International Journal of Ophthalmology and Clinical Research
Inflammation and Oxidative Stress in Retinal Diseases: The Role of Intracellular Signaling in the Retinal Pigment Epithelium
Melina V. Mateos1,2*, Paula E. Tenconi1,2, Norma M. Giusto1,2 and Gabriela A. Salvador1,2
1Instituto de Investigaciones Bioquímicas de Bahía Blanca (INIBIBB), Universidad Nacional del Sur (UNS), Argentina
2Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina
*Corresponding author:
Melina V. Mateos, Instituto de Investigaciones Bioquímicas de Bahía Blanca, CONICET-Bahía Blanca and Universidad Nacional del Sur, Edificio E1, Camino La Carrindanga km 7, 8000 Bahía Blanca, Argentina, Tel: ++54-291-4861201, Fax: ++54-291-4861200, E-mail: mvmateos@criba.edu.ar
Int J Ophthalmol Clin Res, IJOCR-2-033, (Volume 2, Issue 4), Review Article; ISSN: 2378-346X
Received: June 29, 2015 | Accepted: August 19, 2015 | Published: August 23, 2015
Citation: Mateos MV, Tenconi PE, Giusto NM, Salvador GA (2015) Inflammation and Oxidative Stress in Retinal Diseases: The Role of Intracellular Signaling in the Retinal Pigment Epithelium. Int J Ophthalmol Clin Res 2:033. 10.23937/2378-346X/1410033
Copyright: © 2015 Mateos MV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The retinal pigment epithelium (RPE) is essential for the integrity and function of the retina. RPE cells exert key functions to maintain photoreceptors' (PRs) viability and functionality, such as light absorption and protection against photo-oxidation, phagocytosis of photoreceptor outer segments (POS), transport of nutrients and water, secretion of several growth factors and reisomerization of all-trans-retinal. The RPE is also part of the outer blood-retinal barrier (BRB) and can secrete immunomodulatory molecules. This review summarizes signaling events elicited in RPE cells under stress conditions, such as bacterial endophthalmitis, hyperglycemia and oxidative stress (OS). Inflammation and OS participate in the pathogenesis of several retinal diseases that eventually end in vision loss and blindness, such as age-related macular degeneration (AMD), diabetic retinopathy (DR), retinitis pigmentosa and uveitis. Elucidating the molecular events involved in the inflammatory process in the RPE could thus lead to the discovery of new therapeutic targets for the treatment of retinal degenerative diseases.
RPE response to inflammatory situations can mediate retinal damage or survival depending on the inflammatory context and stress duration. Independently of the nature of the stress inductor, intracellular events involved in RPE cell damage could be postulated as therapeutic targets for the treatment of ocular inflammatory diseases, among them: extracellular signal-regulated kinase (ERK) as well as the nuclear transcription factor-κB (NF-κB) activation and increased inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression.
Keywords
Retinal pigment epithelium, Inflammation, oxidative stress, Age-related macular degeneration, Diabetic retinopathy, Lipopolysaccharide
Abbreviations
AMD: Age-Related Macular Degeneration, BRB: Blood-Retinal Barrier, COX: Cyclooxygenase, DAG: Diacylglycerol, DR: Diabetic Retinopathy, ER: Endoplasmic Reticulum, ERK: Extracellular Signal-Regulated Kinase, GLUT: Glucose Transporter, HG: High Glucose, IL: Interleukin, iNOS: Inducible Nitric Oxide Synthase, JNK: Jun Kinase, LPS: Lipopolysaccharide, LPPs: Lipid Phosphate Phosphatases, MAPKs: Mitogen-Activated Protein Kinases, MEK: MAPK kinase, NF-κB: Nuclear Transcription Factor-κB; NO: Nitric Oxide, NPD1: Neuroprotectin 1, OS: Oxidative Stress, PA: Phosphatidic Acid, PC: Phosphatidylcholine, PEDF: Pigment Epithelial-Derived Factor, PGs: Prostaglandins, PI3K: Phosphatidylinositol 3-kinase, PKC: Protein Kinase C, PLD: Phospholipase D, POS: Photoreceptor Outer Segments, PR: Photoreceptor, ROS: Reactive Oxygen Species, RPE: Retinal Pigment Epithelium, SOD: Superoxide Dismutase, VEGF: Vascular Endothelial Growth Factor.
Introduction
Inflammation and oxidative stress (OS) are common factors involved in the pathogenesis of several retinal diseases that eventually end in vision loss and blindness, such as age-related macular degeneration (AMD), diabetic retinopathy (DR), retinitis pigmentosa and uveitis [1-5]. Most of the studies on the above- mentioned diseases have been focused on the neural retina. However, in view of the importance of the retinal pigment epithelium (RPE) in the maintenance of photoreceptors' (PR) viability and visual function, elucidating the effects of the inflammatory process in these cells could lead to the discovery of new therapeutic targets for the treatment of retinal degenerative diseases.
The RPE is located between the Burch's membrane, which separates RPE cells from the choriocapillaris, and the light-sensitive retina, with the apical membrane of RPE cells facing photoreceptor outer segments (POS) [6]. This monolayer of multifunctional pigmented cells is essential for the integrity and function of the retina [6,7]. Among its functions are light absorption and protection against photo-oxidation, POS phagocytosis, nutrient and water transport, secretion of several growth factors and reisomerization of all-trans-retinal [6]. The RPE also behaves as the outer blood-retinal barrier (BRB) participating in the immune privilege of the retina. Tight junctions among neightbouring RPE cells are essential in the control of fluids, solutes as well as toxic molecules that cross the BRB [7].This epithelium is in the unique position to sense the circulating immune system status and has both macrophage and microglia-like activities in the retina [8].
RPE cells can mediate the immune response in the eye because they i) express innate as well as adaptive immune receptors [9,10], ii) secrete immunomodulatory factors, such as interleukins (IL-6, 8, 11), tissue- necrosis factor- (TNF-β), interferon- (INF-β) , complement factor H (CFH) and monocyte chemotactic protein-1 (MCP1) and iii) inhibit T cells and macrophages through the modulation of IL-2 receptor and CD71 surface expression as well as IL-10 and pigment epithelial-derived factor (PEDF) secretion [11,12].
Although the intracellular mechanisms that mediate the RPE response to inflammatory and oxidative injury have not been fully elucidated to date, this review will summarize signaling events elicited in RPE cells under stress conditions and the participation of these signaling pathways in RPE viability and functions.
Lipopolysaccharide (LPS)- Elicited Signaling Pathways in the RPE
Although bacterial endophthalmitis (posterior segment eye infection) is an unusual pathology, it is a vital ocular emergency since it has poor prognosis and usually ends in vision loss [13,14]. This pathology can appear as a consequence of eye surgery, intravitreal injections, trauma or sepsis (endogenous endophthalmitis) [13-16]. Endogenous endophthalmitis is mostly seen in immunocompromised patients [16].
Previous research has demonstrated that RPE cells isolated from human healthy donors and the human RPE cell line (ARPE-19) can respond to the bacterial endotoxin since they express the primary LPS receptor, CD14, and its membrane-linked co-receptor, toll-like receptor 4 (TLR4) [17,18]. LPS induces ARPE-19 cells to secrete high levels of IL-6, IL-8, INF-γ, MCP-1 and intercellular adhesion molecule-1 (ICAM-1) and, to a lesser extent, IL-4, IL-5 and IL-10 [2,15,19]. Furthermore, Leung and collaborators demonstrated that ARPE-19 cells express several cytokine receptors (IL-R) for anti-and pro-inflammatory cytokines. Cell death was also observed to be induced in ARPE-19 cells by LPS, IL-6 or IL-8 treatment [2]. The deleterious effect of these pro-inflammatory cytokines was abolished when IL-6R and IL-8R were simultaneously silenced, thus suggesting that IL-6 and IL- 8 mediate LPS cytotoxicity via autocrine signaling [2]. However, the release of MCP-1 and IL-10 could have potential benefic effects, indicating that the RPE may contribute either to the progression or prevention of retinal degenerative diseases depending on the inflammatory stimulus, namely pro-inflammatory cytokines and/or LPS [2].
It was also demonstrated that LPS induces the activation of mitogen-activated protein kinases (MAPKs), such as p38, the extracellular signal-regulated kinase (ERK) and Jun kinase (JNK) in ARPE-19 cells [18,19]. Jung and collaborators demonstrated that ERK and the nuclear transcription factor-κB (NF-κB) activation are necessary to induce IL-6, MCP-1, and ICAM-1 genes. They also demonstrated that 15-deoxy-δ12,14- prostaglandin J2 (15d-PGJ2) inhibits LPS-induced activation of ERK and NF-κB and production of IL-6, MCP-1, ICAM-1 in ARPE-19 cells. Although this prostaglandin can activate the peroxisome proliferator-activated receptor γ (PPARγ) transcription factor, these effects of 15d-PGJ2 were PPARγ-independent [19].Therefore, leukocyte migration and adhesion mediated by IL-6-, MCP-1-, and ICAM-1 can be reduced by 15d-PGJ2 and may lead to the suppression of ocular inflammation [19].
Peroxisome proliferator-activated receptor α (PPARα) is another transcription factor that is highly expressed in RPE cells [20]. Recent studies have found that PPARα activation ameliorates inflammation by inhibiting NF-κB activity and pro-inflammatory cytokine production in a type I diabetes experimental model [21]. Shen and collaborators demonstrated that LPS exposure induces a twofold increase in TLR4 expression in ARPE-19 cells. They showed that either down-regulation or deletion of PPARα leads to increased TLR4 levels, activation of NF-κB signaling and inflammatory cytokine production in RPE cells. Likewise, PPARα activation decreases TLR4 levels and also inhibits the NF-κB signaling pathway induced by LPS in RPE cells. Moreover, PPARα agonists were observed to be able to reduce the LPS-induced production of TNF-α and ICAM-1 in ARPE-19 cells [20]. In contrast, under our experimental conditions, ARPE-19 cells treated with different Pseudomona aeruginosa LPS concentrations showed no changes in the expression of TLR4 [18]. Differential responses in TLR4 expression and also in RPE cell viability could be due to the different LPS bacterial sources used, which have not been specified in most of the literature available.
Under pathological conditions in which BRB integrity is compromised, complement proteins may leak into the retinal tissue, resulting in local complement activation. However, it has been reported that murine and human RPE express the genes of key complement components of the classical and alternative pathways [22,23]. The expression of complement genes in RPE cells was observed to be up regulated by LPS, IL-6 and, to a greater extent, by IFN-γ and TNF-α treatment [23]. Therefore, the production of inflammatory cytokines by RPE cells under pathological conditions could regulate their own complement genes expression.
We have recently reported that ARPE-19 cells express the classical phospholipase D isoforms (PLD1 and PLD2) and that LPS increases PLD activity in this RPE cell line [18]. PLD catalyzes phosphatidylcholine (PC) hydrolysis to generate the lipid second messenger, phosphatidic acid (PA), and choline. PA generated by PLD can be further hydrolyzed by lipid phosphate phosphatases (LPPs) in order to generate diacylglycerol (DAG), another lipid second messenger [24]. Thus, through the generation of DAG the PLD/LPPs pathway can mediate several cell responses by modulating the activity of DAG-responding proteins, such as classical and novel protein kinases C (PKCs), chimaerins, DAG kinases, protein kinases D, mammalian unc13 and Ras guanine-releasing protein, all of which present at least one DAG-binding C1 domain [25-28]. Previous reports have shown that the PLD pathway plays important roles in macrophage functions, such as chemotaxis, phagocytosis, inducible nitric oxide synthase (iNOS) expression and NO production [29-32]. However, findings from our laboratory demonstrated for the first time that PLD1 and PLD2 exert key functions during the inflammatory process in RPE cells [18]. After sustained stimulation of RPE cells with LPS, the subsequent activation of PLD2, ERK and the enhanced expression of cyclooxygenase 2 (COX-2) and PGE2 production, mediate RPE cell damage, possibly through PGE2 paracrine or autocrine effects. However, the role of PLD1 under inflammatory conditions in the RPE is not yet clear because although this isoform has the ability to promote cell damage through an ERK-independent COX-2 induction it also seems to have the ability to modulate cellular protective mechanisms as well since its inhibition is not able to counteract LPS-induced loss of cell viability [18]. Our results demonstrated that the PLD pathway is a novel player in the inflammatory response of the RPE. These findings, together with the development of new selective PLD isoform inhibitors [18,33-36], lead us to consider that PLD2 and ERK could be potential therapeutic targets for the treatment of inflammatory ocular pathologies. Figure 1 summarizes LPS-induced responses in RPE cells.
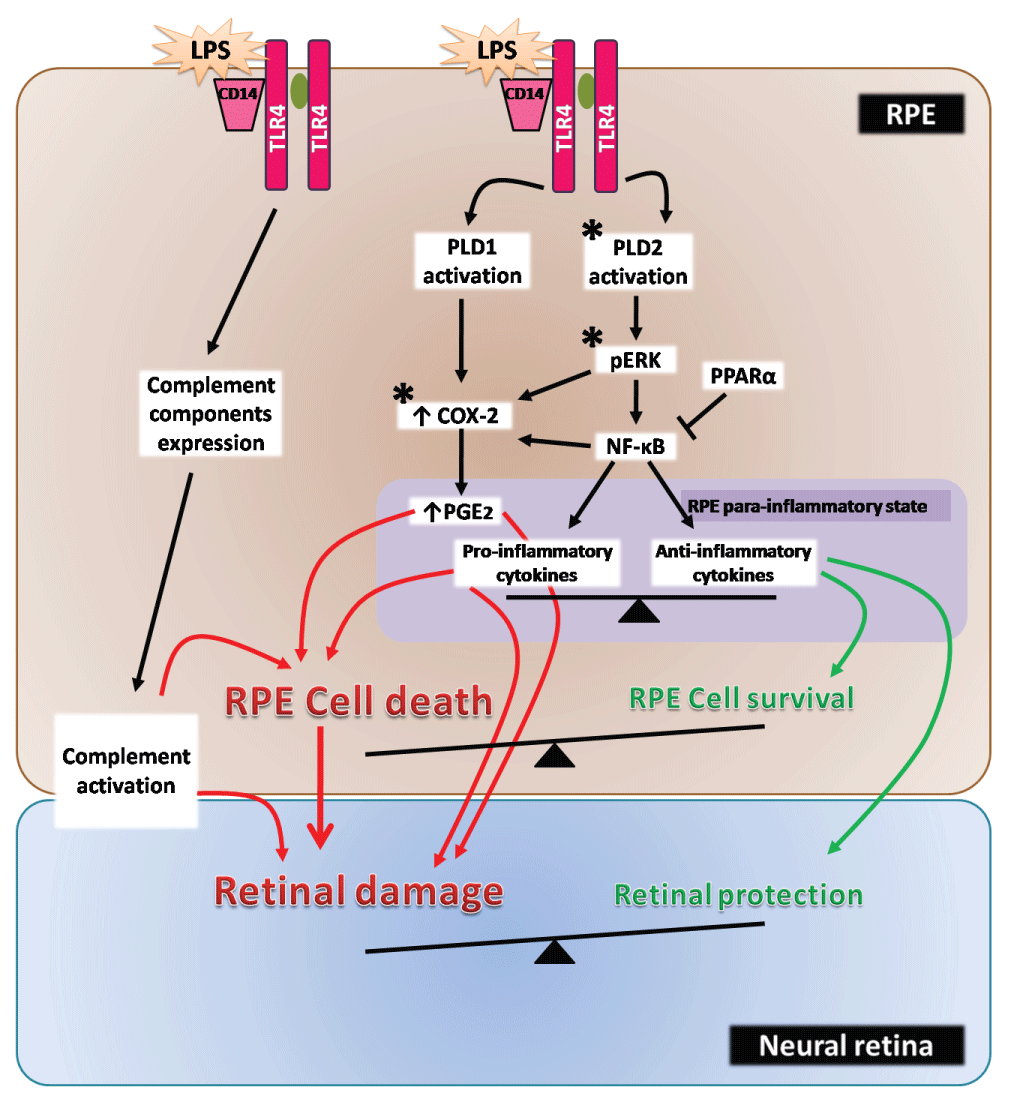
.
Figure 1: LPS-induced responses in RPE cells
Schematic view of the LPS-induced intracellular signaling events in RPE cells. Asterisks indicate potential therapeutic targets for the treatment of retinal inflammatory diseases.
View Figure 1
RPE Responses to High Glucose (HG) Concentrations
Diabetes is now recognized as a global epidemic. This chronic disease causes progressive damage to many organs and tissues and DR is one of the most serious diabetic complications and one of the leading causes of visual dysfunction and blindness of the working-age adults worldwide [7,37-39]. Retinal vasculature is particularly vulnerable to be damaged in patients with diabetes, this damage is characterized by microvascular lesions, impaired blood flow regulation, increased vasopermeability, microaneurysm formation, and eventually widespread non-perfusion and ischemia [40,41]. Chronic hyperglycemia associated with OS, accelerated formation of advanced glycation endproducts (AGEs) and inflammation are key players in the pathogenesis of DR [7,39,42]. DR can be classified into non-proliferative diabetic retinopathy (NPDR) and proliferative diabetic retinopathy (PDR). Neovascularization due to severe hypoxia is the hallmark of PDR whereas vascular leakage produced by BRB breakdown is the main event involved in the pathogenesis of diabetic macular edema (DME) [7].
In mammals, retinal glucose utilization is higher than in any other body tissue and the anatomical position of the RPE is critical to glucose supply to retinal neurons [43]. To satisfy the retina's large requirement of glucose RPE cells express high levels of glucose transporters (GLUT) 1 and 3, GLUT-1 being responsible for the inducible glucose transport based on metabolic demands [43,44]. Moreover, it was reported that GLUT-1 is expressed on both the apical and the basolateral surface of human RPE cells [43].
Several in vitro studies in which glucose concentrations ranging between 25 and 33mM were used to mimic hyperglycemia have been carried out in order to investigate the effect of HG on RPE cells. Some of these studies reported that HG reduces RPE cell viability and induces apoptosis [42,45]. Kim and collaborators showed that HG down regulates GLUT-1 protein expression and activity via a PKC-OS-Akt signaling pathway in ARPE-19 cells [46]. In addition, because GLUT-1 mRNA levels were not affected by HG, the decreased levels of this transporter could be due to an increased degradation rate of the protein under HG conditions [46].
In human RPE cell lines, such as ARPE-19 and D407, HG was observed to induce iNOS expression, NO production and cell injury [45,47-49]. HG causes activation of p38 MAPK and ERK in ARPE-19 cells [42,45] and the expression of ERK was observed to be upregulated in the RPE from streptozotocine-induced diabetic rats [50]. Furthermore, the inhibition of p38 and ERK was found to abrogate the HG-induced increase in iNOS, cell injury and levels of NO and nitrotyrosine [45]. The HG-induced overexpression of iNOS in RPE cells triggers endothelial cell apoptosis through the PKR-like endoplasmic reticulum kinase (PERK) pathway and mediates the decreased expression and function of p-glycoprotein (P-gp) in RPE cells [48,49]. Thus, hyperglycemia effects on RPE cells can mediate vascular endothelium and neural retina injury on account of the fact that P-gp function prevents the accumulation of toxic drugs and metabolites in the subretinal space.
It has also been reported that the loss of RPE cell viability induced by HG is accompanied by a decrease in superoxide dismutase (SOD) activity, the main enzyme for protection against oxidative stress in RPE cells [42]. In line with this, HG promotes vascular endothelial growth factor (VEGF) production in human RPE cells via intracellular reactive oxygen species (ROS) production and STAT3 (signal transducer and activator of transcription-3) activation [51]. Because VEGF is the major angiogenic factor [6,51,52], the effect of hyperglycemia on RPE cells plays an important role in the exacerbation of choroidal neovascularization (CNV).
The VEGF family includes VEGF-A,-B,-C and -D and the placental growth factor. VEGFs play essential roles in vascular development, angiogenesis and lymphangiogenesis in tissues and tumors [53]. It is well known that VEGF-A plays a key role in DR by increasing retinal vascular permeability and inducing neovascularization. However, the inhibition of VEGF-A only partially decreases neovascularization and vessel hyperpermeability, suggesting that other VEGF family members may also be involved in this process [54,55]. VEGF-C and VEGF-D have been focus of research regarding their roles as regulators of lymphangiogenesis but intraocular expression of VEGF-C and VEGF-D has been less studied [53,56]. It was reported that VEGF-C can promote survival of retinal vascular endothelial cells and that this can be regulated by both TNF-α and hyperglycemia [55]. The increased expression of VEGF-C in the retinal vasculature DR patients further supports a role for VEGF-C in DR [55]. In ARPE-19 cells VEGF-A, VEGF-C and VEGF-D expression was regulated by hypoxia. Furthermore, RPE cells markedly expressed VEGF-C and -D, as well as VEGF-Ain AMD [53].
As to RPE VEGF production under HG conditions, Puddu and collaborators demonstrated that AGEs significantly increase the expression of their specific receptor (RAGE) as well as VEGF-A and VEGF-C secretion in ARPE-19 cells [52]. AGEs are a heterogeneous group of molecules that accumulate during aging and in a faster rate in diabetic patients and are also involved in AMD pathogenesis [52,57,58]. Furthermore, apart from inducing protein-cross linking and OS, AGEs can induce the activation of genes involved in OS and inflammation through the interaction with RAGE [58]. While AGEs-induced VEGF-A secretion by RPE cells is dependent on RAGE upregulation, AGEs-induced VEGF-C secretion is independent on this receptor and the blockage of VEGF-A with bevacizumab (anti-VEGF monoclonal antibody) does not affect VEGF-C production by RPE exposed to HG [52]. These authors postulate that RPE VEGF-C production could be responsible for the clinical failure of treatments using monoclonal antibodies against VEGF in several patients and that the control of hyperglycemia could indeed be determinant and responsible for inter individual responses. Moreover, it has recently been reported that human RPE cells treated with AGE-bovine serum albumin show a complex regulation of pro- and anti-inflammatory cytokine secretion. In line with this, anti-inflammatory cytokines (IL-10, IL-1ra and IL-9) were found to be overexpressed and some pro-inflammatory cytokines (IL-4, IL-15, INF-γ) were overexpressed whereas others were underexpressed (IL-8, MCP1), thus suggesting a para-inflammatory state of the RPE under these conditions [57].
As stated above, BRB breakdown produced by the disruption of tight junctions is the main event in DME pathogenesis [59,60]. A differential expression of tight junctions proteins, such as zonula occludens-1 (ZO-1), claudins and occludin was shown in ARPE-19 cells exposed to HG conditions for 2-3 weeks [60], occludin and ZO-1 levels were similar to those observed under normo-glucose concentrations although a clear upregulation of claudin-1 was evidenced, accompanied with an aberrant distribution of these proteins [60,61]. In addition, HG and HG plus IL-1β were found to increase fibronectin and collagen IV expression in RPE cells but only the combination of HG and IL-1β, a condition that better mimics diabetic milieu, dramatically increased the barrier permeability [61]. Nevertheless, inconsistent results were obtained when transepithelial electrical resistance was measured in RPE cells exposed to HG [60,62]. These differences could be due to different RPE cell lines used and to the period of time during which these cells were kept in confluent cultures.
In view of the above, it becomes clear that hyperglycemia can affect RPE cell viability and BRB integrity. Furthermore, under HG conditions RPE secretes growth factors and enters in a para-inflammatory state, which could lead either to retinal damage or to survival depending on the inflammatory scenario and stress duration.
Role of OS in RPE Pathology
As stated above, RPE integrity is necessary for PR cell maintenance and PR renewal. RPE physiology has a central role in the vision process. Both PR and RPE are constantly exposed to light and are therefore continuously under OS. For this reason, many efforts are being currently made to understand how RPE deals with OS and to elucidate the molecular mechanisms that involve OS injury in retinal pathologies.
OS is a condition with increased ROS levels, which can damage membrane lipids, proteins and nucleic acids affecting cell function and, in many cases, when cell anti-oxidants defenses are overpassed, OS provokes cell death. OS has been reported to be one of the leading causes of several neurodegenerative diseases in the elderly [63] and, as stated above, OS is closely related to DR pathogenesis and HG can induce ROS generation and reduced SOD activity [42,51].
4-Hydroxy-2-nonenal (HNE), a highly reactive end product of lipid peroxidation, is a common marker in several human pathologies that occur during aging, such as Alzheimer's disease, Parkinson's disease and arthritis [64,65]. HNE levels in the RPE have been related with aging and the detrimental lipofuscin generation [66,67]. In line with this, HG was observed to increase lipid peroxides generation in ARPE-19 cells [46]. It has also been demonstrated that the antioxidants catalase and met allothioneins are reduced in the RPE of human donor eyes with age and with signs of AMD [68,69].
Furthermore, ARPE-19 cells exposed to the oxidant tert-butylhydroperoxide (t-BHP) resulted in ROS generation, glutathione depletion and apoptosis [70]. The inhibition of the MEK/ERK pathway was found to completely block t-BHP-induced apoptosis. On the contrary, neither JNKs nor p38 inhibition were observed to be able to counteract the deleterious effect of this oxidant [70]. In agreement with this, ERK was postulated as a potential target for the treatment of AMD [71].
On the other hand, cumulative evidence demonstrates that during OS RPE can also trigger the activation of survival signaling pathways for self-protection as well as for PR cell death prevention [72]. The synthesis of neuroprotective compounds from docosahexaenoic acid, such as neuprotectin D1 (NPD1), has been reported in RPE cells [73]. It has been shown that the addition of NPD1 protects human RPE cells in culture from OS injury and also upregulates the expression of anti-apoptotic proteins, such as Bcl-2 and Bcl-xL, and downregulates levels of pro-apoptotic markers, such as Bax and Bad [73]. The inhibition of caspase 3 and a diminished COX-2 expression have also been shown to be triggered by NPD1 during OS events [73] and phosphatidylinositol 3-kinase (PI3K)/Akt activation is involved in the survival induced by NPD1 in RPE cells [74]. NPD1 synthesis has also been related to PR shedding by RPE and this phagocytic process has been shown to induce refractoriness against OS [75].
Autophagy has been proposed as another RPE protective mechanism against OS. RPE autophagy activation and protection against OS has been shown to be regulated by the inhibition of the PI3K-Akt-mTOR pathway [76,77]. Autophagosome activity has, in fact, been observed to be age-dependent in control human donor specimens and mice. In contrast, eyes from AMD patients were found to evidence reduced levels of autophagy proteins [76] and decreased autophagy has been related with lipofuscin accumulation under pathological conditions [78]. In addition, HG-induced autophagy in RPE was shown to be regulated by ROS and endoplasmic reticulum stress signaling mediated by PERK activation [79]. Figure 2 schematizes HG and OS-elicited signaling events in RPE cells.
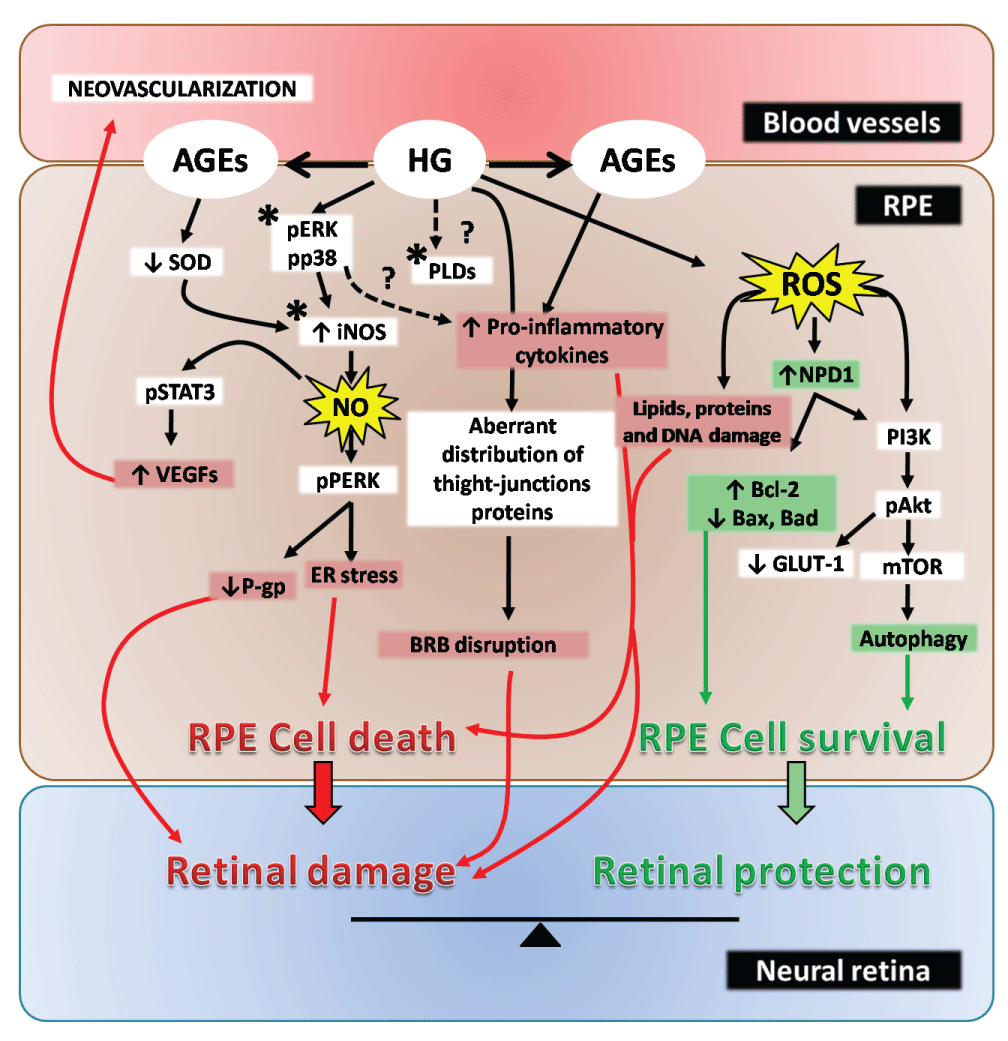
.
Figure 2: HG and OS-induced responses in RPE cells
Schematic view of the signaling pathways elicited in RPE cells under HG and OS conditions. Asterisks indicate potential therapeutic targets for the treatment of retinal degenerative diseases.
View Figure 2
Findings reported in this review clearly show the central role of the crosstalk among OS defenses, RPE and PR survival. The light shed on the above-mentioned neuroprotective mechanisms is mostly proposed as an important topic in the screening of new therapeutic strategies for retinal degeneration diseases.
Concluding Remarks
RPE cells are extremely resistant cells that are faced with different threats, such as toxins, UV light and ROS. Nevertheless, taking into account the different and essential roles exerted by the RPE for proper retina function, minor changes in RPE viability could lead to an important PR damage and, in consequence, to vision loss in vivo. As discussed, RPE response to inflammatory situations can mediate either retinal damage or survival depending on the inflammatory context and stress duration. Even though the response towards the stress could vary between RPE cell lines and primary RPE cell cultures, ARPE-19 cells are broadly used for the study of RPE pathologies. However, some intracellular events, such as ERK and NF-κB activation, as well as iNOS and COX-2 induction seem to mediate RPE cell damage, independently of the nature of the stress inductor. Then, these signaling pathways should be considered as potential therapeutic targets for ocular inflammatory diseases. In addition, the inhibition of the PLD2/LPPs pathway emerges as a novel therapeutic strategy. Still, further experiments are needed to fully elucidate the participation of this DAG generating pathway in retinal and RPE inflammatory conditions. Therefore, the design of selective inhibitors for the above-mentioned pathways opens a promising field for the treatment of retinal diseases (Figures 1,2).
Acknowledgements
Authors are grateful to Translator Viviana Soler for her technical assistance in controlling the use of the English language in this review
Funding
This work was supported by grants from the Universidad Nacional del Sur, the Consejo Nacional de Investigaciones Científicas y Técnicas (PIP-CONICET) and the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT). MVM, GAS and NMG are research members of CONICET, PET is a doctoral fellow of ANPCYT.
References
-
Bazan NG, Molina MF, Gordon WC (2011) Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer's, and other neurodegenerative diseases. Annu Rev Nutr 31: 321-351.
-
Leung KW, Barnstable CJ, Tombran-Tink J (2009) Bacterial endotoxin activates retinal pigment epithelial cells and induces their degeneration through IL-6 and IL-8 autocrine signaling. Mol Immunol 46: 1374-1386.
-
Perez VL, Saeed AM, Tan Y, Urbieta M, Cruz-Guilloty F (2013) The eye: A window to the soul of the immune system. J Autoimmun 45: 7-14.
-
Perez VL, Caspi RR (2015) Immune mechanisms in inflammatory and degenerative eye disease. Trends Immunol 36: 354-363.
-
Viringipurampeer IA, Bashar AE, Gregory-Evans CY, Moritz OL, Gregory-Evans K (2013) Targeting inflammation in emerging therapies for genetic retinal disease. Int J Inflam 2013: 581751.
-
Strauss O (2005) The retinal pigment epithelium in visual function. Physiol Rev 85: 845-881.
-
Simó R, Villarroel M, Corraliza L, Hernández C, Garcia-Ramírez M (2010) The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier--implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol 2010: 190724.
-
McLaren MJ (1996) Kinetics of rod outer segment phagocytosis by cultured retinal pigment epithelial cells. Relationship to cell morphology. Invest Ophthalmol Vis Sci 37: 1213-1224.
-
Detrick B, Hooks JJ (2010) Immune regulation in the retina. Immunol Res 47: 153-161.
-
Sparrow JR, Hicks D, Hamel CP (2010) The retinal pigment epithelium in health and disease. Curr Mol Med 10: 802-823.
-
Kaestel CG, Lovato P, Odum N, Nissen MH, Röpke C (2005) The immune privilege of the eye: human retinal pigment epithelial cells selectively modulate T-cell activation in vitro. Curr Eye Res 30: 375-383.
-
Zamiri P, Masli S, Streilein JW, Taylor AW (2006) Pigment epithelial growth factor suppresses inflammation by modulating macrophage activation. Invest Ophthalmol Vis Sci 47: 3912-3918.
-
Haddock LJ, Ramsey, DJ, Young, LH (2014) Complications of subspecialty ophthalmic care: endophthalmitis after intravitreal injections of anti-vascular endothelial growth factor medications. Semin Ophthalmol 29: 257-262.
-
Holland EJ, McDonald MB, Parekh JG, Sheppard JD (2014) Antibiotic resistance in acute postoperative endophthalmitis. Ophthalmology 121: S1-9.
-
Pollreisz A, Rafferty B, Kozarov E, Lalla E (2012) Klebsiella pneumoniae induces an inflammatory response in human retinal-pigmented epithelial cells. Biochem Biophys Res Commun 418: 33-37.
-
Vaziri K, Schwartz SG1, Kishor K1, Flynn HW Jr1 (2015) Endophthalmitis: state of the art. Clin Ophthalmol 9: 95-108.
-
Elner SG, Petty HR, Elner VM, Yoshida A, Bian ZM, et al. (2005) TLR4 mediates human retinal pigment epithelial endotoxin binding and cytokine expression. Invest Ophthalmol Vis Sci 46: 4627-33.
-
Mateos MV, Kamerbeek CB, Giusto NM, Salvador GA (2014) The phospholipase D pathway mediates the inflammatory response of the retinal pigment epithelium. Int J Biochem Cell Biol 55: 119-128.
-
Jung WK, Lee CM, Lee DS, Na G, Lee DY, et al. (2014) The 15-deoxy-delta12,14-prostaglandin J2 inhibits LPSstimulated inflammation via enhancement of the plateletactivating factor acetylhydrolase activity in human retinal pigment epithelial cells. Int J Mol Med 33: 449-456.
-
Shen W, Gao Y, Lu B, Zhang Q, Hu Y, et al. (2014) Negatively regulating TLR4/NF-?B signaling via PPAR? in endotoxin-induced uveitis. Biochim Biophys Acta 1842: 1109-1120.
-
Chen Y, Hu Y, Lin M, Jenkins AJ, Keech AC, et al. (2013) Therapeutic effects of PPARa agonists on diabetic retinopathy in type 1 diabetes models. Diabetes 62: 261-272.
-
Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, et al. (2010) The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog Retin Eye Res 29: 95-112.
-
Luo C, Chen M, Xu H (2011) Complement gene expression and regulation in mouse retina and retinal pigment epithelium/choroid. Mol Vis 17: 1588-1597.
-
Peng X, Frohman MA (2012) Mammalian phospholipase D physiological and pathological roles. Acta Physiol (Oxf) 204: 219-226.
-
Carrasco S, Mérida I (2007) Diacylglycerol, when simplicity becomes complex. Trends Biochem Sci 32: 27-36.
-
Newton AC (2010) Protein kinase C: poised to signal. Am J Physiol Endocrinol Metab 298: E395-402.
-
Wang QJ (2006) PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol Sci 27: 317-323.
-
Yang C, Kazanietz MG (2007) Chimaerins: GAPs that bridge diacylglycerol signalling and the small G-protein Rac. Biochem J 403: 1-12.
-
Ali WH, Chen Q, Delgiorno KE, Su W, Hall JC, Hongu T, et al. (2013) Deficiencies of the lipid-signaling enzymes phospholipase D1 and D2 alter cytoskelet al organization, macrophage phagocytosis, and cytokine- stimulated neutrophil recruitment. PLoS. One 8: e55325.
-
Kantonen S, Hatton N, Mahankali M, Henkels KM, Park H, et al. (2011) A novel phospholipase D2-Grb2-WASp heterotrimer regulates leukocyte phagocytosis in a two-step mechanism. Mol Cell Biol 31: 4524-4537.
-
Knapek K, Frondorf K, Post J, Short S, Cox D, et al. (2010) The molecular basis of phospholipase D2-induced chemotaxis: elucidation of differential pathways in macrophages and fibroblasts. Mol Cell Biol 30: 4492-4506.
-
Park SY, Cho JH, Ma W, Choi HJ, Han JS (2010) Phospholipase D2 acts as an important regulator in LPS-induced nitric oxide synthesis in Raw 264.7 cells. Cell Signal 22: 619-628.
-
Cheol Son J, Woo Kang D, Mo Yang K, Choi KY, Gen Son T, et al. (2013) Phospholipase D inhibitor enhances radiosensitivity of breast cancer cells. Exp Mol Med 45: e38.
-
Liu Y, Käch A, Ziegler U, Ong AC, Wallace DP, et al. (2013) The role of phospholipase D in modulating the MTOR signaling pathway in polycystic kidney disease. PLoS One 8: e73173.
-
Mateos MV, Giusto NM, Salvador GA (2012) Distinctive roles of PLD signaling elicited by oxidative stress in synaptic endings from adult and aged rats. Biochim Biophys Acta 1823: 2136-2148.
-
Scott SA, Selvy PE, Buck JR, Cho HP, Criswell TL, et al. (2009) Design of isoform-selective phospholipase D inhibitors that modulate cancer cell invasiveness. Nat Chem Biol 5: 108-117.
-
Chen JJ, Wendel LJ, Birkholz ES, Vallone JG, Coleman AL, et al. (2015) The metabolic syndrome and severity of diabetic retinopathy. Clin Ophthalmol 9: 757-764.
-
Simó R, Hernández C (2015) Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog Retin Eye Res 48: 160-180.
-
Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R (2013) Pathophysiology of Diabetic Retinopathy. ISRN. Ophthalmol 343560.
-
Ahsan H (2015) Diabetic retinopathy--biomolecules and multiple pathophysiology. Diabetes Metab Syndr 9: 51-54.
-
Safi SZ, Qvist R, Kumar S, Batumalaie K, Bin Ismail IS (2014) Molecular mechanisms of diabetic retinopathy, general preventive strategies, and novel therapeutic targets. Biomed Res Int 801269.
-
Xie P, Fujii I, Zhao J, Shinohara M, Matsukura M (2012) A novel polysaccharide compound derived from algae extracts protects retinal pigment epithelial cells from high glucose-induced oxidative damage in vitro. Biol Pharm Bull 35: 1447-1453.
-
Senanayake Pd, Calabro A, Hu JG, Bonilha VL, Darr A, et al. (2006) Glucose utilization by the retinal pigment epithelium: evidence for rapid uptake and storage in glycogen, followed by glycogen utilization. Exp Eye Res 83: 235-246.
-
Ban Y, Rizzolo LJ (2000) Regulation of glucose transporters during development of the retinal pigment epithelium. Brain Res Dev Brain Res 121: 89-95.
-
Yuan Z, Feng W, Hong J, Zheng Q, Shuai J, et al. (2009) p38MAPK and ERK promote nitric oxide production in cultured human retinal pigmented epithelial cells induced by high concentration glucose. Nitric Oxide 20: 9-15.
-
Kim DI, Lim SK, Park MJ, Han HJ, Kim GY, et al. (2007) The involvement of phosphatidylinositol 3-kinase /Akt signaling in high glucose-induced downregulation of GLUT-1 expression in ARPE cells. Life Sci 80: 626-632.
-
Rosales MA, Silva KC, Duarte DA, de Oliveira MG, de Souza GF, et al. (2014) S-nitrosoglutathione inhibits inducible nitric oxide synthase upregulation by redox posttranslational modification in experimental diabetic retinopathy. Invest Ophthalmol Vis Sci 55: 2921-2932.
-
Zhang X, Fu Y, Xu X, Li M, Du L, et al. (2014) PERK pathway are involved in NO-induced apoptosis in endothelial cells cocultured with RPE under high glucose conditions. Nitric Oxide 40: 10-16.
-
Zhang Y, Li C, Sun X, Kuang X, Ruan X (2012) High glucose decreases expression and activity of p-glycoprotein in cultured human retinal pigment epithelium possibly through iNOS induction. PLoS One 7: e31631.
-
Al-Hussaini H, Kilarkaje N (2015) Effects of diabetes on retinal pigment epithelial cell proliferation and mitogen-activated protein kinase signaling in dark Agouti rats. Exp Toxicol Pathol 67: 117-124.
-
Li X, Cai Y, Wang YS, Shi YY, Hou W, et al. (2012) Hyperglycaemia exacerbates choroidal neovascularisation in mice via the oxidative stress-induced activation of STAT3 signalling in RPE cells. PLoS One 7: e47600.
-
Puddu A, Sanguineti R, Durante A, Nicolò M, Viviani GL (2012) Vascular endothelial growth factor-C secretion is increased by advanced glycation end-products: possible implication in ocular neovascularization. Mol Vis 18: 2509-2517.
-
Ikeda Y, Yonemitsu Y, Onimaru M, Nakano T, Miyazaki M, et al. (2006) The regulation of vascular endothelial growth factors (VEGF-A, -C, and -D) expression in the retinal pigment epithelium. Exp Eye Res 83: 1031-1040.
-
Castellon R, Hamdi HK, Sacerio I, Aoki AM, Kenney MC, et al. (2002) Effects of angiogenic growth factor combinations on retinal endothelial cells. Exp Eye Res 74: 523-535.
-
Zhao B, Smith G, Cai J, Ma A, Boulton M (2007) Vascular endothelial growth factor C promotes survival of retinal vascular endothelial cells via vascular endothelial growth factor receptor-2. Br J Ophthalmol 91: 538-545.
-
Clarijs R, Schalkwijk L, Ruiter DJ, de Waal RM (2001) Lack of lymphangiogenesis despite coexpression of VEGF-C and its receptor Flt-4 in uveal melanoma. Invest Ophthalmol Vis Sci 42: 1422-1428.
-
Lin T, Walker GB, Kurji K, Fang E, Law G, et al. (2013) Parainflammation associated with advanced glycation endproduct stimulation of RPE in vitro: implications for age-related degenerative diseases of the eye. Cytokine 62: 369-381.
-
Yamagishi S, Ueda S, Matsui T, Nakamura K, Okuda S (2008) Role of advanced glycation end products (AGEs) and oxidative stress in diabetic retinopathy. Curr Pharm Des 14: 962-968.
-
Joussen AM1, Smyth N, Niessen C (2007) Pathophysiology of diabetic macular edema. Dev Ophthalmol 39: 1-12.
-
Villarroel M, García-Ramírez M, Corraliza L, Hernández C, Simó R (2009) Effects of high glucose concentration on the barrier function and the expression of tight junction proteins in human retinal pigment epithelial cells. Exp. Eye Res 89: 913-920.
-
Trudeau K, Roy S, Guo W, Hernández C, Villarroel M, et al. (2011) Fenofibric acid reduces fibronectin and collagen type IV overexpression in human retinal pigment epithelial cells grown in conditions mimicking the diabetic milieu: functional implications in retinal permeability. Invest Ophthalmol Vis Sci 52: 6348-6354.
-
Pavan B, Capuzzo A, Forlani G (2014) High glucose-induced barrier impairment of human retinal pigment epithelium is ameliorated by treatment with Goji berry extracts through modulation of cAMP levels. Exp Eye Res 120: 50-54.
-
Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7: 65-74.
-
Siegel SJ, Bieschke J, Powers ET, Kelly JW (2007) The oxidative stress metabolite 4-hydroxynonenal promotes Alzheimer protofibril formation. Biochemistry 46: 1503-1510.
-
Zarkovic K (2003) 4-hydroxynonenal and neurodegenerative diseases. Mol Aspects Med 24: 293-303.
-
Kaemmerer E, Schutt F, Krohne TU, Holz FG, Kopitz J (2007) Effects of lipid peroxidation-related protein modifications on RPE lysosomal functions and POS phagocytosis. Invest Ophthalmol Vis Sci 48: 1342-1347.
-
Krohne TU, Stratmann NK, Kopitz J, Holz FG (2010) Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp Eye Res 90: 465-471.
-
Liles MR, Newsome DA, Oliver PD (1991) Antioxidant enzymes in the aging human retinal pigment epithelium. Arch Ophthalmol 109: 1285-1288.
-
Tate DJ Jr, Newsome DA, Oliver PD (1993) Met allothionein shows an age-related decrease in human macular retinal pigment epithelium. Invest Ophthalmol Vis Sci 34: 2348-2351.
-
Glotin AL, Calipel A, Brossas JY, Faussat AM, Tréton J, et al. (2006) Sustained versus transient ERK1/2 signaling underlies the anti- and proapoptotic effects of oxidative stress in human RPE cells. Invest Ophthalmol Vis Sci 47: 4614-4623.
-
Dridi S, Hirano Y, Tarallo V, Kim Y, Fowler BJ, et al. (2012) ERK1/2 activation is a therapeutic target in age-related macular degeneration. Proc Natl Acad Sci U S A 109: 13781-13786.
-
Bazan NG (2006) Survival signaling in retinal pigment epithelial cells in response to oxidative stress: significance in retinal degenerations. Adv Exp Med Biol 572: 531-540.
-
Mukherjee PK, Marcheselli VL, Barreiro S, Hu J, Bok D, et al. (2007) Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci U S A 104: 13152-13157.
-
Halapin NA, Bazan NG (2010) NPD1 induction of retinal pigment epithelial cell survival involves PI3K/Akt phosphorylation signaling. Neurochem Res 35: 1944-1947.
-
Bazan NG, Calandria JM, Serhan CN (2010) Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res 51: 2018-2031.
-
Mitter SK, Song C, Qi X, Mao H, Rao H, et al. (2014) Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 10: 1989-2005.
-
Tang B, Cai J, Sun L, Li Y, Qu J1, et al. (2014) Proteasome inhibitors activate autophagy involving inhibition of PI3K-Akt-mTOR pathway as an anti-oxidation defense in human RPE cells. PLoS One 9: e103364.
-
Saadat KA, Murakami Y, Tan X, Nomura Y, Yasukawa T, et al. (2014) Inhibition of autophagy induces retinal pigment epithelial cell damage by the lipofuscin fluorophore A2E. FEBS Open Bio 4: 1007-1014.
-
Yao J, Tao ZF, Li CP, Li XM, Cao GF, et al. (2014) Regulation of autophagy by high glucose in human retinal pigment epithelium. Cell Physiol Biochem 33: 107-116.





