white to white for CT (yellow arrow).
View Figure 3
Results
One eye each from 27 subjects were included from June 2012 to October 2012. Mean (SD) age of the study population was 52.46 (13.10) years old. Four of them (15%) were male. There were 5 angle closure patients as classified by gonioscopy. Mean (SD) CTm and PACDm from 3 observers and ICC are shown in table 1.
![]()
Table 1: Average (SD) of manually corneal thickness (CTm) and peripheral anterior chamber depth (PACDm) pixel count and intraclass correlation coefficient between 3 observers.
View Table 1
Agreement between manual and automated software using Bland and Altman plot was R = 0.991 and 0.818 for PACD and CT, respectively. PACDm was greater than PACDa, mean (SD) difference was 0.7 (5.1) (Figure 4). The limits of agreement were -10.6 and 9.3 pixels. For CT, the mean (SD) difference was -1.5 (4.1) pixels with greater in CTa. The limits of agreement were -9.6 and 6.6 pixels (Figure 5).
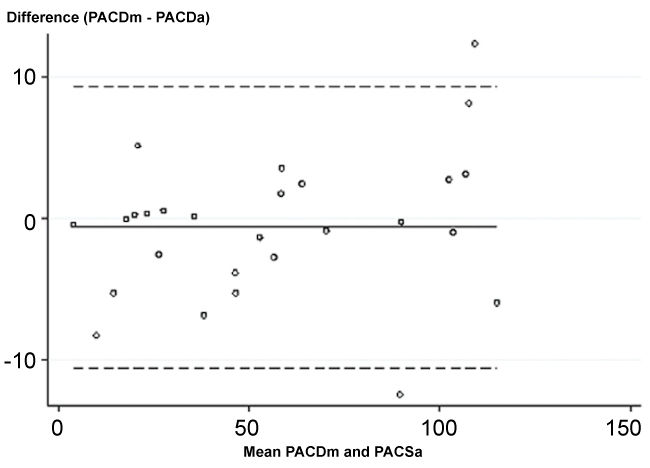
.
Figure 4: Bland and Altman plots of peripheral anterior chamber depth measurement.
PACDm, manually peripheral anterior chamber depth measurement; PACDa, automated peripheral anterior chamber depth measurement.
View Figure 4
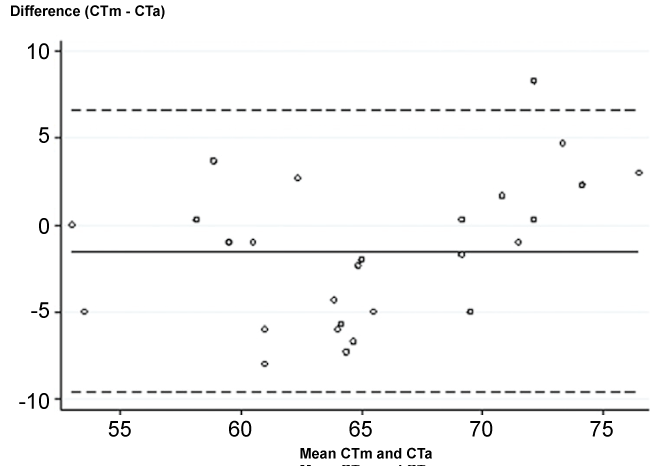
.
Figure 5: Bland and Altman plots of corneal thickness measurement.
CTm, manually corneal thickenss measurement; CTa, automated corneal thickness measurement.
View Figure 5
Area under receiver operator characteristic curve was performed to compare the diagnostic accuracy of the software algorithm and VH method vs. gonioscopy. Using gonioscopy as the gold standard, the AUCs for PACDa, PACDm, VH, CTa, and CTm are shown in table 2.
![]()
Table 2: Area under receiver operator characteristic curve (AUC) for corneal thickness, peripheral anterior chamber depth, and van Herick grading.
View Table 2
We did not find the statistically significant difference of AUCs among all parameters. PACDa and VH showed the greatest sensitivity and specificity of 80.0% and 81.8%. For the PACDa calculation based on the original slit lamp image size of 3.264 × 2.448 pixels, if PACD was less than 27.2 pixels angle closure is likely to occur. (Sensitivity 100.0% and specificity 83.3%).
Discussion
This study investigated the performance of the newly developed ACA screening software that was used to clinically evaluate images taken during routine anterior segment slit lamp examination. Peripheral anterior chamber depth as measured by the software (PACDa) showed very good performance comparable to van Herick grading when gonioscopy was used as a reference (AUC = 0.818 and 0.809 for PACD and VH).
In this study, we developed a robust algorithm to calculate the limbal anterior chamber depth using image-processing technology. This advancement allows healthcare personnel to take pictures and upload them into the software for automatic analysis to assess the possibility of anatomical angle closure. According to our findings, the most reliable and accurate parameter was PACDa. The measurement of the CT was limited by excessively bright corneal reflection that interfered with automated pixel counting. However, when manual counting of CT was performed, the observers excluded areas of extensive brightness; a step that led to observers often arriving at a CT less than that determined by the software. In addition, our algorithm focused on the middle one-fifth area that is centrally located between the upper to lower bounds of the PACD. This programming design aimed to identify the thinnest area of the cornea and widest area of the PACD. From our results and based on the original image size of 3.264 × 2.448 pixels, if PACDa is less than 27.2 pixels, angle closure is likely to happen. In this outcome, we strongly recommend re-evaluation by ophthalmologist using gonioscopy.
In the current clinical environment, there are many tools available for ACA evaluation; however, a limited number of devices are available for screening purposes. What is needed is a low cost, portable, non-contact technique that can be used for mass screening in a non-clinical environment. The simplest method involves the use of a flashlight that is projected at the temporal side of the cornea. Although this is a very simple technique, agreement on observation results between ophthalmologists and healthcare personnel was only 0.42 with low sensitivity, as compared with gonioscopy [5,6,15]. The next step was limbal anterior chamber depth with VH grading. This technique requires trained observers to perform and classify; however, a lack of ophthalmologists in rural areas remains a problem. In addition, the sensitivity and specificity of VH, as compared with gonioscopy, was 61.9% and 89.3%, respectively [6]. Foster et al. proposed modified angle closure grading that used percentage of ratio between PACD and CT, then dividing that outcome into 0%, 5%, 15%, 25%, 40%, 75%, and ≥ 100% [16]. They found that the 5% grade had 91% sensitivity and 93% specificity for the detection of angle closure. However, the grading system proposed in their study is very complicated, requires experienced observers, and is generally not well-adapted for screening.
To identify the ACA, Kashiwagi et al. developed a scanning peripheral anterior chamber depth analyzer (SPAC) for quantitative analysis of the anterior chamber [17]. Their objective was for screening, similar to ours. Their scanning system comprised 21 images with 0.4-mm interval, starting from central to temporal limbus. Values for anterior chamber depth, central corneal thickness, and corneal radius of curvature were then derived. Reproducibility and accuracy were good, but limited generalization in subjects whom difficult to perform [18,19]. In addition, a study in a large community area found that SPAC had limited specificity in narrow angle screening [20].
Our study had some limitations. First, the light of the surrounding environment was controlled, similar to an examination room. However, light may be difficult to control in a rural setting, where a dominant percentage of the screening programs would take place. Secondly, although this software algorithm performed well, the diagnostic accuracy would be further improved if the system eliminate the delay time between the taking and the capture of an image that allows for eye movement to take place. In the near term, an automated ACA screening camera with tracking system will be combined with the screening software to create an angle closure glaucoma screening instrument for the rural setting that does not require an ophthalmologist. Diagnostic ability and reproducibility will then be re-evaluated. Another limitation was observed when conducting an evaluation on a non-uniform anterior chamber, such as a peripheral anterior synechiae. There was also a problem with corneal opacity, with scattering light interfering with the edge detection process.
In summary, this software showed excellent performance in PACD measurement and was found to be comparable with VH. This system could potentially facilitate the screening of the entire population for this disease by imaging each person, evaluating each image with the automated software, and classifying each person as being either open angle or angle closure.
Acknowledgements
The authors would like to thank Dr. Lerprut Mangkornkanokpong for reviewing slit lamp images.
Ethical Statement
This study protocol, including study protocol, patient information sheet and the consent form were approved by the Institutional Review Board of Chulalongkorn University, in accordance with the Declaration of Helsinki. All participants were informed about the study protocol by principle investigator (AM) and gave written informed consents before participate in the study.
Funding Support
Supported by the National Science and Technology Development Agency Fund, Thailand. This paper was delivered as an oral presentation at the 2nd Asia-Pacific Glaucoma Congress, 26-28 September, 2014, Hong Kong.
References
-
Quigley HA, Broman AT (2006) The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 90: 262-267.
-
Congdon NG, He M (2010) Screening and managing eye disease in 2010: an Asian perspective. Am J Ophthalmol 150: 141-143.
-
Maul EA, Jampel HD (2010) Glaucoma screening in the real world. Ophthalmology 117: 1665-1666
-
Yip JL, Foster PJ (2006) Ethnic differences in primary angle-closure glaucoma. Curr Opin Ophthalmol 17: 175-180.
-
Nuriyah Y, Ren XT, Jiang L, Liu XP, Zou YH (2010) Comparison between ophthalmologists and community health workers in screening of shallow anterior chamber with oblique flashlight test. Chin Med Sci J 25: 50-52.
-
Thomas R, George T, Braganza A, Muliyil J (1996) The flashlight test and van Herick's test are poor predictors for occludable angles. Aust N Z J Ophthalmol 24: 251-256.
-
Campbell P, Redmond T, Agarwal R, Marshall LR, Evans BJ (2015) Repeatability and comparison of clinical techniques for anterior chamber angle assessment. Ophthalmic Physiol Opt 35: 170-178.
-
Jindal A, Myint J, Edgar DF, Nolan WP, Lawrenson JG (2015) Agreement among optometrist and ophthalmologists in estimating limbal anterior chamber depth using the van Herick method. Ophthal Physl Opt 35: 179-185.
-
Kraemer C, Gramer E (1997) Long-term follow-up after Nd:YAG laser iridotomy. A pilot study. Invest Ophthalmol Vis Sci 38: s171.
-
Ang LP, Aung T, Chew PT (2000) Acute primary angle closure in an Asian population: long-term outcome of the fellow eye after prophylactic laser peripheral iridotomy. Ophthalmology 107: 2092-2096.
-
Nolan WP, Foster PJ, Devereux JG, Uranchimeg D, Johnson GJ, et al. (2000) YAG laser iridotomy treatment for primary angle closure in east Asian eyes. Br J Ophthalmol 84: 1255-1259.
-
Foster PJ, Buhrmann R, Quigley HA, Johnson GJ (2002) The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol 86: 238-242.
-
Theeraworn C, Kongprawechnon W, Kondo T, Bunnun P, Nishihara A, et al. (2013) Automatic screening of narrow anterior chamber angle and angle-closure glaucoma based on slit-lamp image analysis by using support vector machine. Conf Proc IEEE Eng Med Biol Soc: 5887-5890.
-
Theeraworn C, Kongprawechnon W, Kondo T, Bunnun P, Nishihara A, et al. (2013) Automatic screening algorithm for narrow anterior chamber angle and angle-closure glaucoma based on slit-lamp image analysis. Kasetsart J (Nat Sci) 47: 940-952.
-
Congdon NG, Quigley HA, Hung PT, Wang TH, Ho TC (1996) Screening techniques for angle-closure glaucoma in rural Taiwan. Acta Ophthalmol Scand 74: 113-119.
-
Foster PJ, Devereux JG, Alsbirk PH, Lee PS, Uranchimeg D, et al. (2000) Detection of gonioscopically occludable angles and primary angle closure glaucoma by estimation of limbal chamber depth in Asians: modified grading scheme. Br J Ophthalmol 84: 186-192.
-
Kashiwagi K, Kashiwagi F, Toda Y, Osada K, Tsumura T, et al. (2004) A newly developed peripheral anterior chamber depth analysis system: principle, accuracy, and reproducibility. Br J Ophthalmol 88: 1030-1035.
-
Kashiwagi K, Kashiwagi F, Hiejima Y, Tsukahara S (2006) Finding cases of angle-closure glaucoma in clinic setting using a newly developed instrument. Eye (Lond) 20: 319-324.
-
Kashiwagi K, Tsumura T, Tsukahara S (2006) Comparison between newly developed scanning peripheral anterior chamber depth analyzer and conventional methods of evaluating anterior chamber configuration. J Glaucoma 15: 380-387.
-
Lavanya R, Foster PJ, Sakata LM, Friedman DS, Kashiwagi K, et al. (2008) Screening for narrow angles in the singapore population: evaluation of new noncontact screening methods. Ophthalmology 115: 1720-1727, 1727.





