International Journal of Clinical Cardiology
Evaluation of Left Ventricular Systolic Function Immediately after Pulmonary Vein Isolation in Patients with Chronic Atrial Fibrillation using Speckle Tracking Echocardiography
Hisao Matsuda1*, Tomoo Harada2, Yui Nakayama2, Marika Yamada2, Yasushi Takagi4, Makoto Takano2, Ikutarou Nakajima2, Emi Nakano1, Yukako Takimura3, Satoru Nishio3, Toshiyuki Furukawa2, Hidekazu Miyazaki3 and Yoshihiro J Akashi2
1Division of Cardiology, St. Marianna University school of Medicine, Yokohama city Seibu Hospital, Yokohama, Japan
2Division of Cardiology, Department of Internal Medicine, St. Marianna University school of Medicine, Kawasaki, Japan
3Division of Cardiology, Kawasaki municipal Tama Hospital, Kawasaki, Japan
4Division of Cardiology, St. Marianna University school of Medicine, Toyoko Hospital, Kawasaki, Japan
*Corresponding author: Hisao Matsuda, Division of Cardiology, St. Marianna University School of Medicine, Yokohama City Seibu Hospital, Asahi-ku, Yasashi-cho, 1197-1, Yokohama, Japan, Tel: +81453661111, E-mail: h.matsuda@marianna-u.ac.jp
Int J Clin Cardiol, IJCC-3-081, (Volume 3, Issue 2), Original Article; ISSN: 2378-2951
Received: May 27, 2016 | Accepted: July 14, 2016 | Published: July 18, 2016
Citation: Matsuda H, Harada T, Nakayama Y, Yamada M, Takagi Y, et al. (2016) Evaluation of Left Ventricular Systolic Function Immediately after Pulmonary Vein Isolation in Patients with Chronic Atrial Fibrillation using Speckle Tracking Echocardiography. Int J Clin Cardiol 3:081. 10.23937/2378-2951/1410081
Copyright: © 2016 Matsuda H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: The effect of pulmonary vein isolation (PVI) on left ventricular (LV) function after restoration of sinus rhythm remains unclear in patients with chronic atrial fibrillation (CAF). This study aimed to evaluate LV systolic function in the early phase after PVI using speckle tracking echocardiography.
Methods: Twelve patients with CAF could be restored to sinus rhythm by PVI and maintained sinus rhythm after PVI. Echocardiography was performed before, immediately after and 3 months after PVI. Measurements from conventional echocardiography and speckle tracking imaging were analyzed. Radial strain (RS) was calculated for each of the 6 standard segmental LV regions on left midventricular short-axis images.
Results: In all segments, RS was significantly increased immediately after PVI and showed no significant change after 3 months. As a conventional parameter, LV ejection fraction remained unimproved in the early phase.
Conclusion: LV systolic function could be significantly improved in the early phase after PVI among patients with CAF. These improvements could be quantified using speckle tracking echocardiography.
Keywords
Atrial fibrillation, Catheter ablation, Speckle tracking echocardiography, Pulmonary vein isolation, Left ventricular function
Introduction
Radiofrequency catheter ablation (RFCA) is an accepted option for treating drug-resistant atrial fibrillation (AF) [1]. The most commonly used ablation strategy is circumferential pulmonary vein (PV) isolation (PVI) around the ostium of the ipsilateral PV using RF current. In clinical trials of patients without significant structural heart disease, RFCA is superior to pharmacotherapy in maintaining sinus rhythm for AF [2,3]. In addition, PVI has allowed maintenance of sinus rhythm in patients with chronic AF (CAF) [4]. However, the precise effects of PVI on left ventricular (LV) systolic function in patients with CAF remain unclear. Two-dimensional (2D) speckle tracking imaging (STI) is currently used to noninvasively evaluate left ventricular systolic function [5,6]. This technique enables accurate detection of subtle abnormalities in ventricular function that are not revealed by conventional echocardiographic parameters such as LV ejection fraction (LVEF) [7]. STI has been used to quantitatively evaluate myocardial strain patterns independent of cardiac translation angle [8], overcoming a major limitation of Tissue Doppler Imaging (TDI) [9]. STI is also less time-consuming than TDI and has been validated in both experimental and human studies [8]. Furthermore, reductions in myocardial strain precede significant changes in LVEF [10], and STI has been recommended for early detection of sub-clinical LV systolic dysfunction [11].
The purpose of this study was to assess LV systolic function in the early phase after PVI using 2D STI after RFCA in patients with CAF.
Material and Methods
Study population
Participants in this study comprised 33 patients with drug-resistant CAF who underwent catheter ablation for CAF between April 2007 and April 2009. Written informed consent was obtained from all patients prior to enrolment. Twelve of these 33 patients maintained sinus rhythm with or without use of anti-arrhythmic drugs, and were able to undergo echocardiography before PVI, immediately after PVI (within 1 week after PVI), and 3 months after PVI. The present study retrospectively analyzed echocardiographic data from these 12 patients. This study was approved by St. Marianna University Ethics Committee (Approved Number: 3316).
PVI
Left atrium (LA) and left atrial appendage thrombus were excluded by transesophageal echocardiography before the procedure in all patients. Antiarrhythmic drugs were disrupted at least a week before the procedure. The procedure was performed with deep sedation using midazolam and continuous infusion of dexmedetomidine. A 6-F decapolar catheter was positioned in the coronary sinus through the right internal jugular vein. Through the right femoral vein, two steerable 8.5-F long sheaths (Agilis; St. Jude Medical) and a non-steerable 8.5-F long sheath (RAMP 90; St. Jude Medical) were introduced into the LA using a single, transseptal puncture guided by fluoroscopy.
Directly after transseptal puncture, heparin was administered intravenously at a dose of 100 U/kg body weight. Activated clotting time (ACT) was subsequently checked every 30 min, with administration of additional heparin as necessary to maintain ACT > 300 s.
After transseptal puncture, LA angiography was performed during right ventricular high-rate pacing (200 beats/min). All procedures were guided using a 3D electro anatomical mapping system (CARTO; Biosense Webster). Two spiral mapping catheters (Lasso; Biosense Webster) were positioned in each ostium of the left and right PVs. PVI was performed with an 8-mm tip catheter (Navistar; Biosense Webster) or 3.5 mm tip irrigated catheter (Navistar Thermocool; Biosense Webster) to create a single circular line around 2 ipsilateral PVs and complete block between PVs and the LA. Isolation of each PV was confirmed by bidirectional block (entrance block and exit block) using the spiral mapping catheter. When PVI was not achieved with circumferential ablation, the earliest PV potentials were targeted sequentially until complete isolation was achieved. After the successful PVI, antiarrhythmic drugs were resumed and continued during the follow up period.
Conventional echocardiography and 2D speckle tracking imaging
Echocardiography was performed in the left lateral decubitus position using a Vivid 7 system (GE Medical Systems, Milwaukee, WI). LV end-diastolic and end-systolic volumes were measured at the standard apical two- and four-chamber views using a modification of Simpson's rule and LVEF was derived [12].
STI was performed using an available speckle-tracking system in an EchoPAC workstation (GE Medical Systems). Standard grey-scale 2D images were acquired at a high frame rate to ensure adequate tracking of the speckles equally distributed within the myocardium. Myocardial strain can be calculated by measuring the change in the position of the speckles within a myocardial segment along the cardiac cycle [13]. Radial strain assesses the thickening and thinning of the myocardial wall, as measured in the standard midventricular short-axis view. To maximize reproducibility, parasternal circular midventricular short-axis images were taken at the papillary muscle as an indicator of the measurement level in the left ventricle.
The midventricular short axis of the LV wall was divided into standard 6 segments; Antero-Septal, Anterior, Lateral, Posterior, Inferior, and Septal. STI applied to routine midventricular short-axis images calculated radial strain from multiple circumferential points averaged to the 6 standard segments (Figure 1).
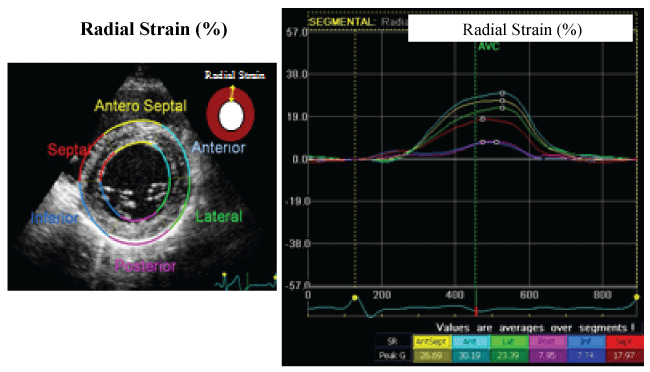
.
Figure 1: Two-dimensional speckle tracking imaging (radial strain).
Myocardial strain can be calculated by measuring changes in the position of speckles within a myocardial segment along the cardiac cycle. Radial strain assesses thickening and thinning of the myocardial wall, measured on the standard midventricular short-axis view, and the value is expressed as the ratio of wall thickening (%). The midventricular short axis of the LV wall is divided into standard 6 segments: Antero-Septal, Anterior, Lateral, Posterior, Inferior, and Septal. Speckle Tracking Imaging applied to routine midventricular short-axis images calculates radial strain from multiple circumferential points averaged to the 6 standard segments.
View Figure 1
Echocardiography was performed before PVI, immediately after PVI (within 1 week after PVI), and 3 months after PVI. LVEF as a conventional echocardiographic parameter and radial strain at each standard segment from the short-axis view were calculated at each time. An example of measurement of the STI is shown in figure 2. To avoid underestimation of LV systolic function during AF before PVI, calculations of all parameters were performed at the preceding RR interval > 500 ms.
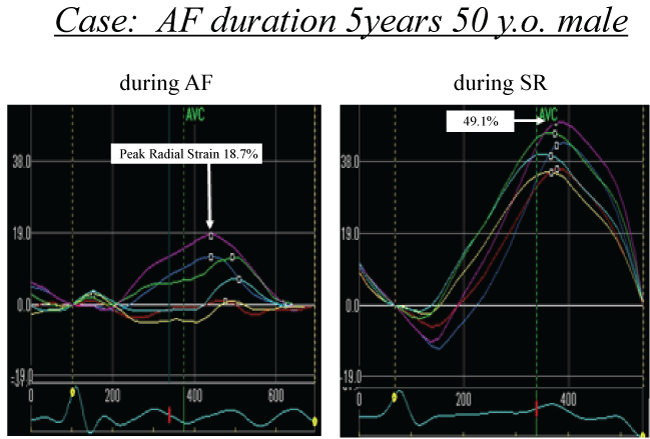
.
Figure 2: An example measurement of radial strain.
The midventricular short axis of the left ventricular (LV) wall is divided into standard 6 segments. Radial strain is calculated at each segment. Yellow line- Antero-Septal, sky blue- Anterior, green- Lateral, purple- Posterior, blue- Inferior and red- Septal. In this case, peak radial strain is 18.7% during AF (left panel). Radial strain improves to 49.1% during SR and radial strain is improved in all segments (right panel).
AF = Atrial Fibrillation, SR= Sinus Rhythm
View Figure 2
Statistical analysis
All continuous variables are expressed as mean ± standard deviation. Student's t test was used for statistical analysis. Two-tailed probability values of p < 0.05 were considered statistically significant. All statistical analyses were performed using Excel 2010 software (Microsoft).
Results
Patient characteristics are described in table 1. All PVs were successfully isolated in all 12 patients. Corresponding to this study protocol, echocardiography before PVI was performed during AF in all patients. Mean preceding RR interval was 927 ± 266 ms before PVI, 928 ± 185 ms immediately after PVI, and 1004 ± 183 ms at 3 months after PVI. No significant differences in the preceding RR interval were apparent.
![]()
Table 1: Patient characteristics.
View Table 1
Although LVEF showed a tendency toward improvement immediately after PVI, the degree of improvement was not significant (58.8 ± 11.2% before PVI, 64.1 ± 9.15% immediately after PVI; p = 0.18). However, LVEF showed significant improvement at 3 months after PVI (66.91 ± 6.90%; p < 0.05) (Figure 3). Left atrial dimension (LAD) showed similar improvement with LVEF. Although, there was no significant improvement immediately after PVI (45.6 ± 7.70 mm before PVI, 43.9 ± 8.27 mm immediately after PVI; p = 0.41), LAD showed significant improvement at 3 months after PVI (41.0 ± 4.85 mm; p < 0.05). Other conventional echocardiographic parameters, Left ventricular end-diastolic dimension (LVDd) and Left ventricular end-systolic dimension (LVDs) showed no significant difference during the follow up periods. (LVDd; 49.1 ± 6.49 mm before PVI, 47.2 ± 5.17 mm immediately after PVI, and 47.6 ± 4.22 mm 3 months after PVI. LVDs; 33.8 ± 5.92 mm before PVI, 30.5 ± 4.73 mm immediately after PVI, and 30.3 ± 4.68 mm 3 months after PVI.) In contrast to these results, radial strain at the midventricular level showed significant improvement immediately after PVI in all 6 standard segments (p < 0.01), and the improvement of radial strain was unchanged 3 months after PVI (Figure 4).
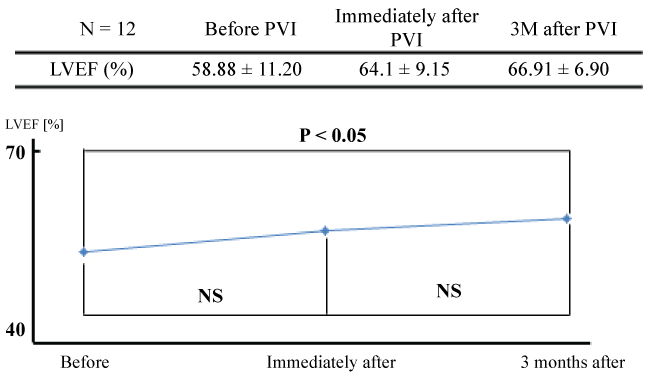
.
Figure 3: LVEF before and after PVI.
Although left ventricular ejection fraction (LVEF) shows a tendency toward improvement immediately after pulmonary vein isolation (PVI), the degree of improvement is not statistically significant (58.8 ± 11.2% before PVI, 64.1 ± 9.15% immediately after PVI; p = 0.18). However, LVEF is significantly improved at 3 months after PVI (58.8 ± 11.2% before PVI, 66.91 ± 6.90% at 3 months after PVI; p < 0.05).
View Figure 3
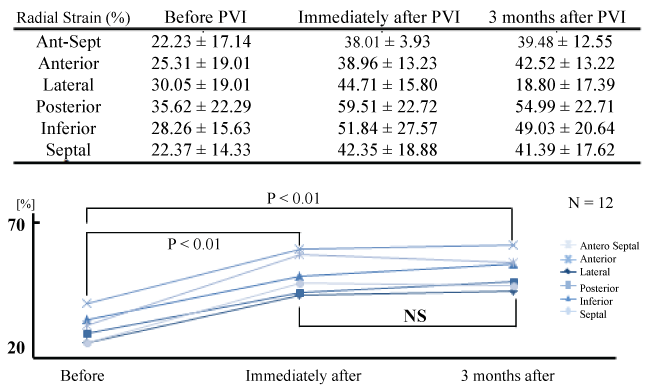
.
Figure 4: Radial strain before and after PVI.
Radial strain is significantly improved immediately after pulmonary vein isolation (PVI) in all 6 standard segments (p < 0.01), and improvement of radial strain is maintained at 3 months after PVI.
View Figure 4
Discussion
Our study demonstrated that: 1) PVI could restore sinus rhythm in some patients with long-lasting CAF; 2) LV systolic function was significantly improved after restoration of sinus rhythm in patients with CAF; and 3) improvement of radial strain on STI precedes the improvement of LVEF. To the best of our knowledge, this study is the first systematic analysis of LV systolic function using STI in the early phase after PVI in patients with CAF.
Radiofrequency catheter isolation of PVs has become a standard and potentially curative treatment for symptomatic, drug-refractory AF [1]. RFCA for AF has been shown to be safe and effective in a large number of cases [1]. Although the rate of AF recurrence after RFCA for CAF remains considerably high and repeat procedures are frequently necessary, RFCA has gained acceptance as a treatment option for CAF. The indication of RFCA for CAF is currently categorized as class 2 [14].
Several previous studies [15-18] have shown that RFCA for AF in patients with LV systolic dysfunction results in significant improvement of LV function. As a conventional parameter of global LV systolic function, LVEF was used in those previous studies. However, observer-dependent subjective parameters, such as LVEF, require experience and have problems with reproducibility. STI is well-known as an angle- and observer-independent tool for detecting abnormalities of wall motion [7]. This method measures myocardial thickening and shortening, providing radial strain. This technique enables accurate detection of subtle abnormalities in ventricular function that remain unrevealed by conventional echocardiographic parameters such as LVEF [7].
We have demonstrated that LVEF was significantly improved after the maintenance of sinus rhythm. The results of our study are in accordance with those of previous studies. In addition to this result, we have also demonstrated that improvement of radial strain precedes the improvements in LVEF. Previous studies [19,20] have shown that subtle changes in the left ventricle were observed in AF patients with preserved systolic function. In those studies, STI was used to assess the improvement of LV systolic function. STI offers substantial advantages for the evaluation of subtle changes in LV systolic function compared with conventional echocardiography.
AF is a common cause of tachycardia-induced cardiomyopathy, which is characterized by decreased LVEF and structural remodeling. Long-standing rapid LV response in patients with CAF leads to LV systolic dysfunction, and this type of cardiomyopathy is a well-known form of reversible myocardial dysfunction [21,22]. The most important aspect of treatment for patients with tachycardia-induced cardiomyopathy is heart rate normalization with adequate rhythm control. Maintenance of sinus rhythm results in increased LVEF and improved exercise tolerance [17]. The mechanisms underlying improved LVEF after RFCA of AF include better control of ventricular rate, rhythm regularization, and restoration of left atrial transport function. Furthermore, synchronized atrioventricular contractility is another important factor in the improvement of LVEF [15]. Greater benefits of RFCA for AF in patients with baseline LV systolic dysfunction suggest that the majority of cases of tachycardia-induced cardiomyopathy involve AF with rapid LV response.
However, mean values of baseline LVEF in the present study were relatively maintained, ranging from 42.2% to 75.4%, with a mean LVEF of 58.8 ± 11.2%. We confirmed that improvement of radial strain precedes improvement of LVEF in the population with relatively preserved LVEF. Although greater benefits of RFCA are seen in patients with baseline LV systolic dysfunction, STI could evaluate subtle changes of LV wall motion in patients with baseline preservation of LV systolic function.
On the other hand, compared to patients with paroxysmal AF, those with CAF have the potential to achieve positive effects on LV systolic function through the restoration and maintenance of sinus rhythm by RFCA [16]. Patients with CAF ordinarily suffer a greater AF burden, such as longer duration and higher frequency, which could result in tachycardia-induced cardiomyopathy and decreased LV systolic function. Patients with CAF could thus benefit more from successful RFCA than those with paroxysmal AF. The present study was intended for patients with CAF, and this could be a factor in the improvement of LV systolic function after successful RFCA, even in patients with relatively preserved LV systolic function. The improvement of LV systolic function could be evaluated by radial strain earlier than by conventional parameters.
We were able to confirm that improvement of cardiac strain precedes the improvement of LVEF in patients with CAF after restoring sinus rhythm. The present study suggests that the evaluation of LV systolic function immediately after PVI using STI may bring important added value to daily clinical examinations.
Study Limitations
Several limitations to this study should be acknowledged. First, the lack of reproducibility of values obtained by 2D STI remains a major problem. As mentioned above, we measured radial strain at the level of the papillary muscle in order to improve reproducibility. Secondly, although the sample size was relatively small to evaluate STI or LVEF, we were still able to identify statistically meaningful differences from these relatively small numbers, suggesting that STI could quantify the improvement of LV systolic function in the early phase after PVI in patients with CAF. Thirdly, the patients who could be restored to sinus rhythm and maintained sinus rhythm were enrolled in the present study. The effective results of improved LV systolic function may be due to only maintenance of sinus rhythm and not related to the direct effect of PVI. In the present study, PVI was just a method to restore and maintain sinus rhythm therefore we could not refer to the direct effect of PVI upon LV systolic function. Furthermore, as the present study was retrospective observation study and was not randomized study, we could not refer to the efficacy of PVI upon LV systolic function. We observed LV systolic function of the patients who could be restored to sinus rhythm retrospectively. Although we could not refer that PVI improves LV systolic function, we could observe the improvement of LV systolic function early after restoration to sinus rhythm. Larger studies are required to confirm our initial results.
Conclusion
LV systolic function could be significantly improved in the early phase after PVI in patients with CAF. Quantification of such improvements can be made using speckle tracking echocardiography.
References
-
Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, et al. (2012) 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Heart Rhythm 9: 632-696.e21.
-
Nair GM, Nery PB, Diwakaramenon S, Healey JS, Connolly SJ, et al. (2009) A systematic review of randomized trials comparing radiofrequency ablation with antiarrhythmic medications in patients with atrial fibrillation. J Cardiovasc Electrophysiol 20: 138-144.
-
Wilber DJ, Pappone C, Neuzil P, De Paola A, Marchlinski F, et al. (2010) Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA 303: 333-340.
-
Kohári M, Zado E, Marchlinski FE, Callans DJ, Han Y (2014) Left atrial volume best predicts recurrence after catheter ablation in patients with persistent and longstanding persistent atrial fibrillation. Pacing Clin Electrophysiol 37: 422-429.
-
Hurlburt HM, Aurigemma GP, Hill JC, Narayanan A, Gaasch WH, et al. (2007) Direct ultrasound measurement of longitudinal, circumferential, and radial strain using 2-dimensional strain imaging in normal adults. Echocardiography 24: 723-731.
-
Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, et al. (2004) Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr 17: 1021-1029.
-
Edvardsen T, Helle-Valle T, Smiseth OA (2006) Systolic dysfunction in heart failure with normal ejection fraction: speckle-tracking echocardiography. Prog Cardiovasc Dis 49: 207-214.
-
Langeland S, D'hooge J, Wouters PF, Leather HA, Claus P, et al. (2005) Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation 112: 2157-2162.
-
Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA (2000) Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation 102: 1158-1164.
-
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, et al. (2014) Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 63: 2751-2768.
-
Plana JC, Galderisi M, Barac A, Ewer MS, Scherrer-Crosbie M, et al. (2014) Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 15: 1063-1093.
-
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, et al. (2005) Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 18: 1440-1463.
-
Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, et al. (2006) Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 47: 789-793.
-
JCS Joint Working Group (2013) Guidelines for Non-Pharmacotherapy of Cardiac Arrhythmias (JCS 2011). Circ J 77: 249-274.
-
Dagres N, Varounis C, Gaspar T, Piorkowski C, Eitel C, et al. (2011) Catheter ablation for atrial fibrillation in patients with left ventricular systolic dysfunction. A systematic review and meta-analysis. J Card Fail 17: 964-970.
-
Wilton SB, Fundytus A, Ghali WA, Veenhuyzen GD, Quinn FR, et al. (2010) Meta-analysis of the effectiveness and safety of catheter ablation of atrial fibrillation in patients with versus without left ventricular systolic dysfunction. Am J Cardiol 106: 1284-1291.
-
Zhu P, Zhang Y, Jiang P, Wang Z, Wang J, et al. (2014) Effects of radiofrequency catheter ablation on left ventricular structure and function in patients with atrial fibrillation: a meta-analysis. J Interv Card Electrophysiol 40: 137-145.
-
Fiala M, Wichterle D, Bulková V, Sknouril L, Nevralová R, et al. (2014) A prospective evaluation of haemodynamics, functional status, and quality of life after radiofrequency catheter ablation of long-standing persistent atrial fibrillation. Europace 16: 15-25.
-
Tops LF, Den Uijl DW, Delgado V, Marsan NA, Zeppenfeld K, et al. (2009) Long-term improvement in left ventricular strain after successful catheter ablation for atrial fibrillation in patients with preserved left ventricular systolic function. Circ Arrhythm Electrophysiol 2: 249-257.
-
Reant P, Lafitte S, Bougteb H, Sacher F, Mignot A, et al. (2009) Effect of catheter ablation for isolated paroxysmal atrial fibrillation on longitudinal and circumferential left ventricular systolic function. Am J Cardiol 103: 232-237.
-
Shinbane JS, Wood MA, Jensen DN, Ellenbogen KA, Fitzpatrick AP, et al. (1997) Tachycardia-induced cardiomyopathy: a review of animal models and clinical studies. J Am Coll Cardiol 29: 709-715.
-
Gopinathannair R, Etheridge SP, Marchlinski FE, Spinale FG, Lakkireddy D, et al. (2015) Arrhythmia-Induced Cardiomyopathies: Mechanisms, Recognition, and Management. J Am Coll Cardiol 66: 1714-1728.





