International Journal of Critical Care and Emergency Medicine
Emerging Concepts in Acute Heart Failure: From the Pathophysiology to the Clinical Case Based Approach
Antonio Giovanni Solimando1,2*#, Antonella Argentiero3#, Anna Ruckdeschel2, MaxBittrich2, Andreas Schneider4, Rodolfo Sbrojavacca5, Angelo Vacca1, Georg Fritz6 and Hermann Einsele2
1Department of Biomedical Sciences and Human Oncology, Section of Internal Medicine "G. Baccelli", University of Bari Medical School, Bari, Italy
2Department of Internal Medicine II, Hematology and Medical Oncology, University Medical Center Würzburg, Germany
3Department of Biomedical Sciences and Human Oncology, Section of Medical Oncology, University of Bari Medical School, Bari, Italy
4Department of Internal Medicine I, Emergency Medicine and Intensive Care, University Medical Center Würzburg, Germany
5Department of Medicine, Emergency Medicine Unit, University Hospital "Santa Maria della Misericordia", Udine, Italy
6Department of Intensive Care and Anesthesiology, Heart Center Brandenburg, Bernau, Germany
#These authors contributed equally
*Corresponding author: Antonio Giovanni Solimando MD, Department of Biomedical Sciences and Human Oncology, Section of Internal Medicine "G.Baccelli", University of Bari Medical School, Policlinico, Piazza Giulio Cesare 11, 70124 Bari, Italy, Tel: +393395626475, E-mail: antonio.solimando@uniba.it
Int J Crit Care Emerg Med, IJCCEM-3-023, (Volume 3, Issue 1), Case Report; ISSN: 2474-3674
Received: October 31, 2016 | Accepted: February 22, 2017 | Published: February 25, 2017
Citation: Solimando AG, Argentiero A, Ruckdeschel A, Bittrich M, Schneider A, et al. (2017) Emerging Concepts in Acute Heart Failure: From the Pathophysiology to the Clinical Case Based Approach. Int J Crit Care Emerg Med 3:023. 10.23937/2474-3674/1510023
Copyright: © 2017 Solimando AG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Acute heart failure (AHF) represents a heterogeneous clinical syndrome, comprising new or worsening signs and symptoms on a background of stable chronic heart failure (HF), as well as new-onset HF. In either clinical picture, urgent care is crucial. Given the variety of clinical scenario, stratifying patient subgroups on a pathophysiologic base can help direct appropriate therapy. This manuscript recapitulates the current indication, with the aim to define a rational basis for a patient-oriented approach to treatment of AHF.
Clinical Case 1
74-year-old man admitted to the emergency care unit (ECU). He had been sitting in a chair all night and called the ambulance because of increasing dyspnea. He had a history of heart failure (HF) for five years (stable for the past year, NYAH II). Three years before the coronary artery disease (CAD) had been ruled out after a coronary angiography. He had hypertension for more than ten years. His last hospitalization was 1.5 years before being admitted to the ECU, because of an acute cardiac decompensation. He had been taking carvedilol 12.5 mg twice daily (discontinued for two days before the admission because of nausea), ramipril 10 mg once daily, hydrochlorothiazide 25 mg once daily, eplerenone, 25 mg once daily. The emergency physician could objectivize dyspnea at rest, a sinus rhythm, tachycardia, blood pressure (BP) 200/95 mmHg and pulmonary rales. Oxygen, IV clonidine 150 g and furosemide 40 mg were administered already in the ambulance and he was admitted to ECU, where he still had dyspnea at rest, pheripheral edema, raised jugular venous pressure (JVP), BP 140/80 mmHg. Lab works showed creatinine 1.5 mg/dL. NT-proBNP 2083 pg/mL, hsTnI 0.053 ng/mL.
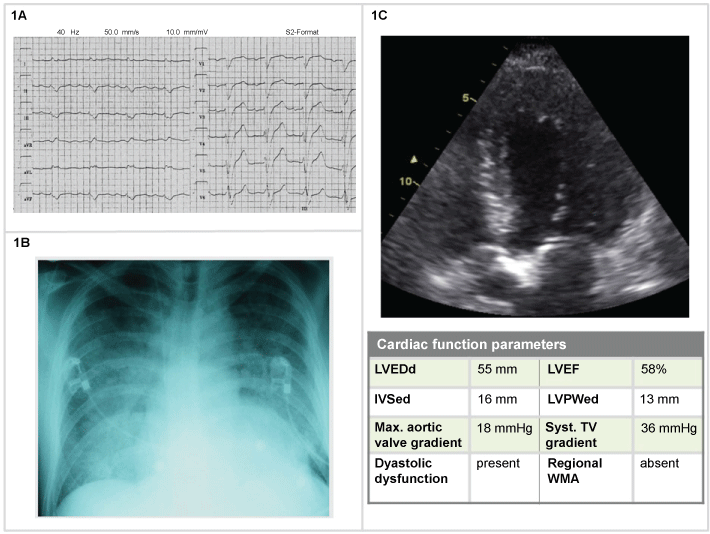
The electrocardiogram (ECG) was abnormal but inconclusive (Figure 1). As in many cases of HF, the ECG does not point to etiology, including presence or absence of ACS. Imaging allows clinicians to exclude aortic stenosis as a cause of AHF. In this patient, moderate pulmonary hypertension was also observed.
.
Figure 1: Man, 74-years-old (1A) Electrocardiogram (ECG): a specific diffuse abnormality, incomplete left bundle branch block; (1B) Chest X-Ray: indistinct vasculature perihilar opacities and peripheral interstitial reticular opacities. Enlarged cardiac silhouette; (1C) 2D echocardiogram: hypertrophic cardiomyopathy with moderate pulmonary hypertension. LVEDd: left ventricular end-diastolic diameter: LVEF: left ventricular ejection fraction; IVSed: Interventricular Septum at end Diastole; LVPWed: left ventricular posterior wall thickness in end-diastole; WMA: wall otion abnormalities; TV: tricuspid valve.
View Figure 1
Thorax RX indicated alveolar edema, enlarged cardiac silhouette, no discrete infiltrates, and cardiac ultrasound allowed to exclude aortic stenosis as a cause of AHF, moderate pulmonary hypertension was observed. The patient received furosemide 80 mg IV, NTG, continuation of previous therapy. The following days the patient was admitted to the cardiac ward and discharged after one week, with a weight loss of 2.5 kg but with residual edema.
Clinical Case 2
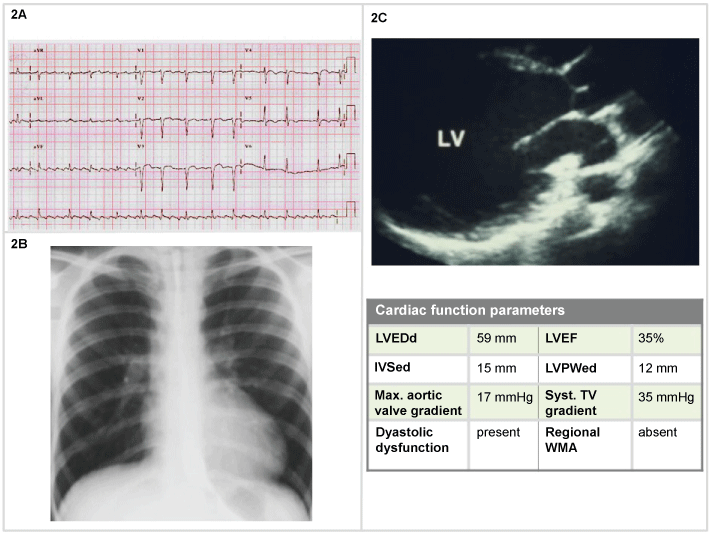
A 60-year-old man with long history of chronic HF, 3 weeks of gradually worsening symptoms, presenting with BP 85/40 mmHg. He was already treated with furosemide 40 mg, aspirin and ramipril. He underwent ECG, thorax RX and echocardiography (Figure 2). Two-dimensional echocardiogram was obtained. Doppler mapping ruled out a left-to-right shunt, aortic regurgitation or tamponade.
.
Figure 2: Man, 60-years-old (2A) ECG: Atrial flutter with variable conduction (mean HR 100 bpm), negative T waves (V1-V5 leads); (2B) Chest X-Ray: increased lucency, moderately flattened diaphragms increased AP diameter, and increased retrosternal clear space. Slightly congested lung hilus and moderate enlarged cardiac silhouette; (2C) 2D echocardiography: systo-diastolic dysfunction with 35% EF.
View Figure 2
Considered his systolic BP, with no clear signs and symptoms of shock, dobutamine 5 g/kg/min was administered, in combination with oxygen therapy 60%. After an initial clinical improvement developed, 3 hours later than the admission he developed increased dyspnea, appeared pale, diaphoretic, mentally confused. The pulse became weak and rapid, often in the range of 90-110 beats/min (bpm). Systolic blood pressure dropped (< 70 mmHg). Rales were audible and the urine output was < 30 mL/h. After endotracheal intubation the patient was admitted in the intensive care unit, where, despite treatment with norepinephrine 10 g/min, dubutamine 20 g/kg/min, he developed refractory hypotension and exitus was confirmed 8 hours later.
Clinical Case 3
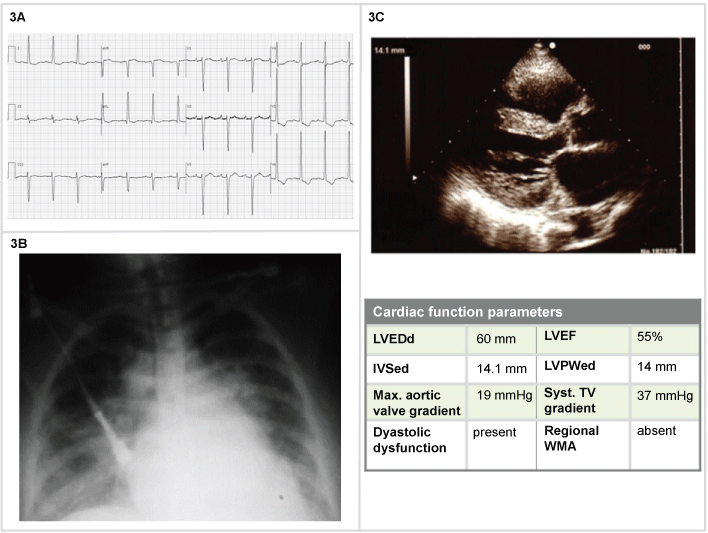
A 80-year-old woman with long history of hypertension, with one hour of sudden onset of dyspnea, headache and a BP 185/120 mmHg. Sat 82%, lactate 2, pH 7.37, PO2 65 mmHg, pCO2 58 mmHg. The chest x-ray showed pulmonary vascular congestion. The heart size was moderately enlarged. The other clinical signs and symptoms and lab findings were irrelevant. Oxygen 60%, IV morfine 4 mg, and furosemide 40 mg were administered and she received nitroglycerin, initially 5 mg/min, then titrated by 5 mg/min at 3-5-min intervals; since no response was seen at 20 mg/min, subsequent increases of 10-20 mg/min were used, until 40 mg/min and BP value of 150/90 mmHg.
After the BP values were stabilized also the symptomatology progressively reversed. The patients were admitted to the normal ward in order to establish the diagnostic-therapeutic approach (Figure 3).
.
Figure 3: Woman, 80-years-old (3A) ECG: signs of atrial abnormality; left ventricle hypertrophy by voltage criteria with left-axis deviation precordial T-waves inversion; (3B) Blurry vasculature perihilar opacities and peripheral interstitial opacities as pulmonary vascular congestion; enlarged cardiac silhouette at chest X-Ray; (3C) 2D echocardiography: left ventricle wall thickening; diastolic dysfunction with preserved EF.
View Figure 3
Clinical Case 4
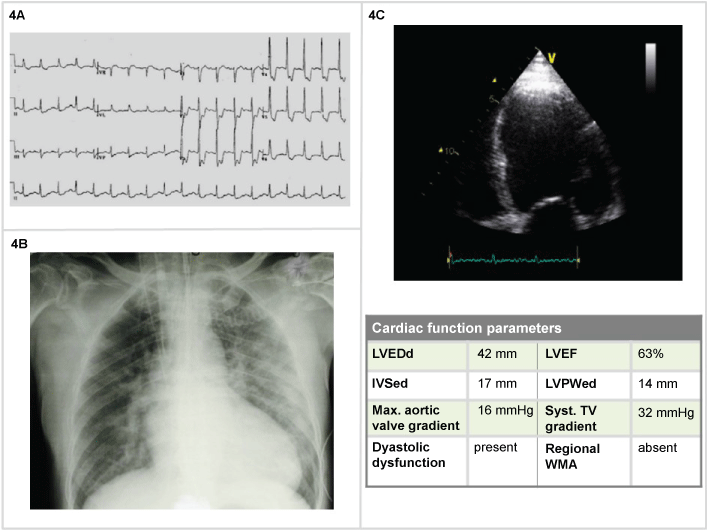
A 68-year-old man with long history of hypertension, diabetes, treated with losartan, amlodipine, metformin, cardioaspirin, presented with progressing worsening dyspnea, BP 200/100 mmHg, HR 130 bpm, BR 28/min, Sat 72%, lactate 3, pH 7,20, PO2 60 mmHg, pCO2 65 mmHg, HCO3- 18, Grace Risk Score 168. The ECG showed sinus tachycardia (130 bpm), signs suggestive for diffuse ischemia (ST-segment depression in most leads (Figure 4A). Those findings were corroborated by the thorax RX, demonstrating diffuse signs of congestion, B kerley lines, enhanced lung hilus, enlarged cardiac silhouette (Figure 4B). The blood gas analysis showed a primary respiratory acidosis, with partial secondary response. The cardiac ultrasound demonstrated clear evidences of dilatative cardiac decompensation, global reduction in contractility with specific apical hypomotility (Figure 4C). In light of those findings and of a Grace Risk Score of 168, the emergency physician could administer Oxygen 60%, IV morfine 4 mg, and furosemide 40 mg. After a nitrate bolus (1 mg followed by continuous infusion 10 g/min - 0,6 mg/h), NIV (BiPAP) with clinical improvement. The coronary angiography demonstrated multiples significant stenosis in the coronary artery (three vascular), the patients received aortocoronary artery bypass (ACBP).
.
Figure 4: Man, 68-years-old (4A) Tachycardia (130 bpm), ECG signs suggestive for diffuse ischemia (multivasal); (4B) Chest X-Ray: diffuse signs of congestion, B kerley lines, enhanced lung hilus, enlarged cardiac silhouette; (4C) 2D echocardiography: evidences of dilatative cardiac decompensation.
View Figure 4
Clinical Case 5
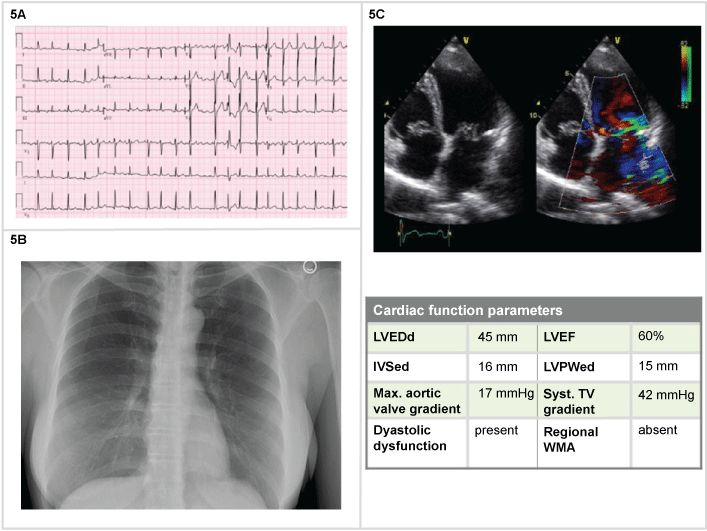
A 28-year-old woman, pregnant at 36th weeks, with long history of systolic murmur, one hour of sudden onset of dyspnea, BP 160/95 mmHg, RR 30/min, Sat 92%, with no ongoing treatment medications. Objectively, she presented a holosystolic murmur, beginning with S1 and continuing through systole to S2, heard at the left ventricular apex and radiates to the axilla. The ECG showed an atrial fibrillation, (150 bpm), ventricular premature complexes, left bundle branch block pattern (Figure 5A).The emergency physician could administer Oxygen 40%, furosemide 40 mg, nitroglycerin, initially 5 mg/min and digoxin 0.25 mg q2h until 1 mg total. After a cesarean delivery the patient was stabilized, a second cardiac ultrasound performed, with the evidence of mitral valve prolapse, a large central mitral jet, Doppler for vena contracta showing 0,72 cm, refilling volume 65 mL/beat (56% of volume), EF 60% and an insufficiency surface area quantified as 0,43 cm2 (Figure 5) and scheduled for valve repair.
.
Figure 5: Woman, 28-years-old (5A) ECG: atrial fibrillation (150 bpm) with left bundle branch block pattern; (5B) Normal chest X-Ray; (5C) 2D echocardiography: mitral valve prolapse, large central jet, enlarged left atrium.
View Figure 5
Working Definition of AHF
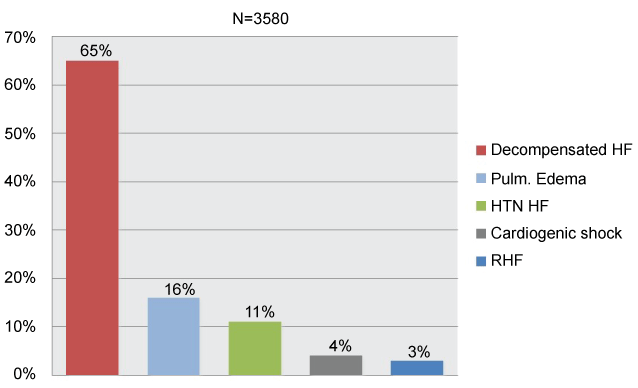
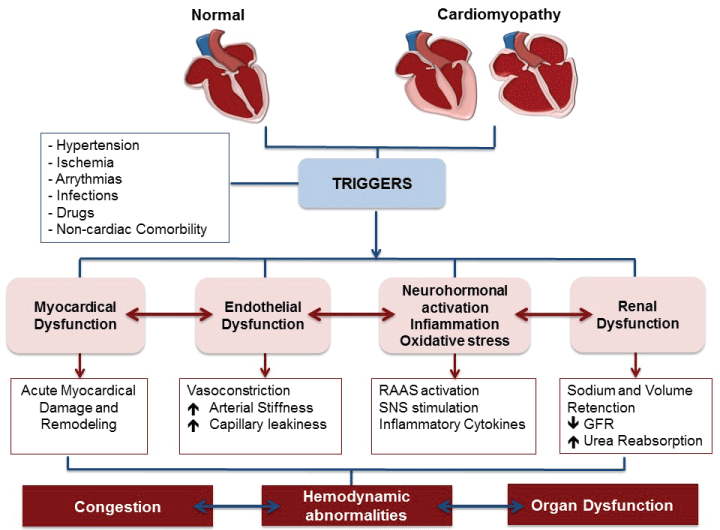
Acute heart failure (AHF) comprises new or worsening signs and symptoms on a background of stable chronic heart failure (HF), as well as new-onset HF. In either scenario, urgent care (i.e. immediate hospitalization) is essential. Patients with AHF are typically elderly: average age is 75 years [1], may have either preserved or reduced ejection fraction. Comorbidities are common and include type 2 diabetes mellitus, chronic kidney disease, and atrial fibrillation. Distinct patient subgroups and disease mechanisms allow for targeted therapy. Indeed, data from the survey indicate that decompensated HF is the most common clinical presentation; pulmonary edema and hypertensive HF are also common presentations (Figure 6) [2]. In acute coronary syndromes (ACS), different pathophysiology helps to stratify patient subgroups and help direct appropriate therapy [3]. In AHF, however, patient subgroups, linked to specific pathophysiologic mechanisms, have not been clearly delineated. AHF combines a substrate, which, for most of patients, is chronic HF, and a trigger, or amplifying mechanism, that changes the clinical milieu. Triggers may be of different kinds: myocardial, renal, neurohormonal, or vascular [1]. A number of mechanisms, operative in cardiovascular disease (CVD), such us vasoconstriction, volume redistribution, after load contractility mismatch, endothelial dysfunction, increased arterial stiffness and capillary leakiness can contribute to the development of AHF (Figure 7) [3,4].
.
Figure 6: Clinical profiles in euroheart failure survey II (AHF): the most common clinical presentation of AHF. Modified from ref [2].
View Figure 6
.
Figure 7: Complex pathophysiological mechanism of AHF: AHF combines a substrate and a triggering factor that may be the myocardial, vascular, neurohormonal, inflammatory and renal, which lead to severe acute hemodynamic abnormalities with congestion and organ dysfunction. RAAS: Renin Angiotensin Aldosterone System; SNS: Sympathetic Nervous System; GFR: Glomerular Filtrate Rate. Modified from ref [1].
View Figure 7
In chronic HF the list of compensatory mechanisms that have been described thus far include activation of the renin-angiotensin-aldosterone (RAA) and adrenergic nervous systems, which are responsible for maintaining cardiac output through increased retention of salt and water, and increased myocardial contractility. In addition, there is activation of a family of countervailing vasodilatory molecules, including the atrial and brain natriuretic peptides (ANP and BNP), prostaglandins (PGE2 and PGI2), and nitric oxide (NO), that offset the excessive peripheral vascular vasoconstriction [4]. Genetic background, sex, age, or environment may influence these compensatory mechanisms, which are able to modulate LV function within a physiologic/homeostatic range so that the functional capacity of the patient is preserved or is depressed only minimally. Although the exact mechanisms that are responsible for acute decompensation are not known, the transition to symptomatic HF is accompanied by increasing activation of neurohormonal, adrenergic, and cytokine systems that lead to a series of adaptive changes within the myocardium collectively referred to as LV remodeling [5].
These changes include hypertrophy and alterations in the contractile properties of the myocytes with progressive loss through necrosis, apoptosis, and autophagic cell death. Moreover, β-adrenergic desensitization, abnormal myocardial energetics and metabolism play a major role in acute emodinamic destabilization in conjunction with reorganization of the extracellular matrix [5]. Remarkably, dissolution of the structural collagen weave surrounding myocytes and subsequent replacement by an interstitial collagen matrix make structural support to the myocytes ineffective. The biologic stimuli for these profound changes include mechanical stretch of the myocyte, circulating neurohormones (e.g., norepinephrine, angiotensin II), inflammatory cytokines [e.g., tumor necrosis factor (TNF)], other peptides and growth factors (e.g., endothelin), and reactive oxygen species (e.g., superoxide) [4,5]. The sustained overexpression of these biologically active molecules is believed to contribute to the progression of HF by virtue of the deleterious effects they exert on the heart and the circulation. Indeed, this insight forms the clinical rationale for using pharmacologic agents that antagonize these systems [e.g., angiotensin-converting enzyme (ACE) inhibitors and beta blockers] in treating patients with HF [6].
In contrast to our understanding of the pathogenesis of HF with a depressed EF, our knowledge of the mechanisms that contribute to the development of HF with a preserved EF is still evolving. That is, although diastolic dysfunction was thought to be the only mechanism responsible for the development of HF with a preserved EF, community-based studies suggest that additional extracardiac mechanisms may be important [6].
Indeed, as shown in the cases presented, clinical presentation of AHF includes severe hypertension, congestion/volume overload, and increased filling pressures even when body weight has not increased [1-3]. Furthermore, in studies with chronic hemodynamic monitoring, there is a disconnect between weight gain and filling pressure, pointing out the role played by vascular redistribution [3,7,8]. Nevertheless, congestion (increased left ventricular end-diastolic diameter - LVEDP), in combination with or without, volume overload are determinant in AHF. Worsening congestion leads to worsening myocardial function and progressive decompensation. Given these evidences and as demonstrated by the cases exposed, is straightforward to notice that incomplete decongestion at discharge is a frequent cause for early rehospitalization. In our representative clinical cases and registry data indicate, the key symptoms of AHF are dyspnea, rales, and peripheral edema [7,9,10]. Despite several investigations among diuretic strategies for acute decompensated heart failure, clinical judgment plays a pivotal role approaching global assessment and therapeutic strategies. Nonetheless, either bolus or continuous infusion of intravenous furosemide as well as low- or high-dose diuretics seems to be effective interventions in symptoms management [11].
In-hospital mortality as well as 60- to 90-day mortality and rehospitalization are increased in the hypotensive patient. Although counterintuitive, it seems to be the case that in AHF, higher systolic blood pressure (SBP) is associated with a better prognosis [7].
AHF etiologies may be distributed among several types of causes, including arterial hypertension, lifestyle indices, drug interactions, worsening renal function and infections [12,13]. Vlachopoulos, et al. studying individuals without HF receiving a routine vaccination, reported a link between the inflammatory response with the vaccination, associated with an increase in vascular stiffness [14]. Such evidences are also important to profile the patient as to underlying cause of AHF [15]. Undeniably, prevention of disease progression in AHF remains a serious unmet need [16].
AHF Leads to Organ Damage: Risk for Death by Early Changes in Surrogate Markers of Organ Function and Indicators of Congestion
In order to achieve improvements in the clinical outcome, early changes in surrogate markers, such as troponin levels, have been associated with organ damage and poor prognosis in AHF [17] and deployed in the clinical practice. Current medications have not demonstrated that they can improve survival and reduce the incidence of hospitalization. The recent serelaxin trial showed a significant reduction in all-cause mortality [18]. Serelaxin appears to address the early organ damage associated with AHF [17]. In AHF patients with heart failure with preserved ejection fraction (HFpEF), compared with those with heart failure with reduced ejection fraction (HFrEF), serelaxin was well tolerated and effective in relieving dyspnea and had a similar effect on short- and long-term outcomes, including survival improvement [19]. With the patient from case 1, medication is straightforward: diuretic, nitroglycerin, and continuation of previous pharmacotherapy. Better management of AHF is needed to address unacceptably high morbidity and mortality, particularly early recognition of cardiac decompensation [13,16]. Nurse-based program involving monitoring inflammation and therapy modification resulted in a 39% relative risk reduction in mortality in patients with AHF after acute decompensation [20]. Moreover, hospitalization appeared to be reduced following use of a wireless monitoring device [21]. Novel HF care model would permit undisrupted continuation of care across different healthcare modalities [22].
ESC HF Long‐Term Registry: All-Cause Mortality
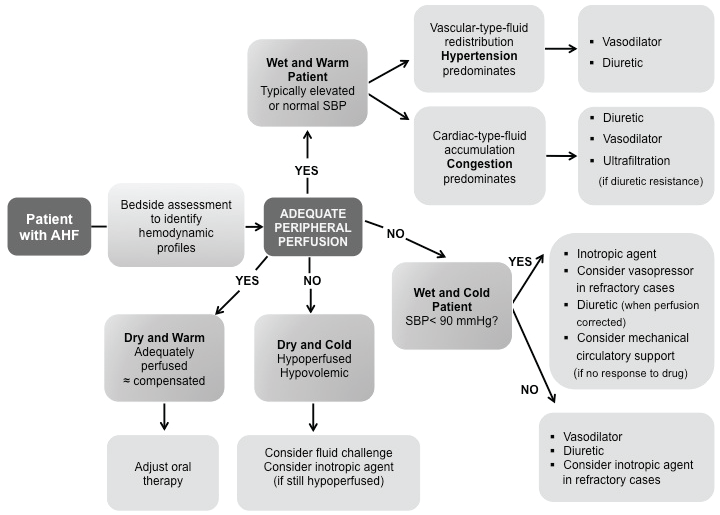
Data from the ESC HF Long-Term Registry: for AHF, there is still a 1-year mortality rate of 24% and rehospitalization rate of 36% [23,24]. The prognosis for hospitalization due to worsening HF is similar to the prognosis for hospitalization with a myocardial infarction or a stroke, as is the prognosis for long-term mortality. The clinical cases presented, confirm that diuretic resistance - lack of response to diuretics - is an independent prognostic variable for mortality in AHF [25]. Organ damage is also a prognostic variable for mortality in AHF [26]. The simple fact is that patients admitted to hospital with AHF have myocardial damage, kidney damage, and perhaps even liver damage. Recommendations on prehospital and early hospital management of AHF were published in 2015. Evidence levels for recommendations are low due to absence of randomized trials. European Society of Cardiology (ESC) guidelines for diagnosis and management of AHF were published in 2016. Use of loop diuretics has a relatively strong recommendation in patients presenting with AHF. This is not the case for other medications [27]. In our small case series guided approach; we can remark the role of clinical judgment in the exclusion of specific causes of instability (Figure 8).
.
Figure 8: 2016 European Society of Cardiology (ESC) guidelines for management treatment of AHF based on four hemodynamic profiles: wet and warm, wet and cold, dry and warm, and dry and cold. Modified from ref [25].
View Figure 8
The guidelines also introduced algorithms, including one for the patient with suspected AHF, where in a number of other potential causes need to be excluded. The current evidences revert to the classification of AHF patients as wet and warm, the most likely categorization, but also as wet and cold, dry and warm, and dry and cold [27]. The two primary hemodynamic determinants of ADHF are elevated LV filling pressures and a depressed cardiac output. Frequently the depressed cardiac output is accompanied by an increase in systemic vascular resistance (SVR) as a result of excessive neurohormonal activation. Because these hemodynamic derangements may occur singly or together, patients with acute HF generally present with one of four basic hemodynamic profiles: normal LV filling pressure with normal perfusion (Profile A), elevated LV filling pressure with normal perfusion (Profile B), elevated LV filling pressures with decreased perfusion (Profile C), and normal or low LV filling pressure with decreased tissue perfusion (Profile L). Most patients can be categorized into one of the four hemodynamic profiles by performing a brief bedside examination that includes examination of the neck veins, lungs, and peripheral extremities. More definitive hemodynamic information may be obtained by performing invasive hemodynamic monitoring, particularly if the patient is gravely ill or if the clinical presentation is unclear. This hemodynamic classification provides a useful guide for selecting the initial optimal therapies for the management of acute HF. Nonetheless, Nowak and his group [28] described three distinct clusters defined using novel noninvasive presenting hemodynamic monitoring technology. Despite such an intriguing approach, further validations are needed to determine whether phenotypic specific therapies based on these clusters can improve outcomes.
Patients with worsening renal function (WRF) but no signs of congestion have a good long-term prognosis compared with patients with WRF and persistent congestion [29]. The recommended approach is aggressive dosing with diuretics [30]. In the ROSE AHF trial, treatment with dopamine or treatment with nesiritide produced no changes in renal parameters or in outcomes [31]. Treatment guidelines recommend vasodilators, but until lately there has not been much new to say about pharmacotherapy [27].
References
-
Felker GM, Teerlink JR (2012) Diagnosis and Management of Acute Heart Failure. In: Bonow RO, Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. (9th edn), Elsevier Saunders, Philadelphia, 484-511.
-
Nieminen M, Brutsaert D, Dickstein K, Drexler H, Follath F, et al. (2006) EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J 27: 2725-2736.
-
Antman EM, Loscalzo J (2011) ST-Segment Elevation Myocardial Infarction. In: Longo DL, Harrison's Principles of Internal Medicine. (18th edn), NY: McGraw-Hill Professional Publishing , New York.
-
Ponikowski Pand, Jankowska EA (2015) Pathogenesis and Clinical Presentation of Acute Heart Failure. Rev Esp Cardiol (Engl Ed) 68: 331-337.
-
Mann DL, Bristow MR (2005) Mechanisms and models in heart failure: The biomechanical model and beyond. Circulation 111: 2837-2849.
-
Kajimoto K, Minami Y, Sato N, Kasanuki H, Investigators of the Acute Decompensated Heart Failure Syndromes (ATTEND) Registry (2016) Etiology of Heart Failure and Outcomes in Patients Hospitalized for Acute Decompensated Heart Failure With Preserved or Reduced Ejection Fraction. Am J Cardiol 118: 1881-1887.
-
Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, et al. (2006) Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 296: 2217-2226.
-
Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, et al. (2005) Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation 112: 2193-2200.
-
Adams KF, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, et al. (2005) Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 149: 209-216.
-
Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, et al. (2003) The EuroHeart Failure survey programme - A survey on the quality of care among patients with heart failure in Europe. Part 1: patientcharacteristics and diagnosis. Eur Heart J 24: 442-463.
-
Felker GM, Lee KL, Bull DA, Margaret M Redfield, Lynne W Stevenson, et al. (2011) Diuretic Strategies in Patients with Acute Decompensated Heart Failure. N Engl J Med 364: 797-805.
-
Fallick C, Sobotka PA, Dunlap ME (2011) Sympathetically mediated changes in capacitance: redistribution of the venous reservoir as a cause of decompensation. Circ Heart Fail 4: 669-675.
-
Angermann CE, Ertl G (2015) Acute heart failure - a unique challenge. Dtsch Med Wochenschr 140: 395-401.
-
Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, et al. (2005) Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation 112: 2193-2200.
-
Oliva F, Mortara A, Cacciatore G, Chinaglia A, Di Lenarda A, et al. (2012) Acute heart failure patient profiles, management and in-hospital outcome: results of the Italian Registry on Heart Failure Outcome. Eur J Heart Fail 14: 1208-1217.
-
Gheorghiade M, De Luca L, Fonarow GC, Filippatos G, Metra M, et al. (2005) Pathophysiologic targets in the early phase of acute heart failure syndromes. Am J Cardiol 96: 11G-17G.
-
Metra M, Cotter G, Davison BA, Felker GM, Filippatos G, et al. (2013) Effect of serelaxin on cardiac, renal, and hepatic biomarkers in the Relaxin in Acute Heart Failure (RELAX-AHF) development program: correlation with outcomes. J Am Coll Cardiol 61: 196-206.
-
Teerlink JR, Cotter G, Davison BA, Felker GM, Filippatos G, et al. (2013) Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet 381: 29-39.
-
Filippatos G, Teerlink JR, Farmakis D, Cotter G, Davison BA, et al. (2014) Serelaxin in acute heart failure patients with preserved left ventricular ejection fraction: results from the RELAX-AHF trial. Eur Heart J 35: 1041-1050.
-
Angermann CE, Störk S, Gelbrich G, Faller H, Jahns R, et al. (2012) Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: the Interdisciplinary Network for Heart Failure (INH) study. Circ Heart Fail 5: 25-35.
-
Adamson PB, Abraham WT, Bourge RC, Costanzo MR, Hasan A, et al. (2014) Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail 7: 935-944.
-
Guder G, Störk S, Gelbrich G, Brenner S, Deubner N, et al. (2015) Nurse-coordinated collaborative disease management improves the quality of guideline-recommended heart failure therapy, patient-reported outcomes, and left ventricular remodeling. Eur J Heart Fail 17: 442-452.
-
Crespo-Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, et al. (2016) European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail 18: 613-625.
-
Kristensen SL, Jhund PS, Køber L, Preiss D, Kjekshus J, et al. (2015) Comparison of outcomes after hospitalization for worsening heart failure, myocardial infarction, and stroke in patients with heart failure and reduced and preserved ejection fraction. Eur J Heart Fail 17: 169-176.
-
Valente MA, Voors AA, Damman K, Van Veldhuisen DJ, Massie BM, et al. (2014) Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J 35: 1284-1293.
-
Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, et al. (2015) Recommendations on pre-hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine. Eur J Heart Fail 17: 544-558.
-
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, et al. (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 37: 2129-2200.
-
Nowak RM, Reed BP, DiSomma S, Nanayakkara P, Moyer M, et al. (2016) Presenting phenotypes of acute heart failure patients in the emergency department: Identification and implications. Am J Emerg Med.
-
Metra M, Davison B, Bettari L, Sun H, Edwards C, et al. (2012) Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail 5: 54-62.
-
Damman K, Testani JM (2015) The kidney in heart failure: an update. Eur Heart J 36: 1437-1444.
-
Chen HH, Anstrom KJ, Givertz MM, Stevenson LW, Semigran MJ, et al. (2013) Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 310: 2533-2543.





