International Journal of Ophthalmology and Clinical Research
Retinal Examination for theDiagnosis of Alzheimer's Disease
Umur Kayabasi1*, Robert C Sergott2 and Marco Rispoli3
1World Eye Hospital, Istanbul, Turkey
2Wills Eye Hospital, Philadelphia, Pennsylvania, USA
3University of Rome, Rome, Italy
*Corresponding author: Umur Kayabasi, World Eye Hospital - Istanbul, Turkey, E-mail: kayabasi@yahoo.com
Int J Ophthalmol Clin Res, IJOCR-1-002, (Volume 1, Issue 1), Research Article; ISSN: 2378-346X
Received: September 29, 2014 | Accepted: October 15, 2014 | Published: October 17, 2014
Citation: Kayabasi U, Sergott RC, Rispoli M (2014) Retinal Examination for the Diagnosis of Alzheimer's Disease. Int J Ophthalmol Clin Res 1:002. 10.23937/2378-346X/1410002
Copyright: ©2014 Kayabasi U, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: To demonstrate AD plaques in retina.
Methods: We examined 30 patients with Mild Cognitive impairment (MCI). In the patients in whom we found hyper or hypo fluorescent lesions on Fundus Auto Fluorescence (FAF), Optical Scanning Tomography (OCT) was performed through these regions to reveal depositions in the retina. Drusen like spots- dots were noticed in different parts of the retina. 20 randomly chosen patients and 20 age-matched healthy controls were given curcumin to check for the changes in FAF and OCT.
Results: In 26 patiens, we were able to find abnormal deposits in different layers. We believe that these plaque-like lesions are related to neuro- degenerative disease. Curcumin caused patchyhypofluorescent FAF to occur and spots seemed brighter on OCT.
Conclusion: We stress that all the middle- aged patients who have family history of Alzheimer's Disease (AD) and/or cognitive defects should have thorough medical examinations including retina exam.
Keywords
OCT, FAF, Retina, Curcumin, Alzheimer's
Introduction
Alzheimer's Disease (AD), first described by German psychiatrist and neuropathologist Alois Alzheimer in 1906, is a chronic neurodegenerative disease characterized by loss of memory and cognitive decline, and is neuropathologically associated with an increase in ß-amyloid (Aß) plaque deposition, Neurofibrillarytangle Formation (NFT), neuronal loss, and inflammation [1,2]. Aß peptides, which are the predominant component of plaques, are the result of sequential cleavage of the Amyloid Precursorprotein (APP). In addition to cognitive impairment, people with AD often develop visual anomalies in color discrimination, stereoacuity and contrast sensitivity [3-5]. These visual abnormalities have been attributed, in part, to AD pathology in central visual pathways plus to retinal dysfunction, such as ganglion cell loss, reduction in the thickness of the retinal nerve fiber layer, and optic nerve degeneration [6].
In the past several years, transgenic mouse models have been engineered to explore different aspects of AD neurodegeneration. These findings have indicated that elevated levels of Aß peptides are associated with dysfunctional neuronal networks both in the brain and eye [1,2]. Scientists recognize Alzheimer's as a disease process that begins many years before symptoms of dementia become evident. New research has found changes in the brain and body up to 20 years before Alzheimer's symptoms arise. The research, published in the journal Lancet Neurology, examined a large extended South American family in Colombia that carried a gene for the early-onset form of Alzheimer's, which typically arises before age 60. Approximately 30 percent of the 5,000 family members carry the defective gene. Inheriting the gene, called presenelin 1, guarantees that the patient will get Alzheimer's at a relative young age [7,8]. If physicians could reliably identify signs that someone is likely to get the disease, they could begin treatment earlier, possibly warding off the onset of Alzheimer's symptoms years down the road.Someone who has no obvious memory problems, might remain problem-free in case of taking a drug that targets beta-amyloid buildup.
Lipofuscin Hypothesis of AD
Although Aß protein has been identified as the main component of senile plaques,triggering factors for the accumulation of Aß protein have not been fully understood. Following the death of neurons during aging, lipofuscin is set free and cannot be rapidly degraded. It may become harmful when it is released into the extracellular space. Lipofuscin contains Aß and its precursor [9,10]. And that its translocation from the intra to the extracellular compartment (together with themicroglia, astrocytes and a neuroinflammatory response) could greatly change its biochemical characteristics. The hydrophobic and insoluble characteristics of lipofuscin may induce an immuneresponse [9]. Mitochondrial autophagocytosis is also a major contributor to lipofuscin formation [10] and finally, the rate of lipofuscin formation is also closely related to oxidative stress [9,10]. Lipofuscin may therefore be the missing link in the pathogenesis of AD (such as oxidative stress,mitochondrial dysfunction and the activation of immune responses) [11]. Recent evidence which indicates that vascular factors play an important role in the genesis of AD may be interpreted in this context because hypoperfusion is a potential cause of damage of neurons and may initiate lipofuscin release [11].
Lipofuscin means 'dark fat' and is also known as 'age pigment' and its distribution is consistent with the release of debris or waste products. Lipofuscin plays a major role in another neuronaldegenerative process that is very common in elderly people: Age-related Macular Degeneration(AMD) [11]. Lipofuscin accumulation in the retinal pigment epithelium is involved in the pathogenesis of AMD, together with the formation of abnormal extracellular deposits (or drusen). It has been shown that drusen contain a number of molecules and, most significantly, Aß protein. Drusen arise from material released by lipofuscin-rich retinal pigmented epithelial cells and contain proteins that are also components of senile plaques. This may suggest an unexpected similarity between the pathogenetic mechanisms of AMD and AD [11].
Retina and Optic Disc in AD
The use of Magnetic Resonance imaging (MRI) and Positron Emission Tomography (PET), have been limited in sensitivity and resolution [3]. Likewise, cerebro spinal fluid analysis is invasive, while neuropsychological testing can be imprecise. But, since the optic nerve and the retina are extensions of the brain,imaging of the eye can be a simple and accessible alternative [8]. Retinal photography, Scanning Laser Ophthalmoscopy (SLO), and Optical Coherence Tomography (OCT) have previously identified retinal NFL loss, retinal blood flow changes, and optic disc changes in AD patients [6]. Fundus Autofluorescence (FAF) examination has been used to determine lipo fuscin accumulation in AMD. Koronyo-Hamaoui et al. Have described increased Aß plaques in the retinas of post mortem eyes from early-stage AD patients, and in vivo in double-transgenic mice, compared to controls. Their imaging methods were facilitated with prior injection of curcumin, a natural extract and fluorochrome that labels Aß plaques. The group was able to demonstrate a correlation of retinal plaque number and size with brain pathology, and have proposed to bring this method into clinical use [12].
There are common pathological associations between AD, glaucoma, and AMD. Deposition of Aß and pTau, combined with other common factors such as oxidative and metabolic stress, directly contribute to neuroinflammatory responses and cell death in these neurodegenerative disorders [12]. Huang et al. showed that AD patients had a specific pattern of retinal nerve fiber layer loss (RNFL)- measured by optical coherence tomography ,narrower venules and decreased blood flow in these venules (measured by a laser Doppler instrument)]. A limitation of the study was the small patient numbers. It should also be noted that retinal vessel widths are influenced by many disorders like hypertension, hyperglycemia, obesity and inflammation [13]. Research on the retinas of AD transgenic mice has demonstrated Aß plaques, hyperphosphorylated tau, increased microvascular deposition of Aß and neuroinflammation [6]. In addition to retinal venular constriction in human AD, Berisha et al. demonstrated significant thinning of the superior RNFL using OCT. This region corresponds with the inferior visual field defects which are common in AD.
Iseri et al. demonstrated macular thinning in AD to be related to the severity of cognitive impairment. The loss of RNFL thickness in AD is linked to a loss of Retinal Ganglion Cells (RGC) and optic nerve axons. A post mortem study by Blanks et al. demonstrated a 25% decrease in RGC at the level of the foveal and parafoveal retina. Retinal photography has also been used to identify RNFL defects (nerve fiber loss) in AD, although one study indicated practical difficulties of this approach.Retinal photography and Scanning Laser Ophthalmoscopy (SLO) have both been used to show opticdisc changes in AD, including optic disc pallor, increased disc cupping and thinning of the neuroretinalrim. Some of the ocular pathologies found in AD are also found in glaucoma [6]. Some studies supported the increased incidence of open-angle glaucoma in AD .A greater than10% year loss in visual field and optic disc cupping were demonstrated in glaucoma patients whowere later diagnosed with AD, whereas an average 3% year loss in visual field was observed in glaucoma patients who did not develop AD, indicating that AD causes the progression of glaucomasymptoms [6]. It is hoped that common neurodegenerative diseases (AD, Glaucoma and AMD) can be targeted simultaneously for treatment and monitoring. In a glaucoma study, lipofuscin accumulation in parapapillary Retinal Pigment Epithelial (RPE) cellswas detected.
FAF in Retinal Diseases
FAF detects lipofuscin in retina
The autofluoresence signal from RPE cells is very much correlated with lipofuscin content and accumulation. FAF is increased with RPE dysfunction due to the accumulation, impaired processing and clearing of lipofuscin. Conversely, the FAF signal may be decreased in the setting of RPE or photoreceptor loss - if there are no photoreceptor outer segments, the source for lipofuscin formation may be lost [12]. The advent of confocal scanning laser ophthalmoscopic technology was an important advance inmaking FAF approaches clinically relevant.
Confocal SLO utilizes a focused, low-power laser that is swept across the fundus in a faster pattern. The confocal nature ensures reflectance and fluoresence are "conjugate" (from same optical plane) [12].
OCT and FAF to Diagnose AD
During the evaluation of patients with mild cognitive impairment or patients with a family history ofAD, FAF test becomes very important. Both hyperfluorescent (regions with excessive lipofuscin) or hypofluorescent (atrophic retina) areas are of concern. To better understand the layer of the defect, OCT can be performed through the abnormal areas on FAF. Some experience is necessary for the differential diagnosis of the lesions [12,13]. To better understand the nature of the lesion, curcumin may be orally or intravenously administered. It binds to B-amyloid plaques in the retina which are most likely related to AD [14].
Material and Methods
We examined 30 patients with MCI at World Eye Hospital/ Istanbul ; none of them had vascular retinal lesions due to hypertension or diabetes. Patients with chronic diseases like glaucoma were also excluded.The diagnosis of MCI was given byneuropsychological testing (including mini mental test) at a differentcenter. Age range was between 66 and 84; 18 women and 12 men.All the patients had FAFand OCT examinations with Heidelberg Spectralis. The regions with hypo or hyperfluorescentlesions on FAF were carefully examined byOCT and the layer of thedefect was detected. The images were examined in a masked fashion. Also, curcumin (Turmeric Phytosome-Meriva) was given to 20randomly selected patients and 20 age-matched healthy controls. Itwas given 80 mg bid for three days and FAF- OCT imagings were repeated. Changes on FAF and OCT after curcumin usage were againdetermined in a masked way. Patients who had retinal stained lesionswith curcumin were sent for brain PET-CT at another center.
Results
In 26 patients, our retina exams with FAF and OCT helped us see abnormal depositions mostly in the outer plexiform , ganglion and nerve fiber layers of the retina (Figures 1-4). The depositions were dot shaped and had different sizes.
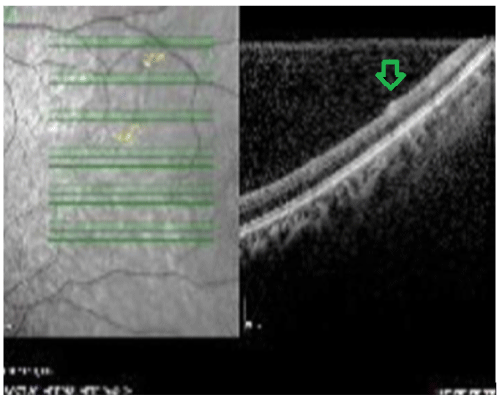
Figure 1: OCT image. Lesion in the nerve fiber and ganglion layers
View Figure 1
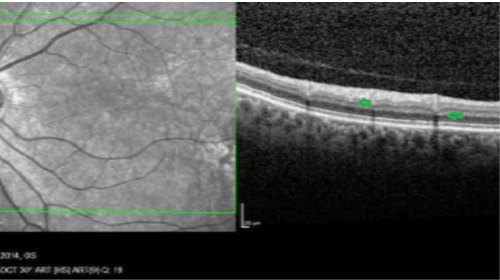
Figure 2: OCT image. Small lesions in the outer plexiform and ganglion layers
View Figure 2
These accumulations were mostly in the perimacular and perivascular areas. We believe that these plauqes are related to neurodegenerative disease. In the patients who used curcumin, it was possible to detect patchy hypofluorescent areas.
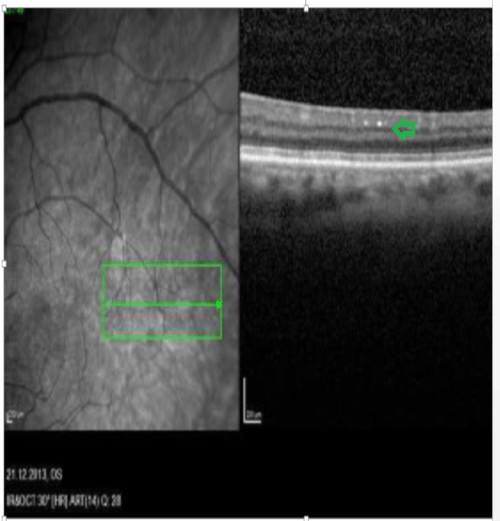
Figure 3: OCT image. Bright plaques in the ganglion layer
View Figure 3
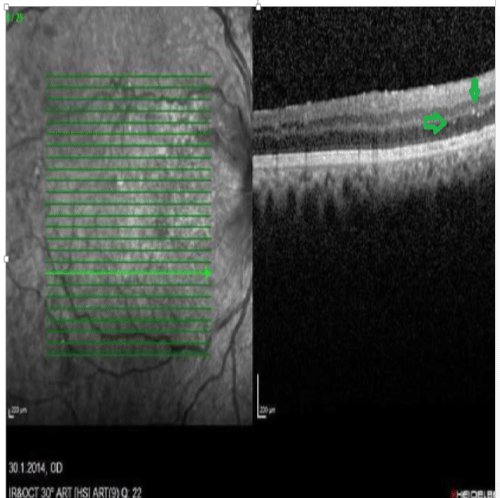
Figure 4: OCT image. Bright plaques in the outer plexiform and outer nuclear layers
View Figure 4
These hypofluorescentare as were in different locations of the retina in a patchyway. The hypofluorescent regions became more apparent after curcumin use (Figures 5-7).
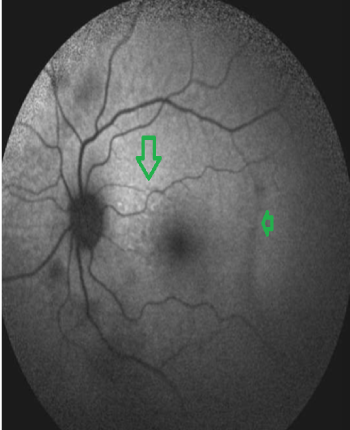
Figure 5: FAF image. Patchy hypo and hyperfluorescent areas
View Figure 5
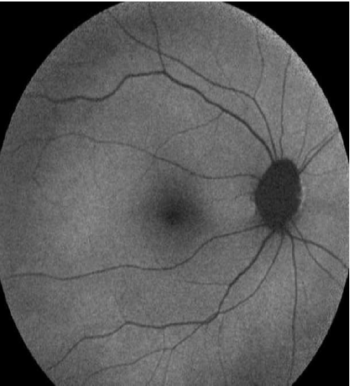
Figure 6: FAF image. Normal control
View Figure 6
No change in FAF was detected in the control group (Figure 8). Since curcumin binded to Bamyloid, it was possible to see hyperintense dot like changes on FAF. This proved that the lesions consisted of beta amyloid.The roundish lesions on OCT also became more prominent. In 26 patients, more than 10 curcumin stained lesions were seen and this was considered as moderate disease.
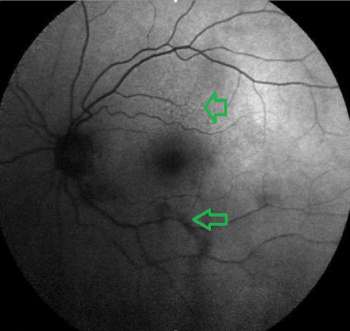
Figure 7: FAF image. Patchy hypo and hyperfluorescent areas
View Figure 7
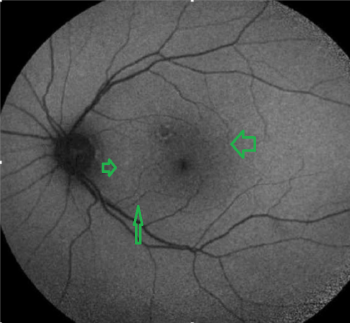
Figure 8: FAF image. Curcumin stained hyperfluorescent lesions between the fovea and the disc
PET- CT: Bilateral temporo-pariet al hypometabolism.
View Figure 8
The brain PET-CTs of 21 patients were consistent with AD and bilateral temporopariet al hypometabolism was reported. (Figure 9) PET- CT was inconclusive in 5 cases. Direct communication with the reporting radiologists was arranged.Brain amyloid PET- CT was not done.
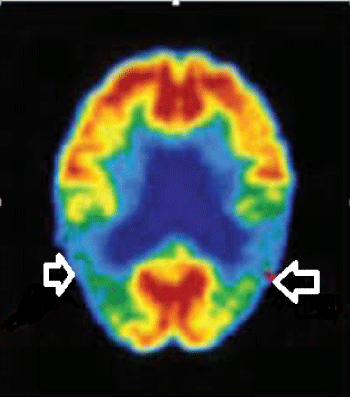
Figure 9: PET- CT revealing bilateral temporo-pariet al hypometabolism
View Figure 9
Conclusion
The OCT images combined with FAF in these patients who have risk factors are very suspicious for early AD plaques in retina. Another interesting finding may be the increased leson sizes on OCT in patients who have hypopigmented areas on FAF. Hypopigmented areas show atrophic retinal changes which may indicate an advanced stage of the disease. Hyperpigmented lesions contain more lipofuscin; but the retina may be less compromised and not atrophic.In the curcumin group, the reason of the increased hypofluorescence may be the thinning in the choroid and degeneration of RPE [15]. Thinning of the choroid and changes in RPE were recently demonstrated by Yuchun Tsai, et al. Our findings about increased hypofluorescence correlate with their observations. Hyperintense dots on FAF which become noticable after curcumin use prove that it has affinity for B-amyloid. Examination of retina with FAF and OCT give us valuable results about neurodegeneration and these tests may be biomarkers for AD. It may be possible to detect the disease and also examine the progression of the lesions. The early detection of AD is of paramount importance. The retina is an excellent model for neurodegenerative diseases. If the plaques start in the retina first, as it has been published [14] this will give us time to stop the disease. Anti- oxidants, healthy moderate exercise and less stress can contribute to the slowing (or reversing ) of the disease. May be, new drugs may halt the disease before the plaques appear in the brain . Much more needs to be done to fight with AD. Detecting the disease early is one of them; so people who have relatives with AD in their families or with MCI should have a thorough medical examination.Our study shows that MCI may be the previous step towards AD. Detailed retina exam with FAF and OCT will contribute to the neurologic evaluation in neurodegenerative diseases.
References
-
Prince M, Bryce R, Ferri C (2011) World Alzheimer report 2011: the benefits of early diagnosis and intervention. Alzheimer's Disease International.
-
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, et al. (2012) National Institute on Aging-Alzheimer's Association guidelines on neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 8: 1-13. .
-
Bloudek LM, Spackman DE, Blankenburg M, Sullivan SD (2011) Review and meta-analysis of biomarkers and diagnostic imaging in Alzheimer's disease. See comment in PubMed Commons below J Alzheimers Dis 26: 627-645.
-
Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, et al. (2012) Clinical and biomarker changes in dominantly inherited Alzheimer's disease. See comment in PubMed Commons below N Engl J Med 367: 795-804.
-
Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, et al. (2008) The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 30: 58-69.
-
Simao LM (2013) The contribution of optical coherence tomography in neurodegenerative diseases. See comment in PubMed Commons below Curr Opin Ophthalmol 24: 521-527.
-
Barnes DE, Yaffe K (2011) The projected effect of risk factor reduction on Alzheimer's disease prevalence. See comment in PubMed Commons below Lancet Neurol 10: 819-828.
-
Blennow K, Hampel H (2003) CSF markers for incipient Alzheimer's disease. See comment in PubMed Commons below Lancet Neurol 2: 605-613.
-
Terman A, Brunk UT (2004) Lipofuscin. See comment in PubMed Commons below Int J Biochem Cell Biol 36: 1400-1404.
-
Seehafer SS, Pearce DA (2006) You say lipofuscin, we say ceroid: defining autofluorescent storage material. See comment in PubMed Commons below Neurobiol Aging 27: 576-588.
-
Giaccone G, Orsi L, Cupidi C, Tagliavini F (2011) Lipofuscin hypothesis of Alzheimer's disease. See comment in PubMed Commons below Dement Geriatr Cogn Dis Extra 1: 292-296.
-
Olsen TW (2008) The Minnesota Grading System using fundus autofluorescence of eye bank eyes: a correlation to age-related macular degeneration (an AOS thesis). Trans Am Ophthalmol Soc 106: 383-401.
-
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, et al. (1991) Optical coherence tomography. See comment in PubMed Commons below Science 254: 1178-1181.
-
Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, et al. (2011) Identification of Amyloid Plaques in Retinas from Alzheimer's Patients and Noninvasive In Vivo Optical Imaging of Retinal Plaques in a Mouse Model . Neuroimage 54: S204-S217.
-
Tsai Y, Lu B, Ljubimov AV, Girman S, Ross-Cisneros FN, et al. (2014) Ocular changes in TgF344-AD rat model of Alzheimer's disease. See comment in PubMed Commons below Invest Ophthalmol Vis Sci 55: 523-534.





