International Journal of Critical Care and Emergency Medicine
To Give or Not To Give - Is that the Question?: The Changing Face of Emergency Oxygen Therapy
Carol Ann Kelly1* and Dave Lynes2
1Postgraduate Medical Institute, Faculty of Health and Social Care, Edge Hill University, UK
2Faculty of Health & Social Care, Edge Hill University, UK
*Corresponding author:
Carol Ann Kelly, Postgraduate Medical Institute, Faculty of Health and Social Care, Edge Hill University, St Helens Road, Ormskirk, Lancashire, L39 4QP, UK, Tel: 0044-1695-657090, E-mail: kellyc@edgehill.ac.uk
Int J Crit Care Emerg Med, IJCCEM-1-005, (Volume 1, Issue 1), Non-systematic Review; ISSN: 2474-3674
Received: July 07, 2015 | Accepted: September 12, 2015 | Published: September 14, 2015
Citation: Kelly CA, Lynes D (2015) To Give or Not To Give - Is that the Question?: The Changing Face of Emergency Oxygen Therapy. Int J Crit Care Emerg Med 1:005. 10.23937/2474-3674/1510005
Copyright: © 2015 Kelly CA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Oxygen's image, together with its reputation, is changing. No longer is it regarded as a benign panacea for all clinical presentations; indeed it is now increasingly evident that oxygen has the potential to contribute to clinical deterioration and mortality.
There is an emerging recognition that oxygen is a drug when administered as a therapeutic intervention and should be used with caution. Contemporary guidelines offer criteria and directives for administration and prescription of oxygen, dependant on the patient's condition, acuity and care setting, yet clinical audit and gathering evidence repeatedly demonstrates that poor practice persists.
There is a need to raise awareness of the importance of maintaining normal oxygen levels and be aware of the detrimental effects of both over and under oxygenation. Clearly more research is needed but in the meantime titration to normal or near normal levels seems a pragmatic solution.
Keywords
Oxygen therapy, Emergency care, Clinical guidelines
Introduction
Oxygen is a colourless, odourless and tasteless chemical element (O). As a therapeutic intervention it is the diatomic molecule, dioxygen (O2), that is commonly referred to as oxygen; the name itself a misnomer. O2 is present as 20.8% of the volume of air, it is essential for cell metabolism in all humans and most forms of life.
Oxygen's image, together with its reputation, is changing. No longer is it regarded as a benign panacea for all clinical presentations; indeed it is now increasingly evident that oxygen has the potential to contribute to clinical deterioration and mortality. But it's not all bad press; in a patient with hypoxaemia or sudden collapse oxygen is still one of the principle treatments, without which emergency care would be primitive. Yet misinformation, misunderstanding, and misplaced beliefs regarding oxygen therapy in clinical practice appear to flourish [1]. This non-systematic review has attempted to highlight the historical context of oxygen, contemporary issues related to its use, and some of the emerging evidence base that has contributed to a paradigm shift in the use of emergency oxygen therapy.
Historical Context and Contemporary Issues
It is interesting to note that caution regarding the use of oxygen therapy isn't new; indeed it has been surrounded by contradiction and controversy since its discovery in the late 18th century. The first reference to the potential detrimental effects of too much oxygen was made by Joseph Priestly himself in 1774 [2], who warned that in the presence of oxygen that:
"as a candle burns out much faster in [oxygen] than in common air, so we might, as may be said, live out too fast ... the air which nature has provided for us is as good as we deserve".
In this citation Priestley seems to be referring to the potential toxicity of oxygen dependant on its concentration, and the fact that the oxygen level provided in atmospheric air is sufficient for health and sustaining life.
In 1849 Lorrain Smith [3] next recognised oxygen's potentially noxious qualities. Smith reported in animal studies that high oxygen tensions act as an irritant, inflaming lung tissue. Advances in physiological understanding were furthered by John Scott Haldane in 1917 [4], when he identified the regulation of the respiratory drive by carbon dioxide and its effects on blood pH and outlined the need for rational use of oxygen. Haldane's cautious advice was to give only as much oxygen as would 'suffice to relieve anoxaemia'[4]. This recognition of judicious employment of oxygen remains a key message in today's guidelines [1].
Experimental work by Meakins in 1920 outlined methods for calculating percentage of oxygen saturation of haemoglobin and its relationship to the patient's condition [5]. setting the scene for the clinical application of the physiological discoveries of the early 20th century.
Juxtaposition to these warnings regarding cautious administration of oxygen was the widespread introduction of the use of 100% oxygen therapy for most acute clinical presentations. The ability to administer high flow oxygen economically, efficiently and comfortably was widely endorsed [6] and although claims were cautioned by those aware of oxygen toxicity, current practice suggests this caveat was seldom heeded and the practice of administrating high flow oxygen to treat most clinical presentations was established and persists today [7,8].
Given the frequency of its use in clinical interventions, there is widespread potential for the misuse of oxygen therapy. For example one UK audit of oxygen use in ambulances recorded use in 34% of patients [7]. For patients with conditions such as COPD (Chronic Obstructive Pulmonary Disease), this prevalence rises to 82% in the emergency department [9]. A further audit found that 88.7% of oxygen administered to patients presenting with an acute exacerbation of COPD in the pre-hospital setting was 'inappropriate' [10]. This seems unsurprising given that the most common reason cited for the administration of emergency oxygen in the acute clinical setting is not hypoxemia but shortness of breath [9].
There is an emerging cognisance that oxygen should be used with caution, and oxygen is regarded as a drug when administered as a therapeutic intervention [11]. Contemporary guidelines offer criteria and directives for administration and prescription of oxygen, dependant on the patient's condition, acuity and care setting [1,12-14]. Guidelines generally suggest that oxygen should be prescribed for hypoxaemia, as either an acute or chronic therapy; the condition being treated normally determining the concentration and duration of oxygen administered. Yet despite these widely accepted guidelines it is evident that poor practice persists [1,15-17]. The reasons why this may be the case remains elusive, but conjecture implies an ingrained culture of 'more is better' [15] and a belief that oxygen alleviates breathlessness [1].
Can Oxygen Therapy Harm?
It has long been established that oxygen can be toxic to the body; evolutionary processes have resulted in the development of protective measures to defend against hyperoxic damage. The ability to harness oxygen as a fuel was developed through the acquisition of mitochondria, which are the inherited legacy from photosynthetic ancestors [18]. The appearance of oxygen in Earth's atmosphere allowed the highly efficient recovery of energy through aerobic respiration allowing oxygen to be harnessed as an economic fuel [19]. This then further supported the evolution of complex organisms with high energy demands.
Paradoxically the adaptation to aerobic respiration required additional adjustment to offer protection against the toxic effects of oxygen. The structure of the mitochondria themselves seems central to this protection; it is thought that the primary evolutionary function of compartmentalisation of mitochondria is to protect the cells' nuclei from the damaging toxic side effects of oxygen metabolism [20].
Oxygen toxicity arises from oxygen's tendency to radicalise, forming incompletely reduced free radicals also known as reactive oxygen species (ROS). ROS can be regarded as unstable molecules produced during normal metabolism that can, in excess, be damaging. An excess of ROS results in oxidative stress.
Not all free radicals are detrimental and some have important roles such as cell signalling, homeostasis and defence mechanisms such as phagocytosis [19]. The presence of free radicals then is essential to normal metabolism and their presence must be tolerated, an anti-oxidant defence system is one way that the body prevents free radical damage [21]. It is these complex metabolic processes that maintain a fine balance in health but which pathological disease states can often tip. When this balance is upset, cumulative oxidative stress at cellular level can cause genetic degeneration and physiological dysfunction leading to progressive aging of a cell and cell death [22].
Although still not fully understood it is now generally accepted that living organisms have evolved to not only utilise oxygen but also to protect themselves from the potential damaging side-effects [19,21,22]. The sensitive equilibrium that the mitochondria aim to achieve is to keep the cellular oxygen concentration high enough to meet the tissue's demands but low enough to minimise or control ROS formation.
Cytotoxic levels of ROS caused by hyperoxygenation are thought to be detrimental to metabolism and cause damage at cellular level [23]. When an excess of ROS or a depletion of antioxidants occurs oxidative stress results, causing damage and cell injury. These free radicals in excess are known to cause mutations and cancers through damage to DNA and protein, and lipid damage causes loss of functionality and an excess of toxic by-products in the cells. They are implicated in inflammation; carcinogenesis; ischeamia-reperfusion injury; atherosclerosis; aging and death [24]. Seemingly the evolutionary irony of oxygen is that in addition to sustaining life it is also what limits lifespan and ultimately kills.
The Danger of Oxygen during Emergency Therapy
For several decades now it has been recognised that oxygen administration can cause significant increases in CO2 levels that can be detrimental to some patients, particularly those with hypercapnic respiratory failure [25]. Advice was proffered by Campbell in 1960 who advocated small increments of oxygen to correct hypoxaemia in order to avoid the risk of worsening hypercapnia [26], referring to the need for 'care and precision' rather than 'haphazard and imprecise dosage'.
In 1962 the detrimental effects of withdrawing oxygen abruptly from patients with hypercapnic respiratory failure was highlighted, cautioning that such practice could worsen hypoxia and hypercapnia and further lower pH. This finding further supported a growing consensus that continuous administration of low flow oxygen with graded increases avoids deterioration of respiratory failure whilst maintaining acceptable oxygen tensions [27]. This philosophy still underpins contemporary emergency oxygen guidelines [1].
Further evidence advocating judicious use of oxygen therapy was published in 1964 [28], suggesting that an arterial oxygen tension of 50 mmHg (7kPa) would prevent death from hypoxia in patients with respiratory failure. The use of arterial blood gases was promoted to distinguish breathlessness from hypoxaemia, and, recognising the dangers of hypoxaemia over hypercapnia, advice was given never to withdraw oxygen completely.
The importance of titrating oxygen to achieve target saturation ranges has become increasingly apparent, with a number of randomised controlled trials indicating that failure to do so, instead over-oxygenating patients presenting with respiratory conditions, can lead to life threatening, if not fatal, respiratory acidosis [15,29-31].
The acidosis resulting from injudicious use of oxygen, often in the pre-hospital setting, has been attributed to a number of mechanisms [1] arguably one of the most important being that of hypoxic pulmonary vasoconstriction. When 'at risk' individuals, namely those with hypercapnic respiratory failure, are administered with high flow oxygen, release of hypoxic pulmonary vasoconstriction occurs, resulting in reduced pulmonary arterial pressure and worsening of ventilation perfusion matching. A mismatch is then created, resulting in increased dead space, further hypercapnia and respiratory acidosis. Other risks to patients receiving excessive oxygen include absorption atelectasis, reduced cardiac output, damage from oxygen free radicals and increased systemic vascular resistance [1].
The evidence is now accumulating for the detrimental use of high flow oxygen and hyperoxaemia in patients with acute respiratory presentations. Austin [15] in a randomised controlled trial assessed the effect of high flow versus titrated O2 on mortality in a population of presumed COPD patients with an acute exacerbation in the prehospital setting in Tasmania. Overall mortality was 9% (21 deaths) in the high flow arm compared with 4% (7 deaths) in the titrated arm. In a sub group of patients with confirmed COPD the mortality was 9% (11 deaths) in the high flow arm compared with 2% (2 deaths) in the titrated arm. This represented a reduced mortality of 58% for all patients and 78% for patients with confirmed COPD.
The dangers of injudicious use of oxygen therapy for patients with a respiratory condition are not restricted to those with COPD. For example a New Zealand study recorded the effect of high concentration oxygen therapy on asthma patients in the emergency department [29]. Through transcutaneous monitoring of CO2 (PtCO2) blood levels, the researchers set out to estimate the proportion of patients with a clinically significant rise in PtCO2. In patients in the high concentration oxygen group the rise in PtCO2 of ≥ 4mm Hg at 60 minutes was 44% versus 19% in the titrated arm. This supports the use of titrated oxygen in patients presenting with acute severe asthma rather than the traditional high flow administration.
There is also evidence that those with suspected CAP (community acquired pneumonia) will be endangered through the use of high concentration oxygen therapy. In one randomised study, 50% of those receiving it showed a clinically significant rise in PtCO2 50% which compares with 14.7% of those in the titrated arm [30]. Amongst the patients with confirmed CAP (radiological confirmation) the PtCO2 rise affected 57.1% (high flow) versus 12.8% (titrated). This suggests that any respiratory disorder with abnormal gas exchange risks increasing hypercapnia with high concentrations of oxygen. In OHS (obesity hypoventilation syndrome) a similar message supports the use of oxygen titrated to saturations. Hollier et al [31] in a double blind randomised crossover study demonstrated that breathing moderate concentration of supplemental oxygen in stable OHS worsened hypercapnia and induced acideamia.
The dangers of inappropriate use of high concentration oxygen therapy are not restricted to patients with respiratory conditions. Indeed recent debate in the cardiology literature has challenged the long established practice of administering 100% oxygen to acute angina and myocardial infarction (MI). Yet the first caution that administering 100% oxygen may be detrimental to ischemic myocardium in the normoxic patient was issued as long ago as 1950 [32]. In 1969 Bourassa et al [33] reported from experimental studies that high flow oxygen can reduce coronary blood flow sufficiently to worsen outcomes. More recently McNulty et al [34] provided evidence that high flow oxygen reduces coronary artery blood flow and confirmed the previously evidence base. Yet the practice of using high flow oxygen persisted and was endorsed for years, and despite subsequent changes in guidelines [1,12], there is evidence that the ritual still persists [35]. Possibly the spotlight on advances in pharmacological treatments and surgical interventions was sufficient distraction to overlook the growing evidence of concerns regarding hyperoxygenation. Moreover a Cochrane Review summarised that there is no conclusive evidence to support the use of oxygen therapy in acute MI unless hypoxaemia is present, and indicated that further clinical trials are urgently needed [36]. A more recent literature review concluded that although the level of evidence is insufficient there is confirmation that this therapy is ineffective and hazardous [37]. A Large RCT is currently underway and due to be reported on in 2014 [38].
Confusion regarding whether to administer oxygen routinely following ischeamic brain injury (stroke) is also apparent. Hypoxia is common immediately following stroke, yet fear of exacerbating oxygen free radical injury and an absence of a positive benefit regarding whether oxygen effects survival or disability in experimental studies [39] has led to controversy. A BMJ editorial outlines the fact that clinical guidelines differ across countries and are indeed contradictory, having changed over time with apparently no justification [40]. Evidence also is conflicting, with some studies suggesting that hyperoxia is harmful to the ischeamic brain, whilst other studies report a neuroprotective effect, and it has been suggested that hyperoxia could indeed narrow the therapeutic window time for thrombolysis [38]. So the jury remains out with regards to the use of oxygen in stroke although current UK guidelines endorse its use only in hypoxic patients [1]. Major on-going clinical trials are anticipated to yield results but the consensus appears to be only treat when hypoxia is present until further evidence is available [41].
Other Areas of Use
Oxygen is also used routinely in the absence of any robust evidence in routine surgery, post-operative care and indeed has been found to mask hypoventilation caused by respiratory depression in sedated patients [42]. On-going clinical trials continue to seek to establish the efficacy of oxygen on mortality, surgical site infections, nausea and atelectasis with varying results [43-45]. Likewise on-going research is attempting to position the use of hyperoxygenation in cluster headache [46] and the use of hyperbaric oxygen for various chronic wounds [47,48].
Can oxygen be prescribed to correct hypoxemia in the emergency situation whilst avoiding the dangers associated with oxygen therapy?
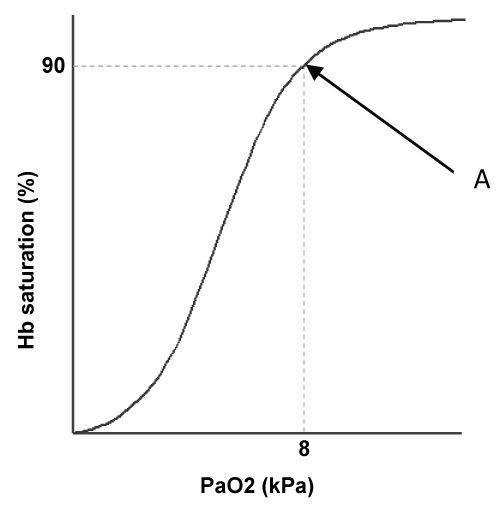
Oxygen therapy should not be prescribed for breathlessness, but for hypoxemia; there is no benefit in providing emergency oxygen therapy if the patient is already adequately oxygenated. The objective is to achieve adequate haemoglobin saturation without over-oxygenating the patient. The major source of transport for oxygen is haemoglobin, and there is a direct relationship between the partial pressure of oxygen available and the percentage of haemoglobin that will be saturated, represented by the oxyheamoglobin dissociation curve. Importantly this curve is not linear but sigmoid in shape: a unique property that influences saturation and desaturation (Figure 1).
Figure 1, point A, illustrates a clinically critical point on the curve; this is the point, 8kPa/90% saturation, below which respiratory failure is defined. Above this point it can be seen that the curve plateaus demonstrating that with further considerable increases in arterial oxygen tension (normal PaO2 is 11-13 kPa) relatively small gains in saturation will result. Contrary to this if the arterial oxygen tension falls below 8 kPa the saturation of the haemoglobin falls rapidly. The principles of this curve contribute to the rationale for the titration of oxygen to within set ranges according to the presenting clinical condition, and illustrate the futility of over-oxygenation.
The patient should, therefore, receive enough oxygen to achieve adequate haemoglobin saturation, but should not exceed the saturation expected in a normal healthy person. There is consensus that oxygen should be prescribed according to a target saturation range, and for those who provide and administer the therapy to monitor the affect of the therapy and the patient and keep within the target saturation range. Indeed the British Thoracic Society (BTS) guidelines for emergency oxygen therapy [1] describe this recommendation as the essence of the guideline.
The evidence base related to emergency oxygen therapy is rapidly changing, and newer BTS guidelines are expected in 2015; the clinician who prescribes oxygen therapy is beholden to remain cognisant of such changes. However key principles are likely to remain constant, which are that the aim of therapy is to achieve normal or near-normal oxygen saturation for all acutely ill patients apart from those at risk of hypercapnic respiratory failure. Target saturation ranges have been recommended, and for most patients these are 94 - 98% but for those at risk of hypercapnic respiratory failure, 88 - 92% or patient specific saturation [1].
Summary
The history of oxygen clearly contributed to the evolvement of evidence based medicine and oxygen does and will continue to play a central role in contemporary healthcare. To date however it seems that empirical knowledge has been overshadowed by erroneous beliefs and a culture of using oxygen as a cure for all. Yet empirical evidence for oxygen is still lacking. It seems that perhaps oxygen therapy has evolved on a trial and error basis founded on the historical perspective. A viewpoint further observed in other specialist areas where the sluggish application of gathering evidence has undoubtedly inflicted further harm and cost lives. Despite this retrospective analysis and conjecture however, firm evidence now exists that does not condone this haphazard approach or negate current poor practice.
Implementation of changes in the pre-hospital setting may be easier as this is protocol driven but practice varies elsewhere. A new gold standard of titration must be adopted urgently to prevent unnecessary morbidity and mortality. Withholding oxygen from a patient presenting with acute shortness of breath, chest pain or stroke may seem counter-intuitive or irrational, but the hazardous properties of oxygen must be remembered and consideration of whether administering oxygen could do more harm than good needs to be at the forefront of clinicians' plan for clinical management.
There is a need to raise awareness of the importance of maintaining normal oxygen levels and be aware of the detrimental effects of both over and under oxygenation. Clearly more research is needed but in the meantime titration to normal or near normal levels seems a pragmatic solution.
References
-
O Driscoll BR, Howard LS, Davison AG, British Thoracic Society (2008) Guideline for Emergency Oxygen use in Adult Patients. Thorax 63.
-
Priestly J (1775) The Discovery of Oxygen Part I: Experiments by Joseph Priestley, LLD. On Dephlogisticated Air. From The Alembic Club Reprints of the University of Chicago Press in 1912. Milton Keynes: Bibliolife Reproduction Series.
-
Smith JL (1899) The pathological effects due to increase of oxygen tension in the air breathed.J Physiol 24: 19-35.
-
Haldane JS (1917) The Therapeutic Administration of Oxygen.Br Med J 1: 181-183.
-
Meakins JC (1920) The therapeutic value of oxygen in pulmonary lesions: preliminary note. Br Med J 1: 324-326.
-
Boothby WM, Mayo CW, Lovelace WR (1939) One hundred percent oxygen: indications for its use and methods of its administration. JAMA 113: 477-482.
-
Hale KE, Gavin C, O'Driscoll BR (2008) Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J 25: 773-776.
-
Eastwood GM, Peck L, Young H, Prowle J, Jones D, et al. (2011) Oxygen administration and monitoring for ward adult patients in a teaching hospital. Intern Med J 41: 784-788.
-
Considine J, Botti M, Thomas S (2012) Descriptive analysis of emergency department oxygen use in acute exacerbation of chronic obstructive pulmonary disease. Internal Medicine Journal 42: e38-e47.
-
Ringbaek TJ, Terkelsen J, Lange P (2015) Outcomes of acute exacerbation in COPD in relation to pre-hospital oxygen therapy. European Clinical Respiratory Journal 2.
-
www.bnf.org/bnf
-
JRCALC (Joint Royal Collages Ambulances Liaison Committee) (2013) Clinical Practice Guidelines. Class Professional Publishing, Bridgewater.
-
NICE (National Institute for Clinical Excellence) (2010) Management of chronic obstructive pulmonary disease in adults in primary and secondary care (partial update).
-
GOLD (Global Initiative for Chronic Obstructive Lung Disease) (2014) Global Strategy for the Diagnosis, Management and Prevention of COPD.
-
Austin MA, Wills KE, Blizzard L, Eugene Walters, Richard Wood-Baker (2010) Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ 341: c5462.
-
Davidson AC, Williams S, Baxter N, E Morris, L Restrick (2011) P102: A Survey of Oxygen Provision Across London. Thorax 66: A108-A109.
-
Lee C, McDonnell L, Davidson C (2013) Has the New Contract Delivered Better Ambulatory Oxygen Devices for Patients? A London Perspective. Thorax 68: A205-A206.
-
Freeman BA (2000) Oxygen: the air-borne nutrient that both sustains and threatens life. Nutrition 16: 478-480.
-
Lane N (2002) Oxygen: The Molecule that made the World. Oxford University Press.
-
Abele D (2002) Toxic oxygen: the radical life-giver. Nature 420: 27.
-
Monaghan P, Metcalfe NB, Torres R (2009) Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12: 75-92.
-
Dowling DK, Simmons LW (2009) Reactive oxygen species as universal constraints in life-history evolution. Proc Biol Sci 276: 1737-1745.
-
Bryan CL, Jenkinson SG (1988) Oxygen toxicity.Clin Chest Med 9: 141-152.
-
Van der Walt J (2006) Oxygen - elixir of life or Trojen horse? Part 1: oxygen and neonatal resuscitation. Paediatric Anaesthesia 16: 1107-1111.
-
Kenneth Donald, Thomas Simpson, John Mcmichael, Bernard Lennox (1949) Neurological Effects of Oxygen. The Lancet 254: 1056-1057.
-
Campbell EJ (1960) Respiratory failure: the relation between oxygen concentrations of inspired air and arterial blood. Lancet 2: 10-11.
-
Massaro DJ, Katz S, Luchsinger PC (1962) Effect of various modes of oxygen administration on the arterial gas values in patients with respiratory acidosis.Br Med J 2: 627-629.
-
Hutchison DC, Flenley DC, Donald KW (1964) Controlled oxygen therapy in respiratory failure. Br Med J 2: 1159-1166.
-
Perrin K, Wijesinghe M, Healy B, Wadsworth K, Bowditch R, et al. (2011) Randomised controlled trial of high concentration versus titrated oxygen therapy in severe exacerbations of asthma. Thorax 66: 937-941.
-
Wijesinghe M, Perrin K, Healy B, Weatherall M, Beasley R (2012) Randomized controlled trial of high concentration oxygen in suspected community-acquired pneumonia. J R Soc Med 105: 208-216.
-
Hollier CA, Harmer AR, Maxwell LJ, Menadue C, Willson GN, et al. (2014) Moderate concentrations of supplemental oxygen worsen hypercapnia in obesity hypoventilation syndrome: a randomised crossover study. Thorax 69: 346-353.
-
Russek HI, Regan FD, Naegele CF (1950) One hundred percent oxygen in the treatment of acute myocardial infarction and severe angina pectoris.J Am Med Assoc 144: 373-375.
-
Bourassa MG, Campeau L, Bois MA, Rico O (1969) The effects of inhalation of 100 percent oxygen on myocardial lactate metabolism in coronary heart disease.Am J Cardiol 24: 172-177.
-
McNulty PH, King N, Scott S, Hartman G, McCann J, et al. (2005) Effects of supplemental oxygen administration on coronary blood flow in patients undergoing cardiac catheterisation. Am J Physiol Heart Circ Physiol 288: H1057-H1062.
-
Burls A, Emparanza JI, Quinn T, Cabello JB (2010) Oxygen use in acute myocardial infarction: an online survey of health professionals' practice and beliefs. Emerg Med J 27: 283-286.
-
Cabello JB, Burls A, Emparanza JI, Bayliss S, Quinn T (2010) Oxygen therapy for acute myocardial infarction. Cochrane Database Syst Rev.
-
Shuvy M, Atar D, Gabriel Steg P, Halvorsen S, Jolly S, et al. (2013) Oxygen therapy in acute coronary syndrome: are the benefits worth the risk? Eur Heart J 34: 1630-1635.
-
http://clinicaltrials.gov/ct2/show/NCT01272713
-
Singhal AB (2006) Oxygen therapy in stroke: past, present, and future.Int J Stroke 1: 191-200.
-
Godlee F (2012) Oxygen and publicity. BMJ 345.
-
Pountain SJ, Roffe C (2012) Does routine oxygen supplementation in patients with acute stroke improve outcome? BMJ 345: e6976.
-
Kopka A, Wallace E, Reilly G, Binning A (2007) Observational study of perioperative PtcCO2 and SpO2 in non-ventilated patients receiving epidural infusion or patient-controlled analgesia using a single earlobe monitor (TOSCA). Br J Anaesth 99: 567-571.
-
Togioka B, Galvagno S, Sumida S, Murphy J, Ouanes JP, et al. (2012) The role of perioperative high inspired oxygen therapy in reducing surgical site infection: a meta-analysis.Anesth Analg 114: 334-342.
-
Meyhoff CS, Jorgensen LN, Wetterslev J, Christensen KB, Rasmussen LS, et al. (2012) Increased Long-term Mortality After a High Perioperative Inspiratory Oxygen Fraction During Abdominal Surgery: Follow-Up of a Randomised Clinical trial. Anesth Analg115: 849-854.
-
Hovaguimian F, Lysakowski C, Elia N, Tramèr MR (2013) Effect of intraoperative high inspired oxygen fraction on surgical site infection, postoperative nausea and vomiting, and pulmonary function: systematic review and meta-analysis of randomized controlled trials.Anesthesiology 119: 303-316.
-
Jürgens TP, Schulte LH, May A (2013) Oxygen treatment is effective in migraine with autonomic symptoms. Cephalalgia 33: 65-67.
-
Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, et al. (2012) Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev 4.
-
Nakamura A, Osonoi T, Terauchi Y (2010) Relationship between urinary sodium excretion and pioglitazone-induced edema. J Diabetes Investig 1: 208-211.