International Journal of Clinical Cardiology
Temporal Trends in Insertion of Implantable Cardioverter Defibrillators from an Australian Regional Tertiary Centre’s Perspective
Harsh V Thakkar1* and John V Amerena2
1Medical Registrar, The University Hospital Geelong - Barwon Health, Australia
2Director of Geelong Cardiology Research Department, Deakin University, Geelong VIC 3220, Australia
*Corresponding author: Harsh V Thakkar, Medical Registrar, Department of medicine, The University Hospital Geelong, 8 Arnold Street Box Hill VIC 3128, Australia, Tel: 1300342255, Fax: +61 03 86772561, E-mail: harsh.v.thakkar@gmail.com
Int J Clin Cardiol, IJCC-3-075, (Volume 3, Issue 1), Original Article; ISSN: 2378-2951
Received: December 29, 2015 | Accepted: April 16, 2016 | Published: April 20, 2016
Citation: Thakkar HV, Amerena JV (2016) Temporal Trends in Insertion of Implantable Cardioverter Defibrillators from an Australian Regional Tertiary Centre’s Perspective. Int J Clin Cardiol 3:075. 10.23937/2378-2951/1410075
Copyright: © 2016 Thakkar HV, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Implantable Cardioverter Defibrillator (ICD) therapy is now standard therapy for prevention of Sudden Cardiac Death (SCD) in high-risk patients. In 2006, the Medical Services Advisory Committee (MSAC) reviewed the role of ICDs and approved them to be used for both primary and secondary prevention. This study reviews the temporal trends in indications for insertion of ICD at Geelong Cardiology Practice (GCP).
Method: We retrospectively audited the files of GCP patients receiving their first ICD between 1990 and 2013.
Results: 393 patients received their device within the study period. The mean age at insertion was 66. The majority of the devices inserted were stand-alone ICDs 79% (n = 311) of which 138 were for primary prevention and 173 for secondary prevention. After their approval in 2006 there was significant increase in number of devices inserted for primary prevention, however the annual numbers for secondary prevention did not show any major changes (p = 0.0001). In 2006, the ICD and CRT first implant rate was 46 per 100,000 inhabitants, while in 2011 the first implant rate increased to 120 per 100,000 inhabitants. The majority of devices for primary prevention were for Ischaemic Cardiomyopathy (24.4%) and the most common arrhythmia for secondary prevention was Ventricular Tachycardia (28.5%) of which 71.4% was of ischaemic origin and 28.5% was of non-ischaemic origin. All-cause mortality over the study period was low at 7.88% (n = 31).
Conclusion: After approval for primary prevention in 2006, there was a dramatic increase in the number of ICDs inserted for this indication. Ischaemic Cardiomyopathy was the most common indication for primary prevention and Ventricular Tachycardia associated with ischaemic LV dysfunction was the most common arrhythmia in the secondary prevention group.
Keywords
Cardiomyopathy, Sudden cardiac death, Implantable cardioverter defibrillator, Heart failure
Background
Despite advances in emergency care and major advances in resuscitation methods, sudden cardiac death remains a major burden on public health. Majority of patients who have an Out of Hospital Cardiac Arrest do not survive [1,2]. Those who are successfully resuscitated may have residual cognitive and motor deficits, which are variable depending on the time required to achieve a stable cardiac rhythm. In the 1970s, motivated by the death of a colleague, Drs Michel Mirowski and Morton Mower and colleagues conceptualised the first idea of an implantable device that can monitor the cardiac rhythm and deliver a shock if ventricular arrhythmia was detected [3,4]. In 1980 the first defibrillator was inserted in a young woman after a recent ventricular fibrillation arrest [5]. Subsequently the designs of the ICDs have improved and a series of observational studies demonstrated that the ICD appeared to be clinically useful. Following these observational studies, large randomised controlled trials showed that in patients with previous or symptomatic ventricular arrhythmias, mortality was significantly reduced by the ICDs as compared to optimal medical therapy [6,7].
A second series of trials were conducted and they showed that the ICDs also reduced mortality in patients with impaired left ventricular function due to previous myocardial infarction or heart failure [8]. There is now a widespread agreement that ICD is standard of care for reducing the mortality in a wide range of patients with increased risk factors for sudden cardiac death. This has led to development of standardised guidelines for insertion of ICD [9,10].
On review of the strength of the evidence for safety and cost effectiveness of the devices for prevention of Sudden Cardiac Death, in 2006, the Medical Services Advisory Committee of the Australian Government published its recommendations for the use of ICD in prevention of primary as well as secondary prevention [11].
While the clinical indications for the use of ICDs are now widely accepted, it is not clear that the clinical practice is consistent with the current guidelines [12-14]. Therefore in order to realise the true potential of reduction in sudden cardiac death that is offered by ICDs it is pertinent to audit current practice and outcomes in patients receiving ICDs and to identify barriers to ICDs. This study was conducted to address the first of these goals by reviewing the temporal trends in insertion of ICDs at Geelong Cardiology Practice, part of The University Hospital Geelong one of the large Victorian regional Tertiary centres. This study reviewed the devices inserted between 1990 and 2013.
Methods
This was a retrospective observational study. We reviewed all the patient files receiving their first ICD at The University Hospital Geelong from the period of 1st January 1990 to 31st December 2013, and included clinical data from this time period. Population data was obtained from the Australian Bureau of Statistics 2006 and 2011 census of Population and Housing for the Geelong region. This study was reviewed by the Barwon Health Research Office and was advised that this project is exempt from ethical review as outlined in the National Statement on Ethical conduct in Human Research Section 5.
We collected data on the indications for ICD implantation, details of the type of device and the patient demographics and mortality. This was done by reviewing the implant records, cardiologist's letters and hospital records for each patient. The ICD indications were classified into primary and secondary prevention.
The primary prevention included patients with Ischaemic cardiomyopathy, Dilated cardiomyopathy, Hypertrophic Cardiomyopathy and Miscellaneous category which has been classified as “Other” which include Cardiomyopathy secondary to Wolff-Parkinson-White, complete heart block, hemochromatosis, Denon's Cardiomyopathy, Long QT syndrome and idiopathic cardiomyopathy.
The secondary prevention group were the ones who had a previous ventricular tachycardia (VT) or ventricular fibrillation (VF) cardiac arrest, syncopal VT or sustained VT. Non-sustained non-syncopal VT was classified as primary prevention, as well as all other indications in which VT/VF had not occurred prior to implantation.
To quantify the trends of devices used after MSAC approval, primary outcome was defined as trend of rate of devices used before and after MSAC approval. Secondary outcomes were defined as trend of rates of primary/secondary prevention and types of devices used.
a) Inclusion Criteria: All the patients who had their first device inserted at the University Hospital Geelong between 1st January 1990 and 31st December 2013 were included in this study. b) Exclusion Criteria: Any Presentation for battery or a generator replacement was excluded from the study.
Data Analysis - Discrete variables were compared using the Pearson Chi Square test. All statistical tests were performed using the SPSS software v22.0.0.0 IBM Corporation.
Results
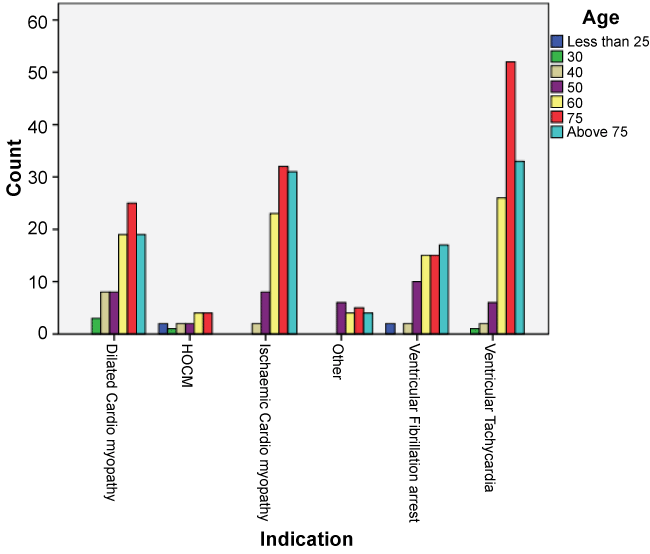
Over the twenty-three years of the study period, 393 patients received their first device. The mean age of insertion was 66yrs (SD of 12.6; Range 19-90) and patients were predominantly male (n = 303, 77%). The demographic details of all the patients are given in Table 1. Figure 1 shows the age groups across all the indications.
![]()
Table 1: Patient demographics and treatment details.
View Table 1
In 2006, the ICD and CRT first implant rate was 46 per 100,000 inhabitants, while in 2011 the first implant rate increased to 120 per 100,000 inhabitants [15].
In 2006 the first implant rate per 100,000 inhabitants for ICD was 45, BiV/Defib was 0.5 and BiV was 0, respectively. In 2011 the rates increased to 88 for ICD, 24 for BiV/Defib and 7 for BiV [15].
![]()
Table 2: Gender distribution for indications in insertion of ICDs.
View Table 2
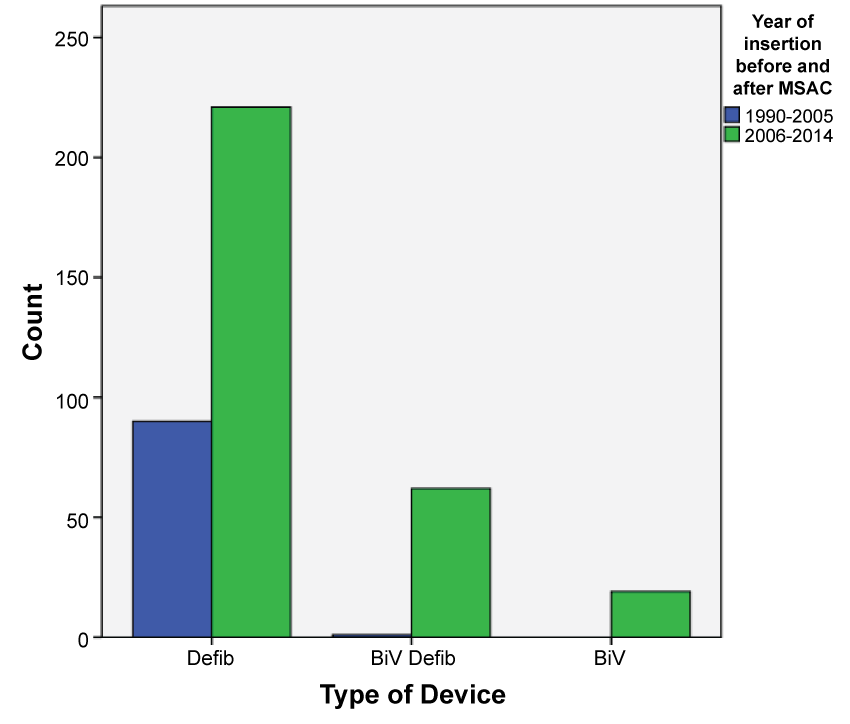
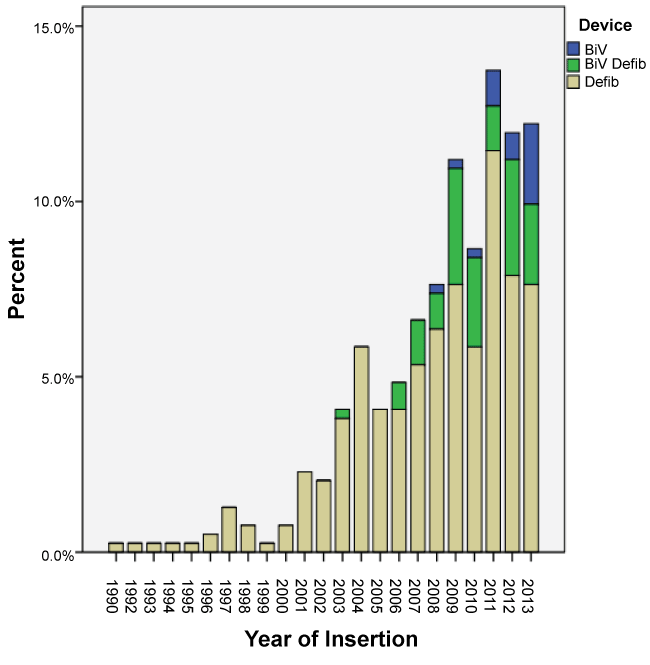
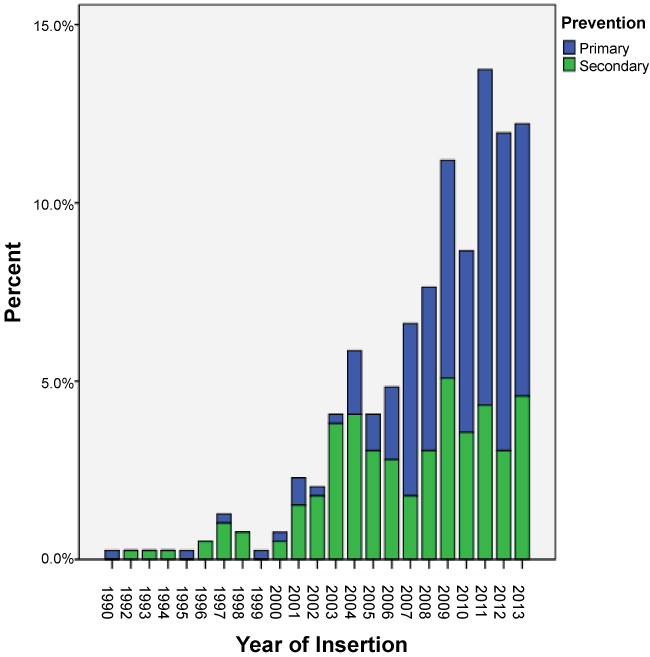
The majority of devices inserted were stand alone ICDs (79%, n = 311) of which 138 were for primary prevention and 173 were for secondary prevention. The remainder of the devices were cardiac resynchronisation devices (CRT) - Bi-ventricular pacing with the defibrillator (BiV/Defib) i.e. CRT-D or Bi-ventricular pacing (BiV) only i.e. CRT-P. The BiV devices were first implanted in 2003. While the percentage of these devices being implanted increased after their approval by MSAC, they still accounted for less than 5% of the new devices being implanted. Over the study period, the majority of devices inserted were for primary prevention (n = 212). As depicted in the Table 1 there was no statistical difference between males and females getting a device before and after MSAC approval (p = 0.601). There was a significant statistical difference between the devices inserted for primary vs. secondary prevention before and after MSAC approval (p = 0.0001). Figure 2 shows the trends in rates of devices used before and after MSAC approval, which is the primary outcome of the study. It was also noted that there was a significant difference in the types of devices used before and after MSAC with ICDs being the most commonly used device (p = 0.0001). Figures 3 and Figure 4 depict secondary outcomes. Figure 3 illustrates the trends in the types of devices implanted over the study period and Figure 4 depicts the trends in primary and secondary prevention across the study period.
The indications for the insertion of the devices were spread out evenly between the primary and secondary prevention (n = 212 and n = 181 respectively). The most common indication for primary prevention was Ischaemic Cardiomyopathy (24.4%) and the most common arrhythmia associated with secondary prevention was Ventricular Tachycardia (28.5%) of which 71% was ischaemic in origin and 29% was non-ischaemic in origin. The indication and gender cross tabulation is shown below in Table 2, overall indications for insertion of devices is shown in Figure 5. It was noted that new devices were much more common in males in all indications. There was a statistically significant association between the indication for devices and the gender of the population with a significant Chi square value of 14.890 and a p value of 0.011.
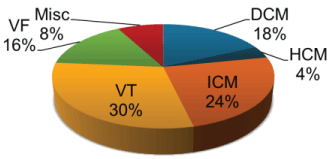
.
Figure 5: Indications in insertion of ICDs.
VF = Ventricular Fibrillation; Misc = Miscellaneous (other in the tables); DCM = Dilated Cardiomyopathy; HCM = Hypertrophic Cardiomyopathy; ICM = Ischaemic Cardiomyopathy; VT = Ventricular Tachycardia.
View Figure 5
Mortality
It was noted that mortality over the study period was low at 7.88% (n = 31), however 72 patients were lost to follow up as they were not followed up at this practice after their device implantation hence their vital status is unknown.
Discussion
The use of ICD therapy to prevent Sudden Cardiac Death increased across the study period. The proportion of devices inserted for primary prevention increased from 23% to 63% after the MSAC approved the use of ICD for primary as well as secondary prevention. This is consistent with expanding indications for ICD therapy [10], and international trends [16,17]. The Swedish registry in 2012 reported 59% of devices were inserted for primary prevention [16] and the Italian registry in 2004 reported that 24% were for primary prevention [17].
Our patient group had a mean age of 66 yrs, which is similar to the Swedish (mean age 63 yrs) [16], Danish (mean age 66 yrs) [18] and Italian (mean age 68 yrs) [17] populations receiving this therapy, while one group from New Zealand quoted a mean age of 53 yrs [19]. It was also noted that majority of individuals receiving the device were males (77%) which is similar to the Swedish study [16]. Reviewing the results of Table 2 with the statistically significant Chi square value we can assume that being male with a cardiomyopathy was associated with an increased device insertion rate. However this study had a small sample size as compared to the various other studies hence further research may be required to answer this question from an Australian perspective. Many studies over the past decade and one recent study has shown that majority of ICDs are inserted in men as compared to women [20-22].
The first implant rates for ICD in 2011 for our study was 120 per 100,000 inhabitants which is in keeping with the European trends and the Swedish study which had 136 per million inhabitants [16,23].
The possible reasons why the trends were similar to the other countries and there was a significant increase in devices for primary prevention is possibly due to increased funding by the government for implantation and also for remote monitoring of ICD devices [11,24,25]. With the increased funding there was an increase in the access to healthcare especially for people living in the rural areas [25]. This helped in making Geelong a referral centre for rural Western Victoria.
There is evidence that patients over the age of 75 receive the same benefit as younger patients from ICD therapy in terms of preventing sudden cardiac death, however the older population have higher all cause mortality rates [26]. For this reason, it is possible that the older population with multiple co-morbidities are less likely to receive ICD therapy.
The overall mortality rate in our study group was low at 7.88% as compared to the other series, which ranged from 19% and 36% [26,27]. However, given that The University Hospital Geelong serves as a referral centre for a major part of rural western Victoria a number of patients were lost to follow up hence their vital status is not known.
This study is a retrospective observational audit, hence suffers from a number of inherent limitations. Gaps exist in the data, as The University Hospital Geelong acts as a referral centre for a major part of rural western Victoria, number of patients were lost to follow up after their device implantation. As well, data may not have been always recorded according to the tightly defined criteria of this study. The results of this study do not represent the trends in the practice in Australia. This study was limited to procedures done in a large public hospital and did not capture the number of ICDs that were implanted within the private sector; hence it is difficult to say whether the trends described in the public sector were same in the private.
It was observed that there was an increasing rate of ICD implantation across the study period, with an increase in proportion of patients receiving this therapy for primary prevention. The previous large multicentre trials in heart failure patients receiving device therapy have shown higher mortality rates [28,29], so there is a possibility that this therapy is still not available to a wide enough patient group locally. This is supported by the young average age of implant recipients and the low overall mortality rate we found in this study. Further work on making this potentially lifesaving therapy more widely available in the Australian context is required.
Conclusion
After ICDs were approved for primary prevention in 2006 there was a dramatic increase in the number of devices inserted for this indication. Ischaemic Cardiomyopathy was the most common indication for primary prevention and Ventricular Tachycardia associated with ischaemic LV dysfunction being the most common arrhythmia in the secondary prevention group.
Acknowledgement
We would like to thank the staff at The Geelong Cardiology Practice and The University Hospital Geelong in helping us obtain the patient files for the purpose of this study.
Ethical Statement
This study was reviewed by the Barwon Health Research Office and was advised that this project is exempt from ethical review as outlined in the National Statement on Ethical conduct in Human Research Section 5.
References
-
Zipes DP, Wellens HJ (1998) Sudden cardiac death. Circulation 98: 2334-2351.
-
Huikuri HV, Castellanos A, Myerburg RJ (2001) Sudden death due to cardiac arrhythmias. N Engl J Med 345: 1473-1482.
-
Mirowski M, Mower MM (1973) Transvenous automatic defibrillator as an approach to prevention of sudden death from ventricular fibrillation. Heart Lung 2: 867-869.
-
Mirowski M, Mower MM, Langer A, Heilman MS, Schreibmen J (1978) A chronically implanted system for automatic defibrillation in active conscious dogs: experimental model for treatment of sudden death from ventricular fibrillation. Circulation 58: 90-94.
-
Mirowski M, Reid PR, Mower MM, Watkins L, Gott VL, et al. (1980) Termination of malignant ventricular arrhythmias with an implantable automatic defibrillator in human beings. N Engl J Med 303: 322-324.
-
Cannom DS, Prystowsky EN (2004) The evolution of the implantable cardioverter defibrillator. Pacing Clin Electrophysiol 27: 419-431.
-
Connolly SJ, Gent M, Roberts RS, Dorian P, Roy D, et al. (2000) Canadian implantable defibrillator study (CIDS) : a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation 101: 1297-1302.
-
Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, et al. (2005) Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 352: 225-237.
-
Steinbeck G, Andresen D, Seidl K, Brachmann J, Hoffmann E, et al. (2009) Defibrillator implantation early after myocardial infarction. N Engl J Med 361: 1427-1436.
-
Epstein AE, Dimarco JP, Ellenbogen KA, Freedman RA, Gettes LS, et al. (2008) ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. J Am Coll Cardiol 51: e1-e62.
-
(2006) Implantable Cardioverter Defibrillators for prevention of Sudden Cardiac Death. Feb/Mar 2006 Assessment report MSAC reference 32 Online ISBN: 1 74186 027 X.
-
Hohnloser SH, Israel CW (2013) Current evidence base for use of the implantable cardioverter-defibrillator. Circulation 128: 172-183.
-
Chase D, Roderick PJ, Burnley H, Gallagher PJ, Roberts PR, et al. (2008) Is there unmet need for Implantable Cardioverter Defibrillators? Findings from a post mortem series of Sudden Cardiac Death. Europace 10: 741-746.
-
Plummer CJ, John Irving R, McComb JM (2005) The incidence of Implantable Cardioverter Defibrillator indications in patients admitted to all coronary care units in a single district. Europace 7: 266-272.
-
Australian bureau of Statistics 2006 and 2011 Census of Population and Housing.
-
Gadler F, Valzania C, Linde C (2015) Current use of implantable electrical devices in Sweden: data from the Swedish pacemaker and implantable cardioverter-defibrillator registry. Europace 17: 69-77.
-
Proclemer A, Ghidina M, Cicutinni G, Gregori D, Fioretti PM (2006) Impact of the main Implantable Cardioverter Defibrillator trials for primary and secondary prevention in Italy: a survey of the national activity during the years 2001-2004. Pacing Clin Electrophysiol 2: S20-28.
-
(2006) The Danish Pacemaker and ICD Registry.
-
Larsen PD, De Silva P, Harding SA, Woodcock E, Lever NA (2010) Use of implantable cardioverter defibrillators in the New Zealand Context from 2000 to 2007. N Z Med J 123: 76-85.
-
Peterson PN, Daugherty SL, Wang Y, Vidaillet HJ, Heidenreich PA, et al. (2009) Gender Differences in Procedure-Related Adverse Events in Patients Receiving Implantable Cardioverter-Defibrillator Therapy. Circulation 119: 1078-1084.
-
Bradshaw PJ, Stobie P, Knuiman MW, Briffa TG, Hobbs MS (2014) Trends in the incidence and prevalence of cardiac pacemaker insertions in an ageing population. Open Heart 1: e000177.
-
Patel NJ, Edla S, Deshmukh A, Nalluri N, Patel N, et al. (2016) Gender, Racial, and Health Insurance Differences in the Trend of Implantable Cardioverter-Defibrillator (ICD) Utilization: A United States Experience Over the Last Decade. Clin Cardiol 39: 63-71.
-
Valzania C, Torbica A, Tarricone R, Leyva F, Boriani G (2016) Implant rates of cardiac implantable electrical devices in Europe: A systematic literature review. Health Policy 120: 1-15.
-
(2011) The Business case for Public funding of remote monitoring of Cardiac Implantable Electronic devices. Medical Technology Association of Australia Limited.
-
(2011) Connecting health services with the future: modernising medicare by providing rebates for online consultations - a discussion paper from the australian government. Telemetric remote monitoring: Using telehealth to manage patients with an Implantable Cardiac Device.
-
Grimm W, Stula A, Sharkova J, Alter P, Maisch B (2007) Outcomes of elderly recipients of implantable cardioverter defibrillators. Pacing Clin Electrophysiol 30 Suppl 1: S134-138.
-
Gold MR, Shih HT, Herre J, Breiter D, Zhang Y, et al. (2007) Comparison of defibrillation efficacy and survival associated with right versus left pectoral placement for implantable defibrillators. Am J Cardiol 100: 243-246.
-
Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, et al. (2005) The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 352: 1539-1549.
-
Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, et al. (2002) Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346: 877-883.