International Journal of Clinical Cardiology
Increased Corrected QT Interval (QTc) in First Nations Women of Northern British Columbia with Systemic Lupus Erythematosus (SLE)
Fritha A Munday1, Sirisha Asuri2, Sarah McIntosh2, Heather Jackson3, Anthony Tang4 and Laura Arbour2*
1Division of Medical Sciences, University of Victoria, Victoria, BC, Canada
2Department of Medical Genetics, and the Island Medical Program, University of British Columbia, Victoria, BC, Canada
3Heart Rhythm BC at Cardiac Services BC, Vancouver BC, Canada
4Department of Medicine, Western University, London, Ontario, Canada
*Corresponding author: Laura Arbour, UBC Island Medical Program, Medical Sciences Building, Rm 104, University of Victoria, P.O. Box 1700, STN CSC, Victoria, BC, Canada, V8W 2Y2, Tel: 250-472-5544, Fax: 250-472-4283, E-mail: larbour@uvic.ca
Int J Clin Cardiol, IJCC-3-072, (Volume 3, Issue 1), Original Article; ISSN: 2378-2951
Received: November 06, 2015 | Accepted: January 27, 2016 | Published: January 30, 2016
Citation: Munday FA, Asuri S, McIntosh S, Arbour L, Jackson H, et al. (2016) Increased Corrected QT Interval (QTc) in First Nations Women of Northern British Columbia with Systemic Lupus Erythematosus (SLE). Int J Clin Cardiol 3:072. 10.23937/2378-2951/1410072
Copyright: © 2016 Munday FA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Long QT Syndrome (LQTS), a genetic predisposition to sudden cardiac death is defined by a prolonged corrected QT interval (QTc) measured on electrocardiogram (ECG). A participatory research project has been underway with the Gitxsan of Northern BC for more than ten years where the condition (LQTS1) is common, due to a founder mutation (V205M) in KCNQ1. It is effectively treated with beta blockers and avoidance of QT prolonging drugs. Some chronic conditions are also known to increase the QTc, which is an independent risk factor for sudden cardiac death. Autoimmune disease is also common in BC First Nations, and among our study population we noted an enrichment of women with Systemic Lupus Erythematosus (SLE) in categories with a high QTc. Here we assessed whether persons with SLE and without known LQTS mutations, have a higher than expected QTc with implications for clinical management. Our results support an increase in QTc associated with SLE. In addition to congenital LQTS, physicians should be alerted to the risk for an increased QTc with SLE and consider avoidance of QT prolonging medications when possible.
Keywords
Long QT Syndrome, QTc, Autoimmune disease, Systemic lupus erythematosus, First Nations
Introduction
Long QT Syndrome (LQTS), congenital or acquired, is characterized by a prolonged QT interval identified on an electrocardiogram (ECG) predisposing to arrhythmias, ventricular fibrillation, cardiac arrest and sudden death [1]. Congenital LQTS is inherited due to abnormalities in at least 13 known genes (LQTS1-LQTS13) predominantly responsible for ion channel function [2]. Acquired LQTS occurs without a molecularly defined LQTS mutation and may be caused by drugs [3], some cardiac diseases [4] as well as other chronic diseases such as diabetes [5], and rheumatic/autoimmune diseases including systemic lupus erythematosus (SLE) [6] and rheumatoid arthritis [7].
Congenital LQTS is relatively rare, affecting an estimated 1:2000 [8], but it is disproportionately prevalent (~1:120) in a Canadian First Nations community in northern British Columbia (the Gitxsan). Through community initiation, individuals diagnosed with LQTS and their relatives participated in our early studies, which led to the identification of a novel c.613 G > A missense mutation resulting in a valine to methionine substitution at position 205 (V205M) in KCNQ1. KCNQ1 is the gene most commonly associated with LQTS (LQTS1) [9] and encodes the α subunit of the slow delayed rectifier potassium channel, Iks. This novel mutation was shown in mechanistic studies to alter Iks by slowing activation and accelerating deactivation [10]. Furthermore, a prolonged QTc, cardiac arrest and sudden cardiac death correlates with mutation positive status, supporting that it predisposes individuals to LQTS [10].
A normal QTc is generally considered to be within 390-450 ms for men and within 390-460 ms for women [11]. A prolonged QTc is an independent risk factor for sudden death reflecting repolarization instability, predisposing individuals to cardiac arrhythmias [12,13]. Although the mutation positive patients in our study cohort clearly show a prolonged QTc, an increase in the baseline QTc in the mutation negative group has also been observed [10,14] suggesting other factors, genes and QT prolonging circumstances [15], may contribute to a prolonged QT interval. Further identification of mutations responsible for LQTS, as well as the elucidation of non-genetic contributing factors, is important in the delivery of appropriate clinical management in this community. For this study, we noted an enrichment of women with a diagnosis of SLE, in our category of those with a QTc over 470 ms. This was of interest since SLE has been previously found to be prevalent in British Columbia and in other aboriginal populations [16-18].
SLE is an autoimmune disease of unknown cause that primarily affects young adult women. It causes widespread, nonspecific organ inflammation, primarily in the kidneys and skin, and more than half of SLE patients exhibit cardiac involvement [19,20]. The inflammatory effects of SLE can impact all parts of the heart including valves, blood vessels, the pericardium and the myocardium. Arrhythmia may result and the QT interval has been noted to be affected [21-23].
The objective of this study was to determine whether SLE patients enrolled in our LQTS study, without the V205M LQTS founder mutation, have an elevated QTc, with implications for management.
Materials and Methods
Long QT syndrome study participants
Community based participatory methods as previously described [10] were adhered to. Community members were invited to participate if they had a diagnosis of LQTS, a possible diagnosis (designated by an increased corrected QT interval) or had a known relative with LQTS. Ethics approval was granted by the UBC and Northern Health Authority ethics boards. The Gitxsan Health Society reviewed and participated in protocol development. Multi-generation family and medical histories were documented by trained genetic counsellors. Medical records were reviewed, an ECG was carried out, and all other ECGs on file were collected for review. As previously reported, blood or saliva samples were collected from the participants under the stipulation of DNA on Loan [24]. DNA was extracted by standard protocols. Genotyping was carried out for the known KCNQ1 V205M mutation and a second KCNQ1 mutation (R591H) documented in an adjacent community [14] in the BC Provincial Health Services Authority (PHSA) lab of the BC Children's Hospital, Vancouver. In addition, three individuals with SLE with a documented prolonged QTc > 470 ms, but negative targeted V205M mutation testing underwent expanded testing to exclude mutations in the 5 genes most commonly responsible for LQTS (KCNQ1, KCNE1, KCNH2, KCNE2, and SCN5A). No other mutations were detected.
Participants also completed an interview which documented the presence of chronic disease diagnoses [10]. All participants with a documented previous medical diagnosis of SLE were considered to be 'affected with SLE' and eligible for this sub-study. Those thought to have a 'possible' diagnosis of SLE were excluded. Further eligibility included at least one ECG and completion of targeted KCNQ1 mutation testing.
Four groups for comparison were established:
1) Those with a documented V205M mutation, n = 26,
2) Those with a confirmed medical diagnosis of SLE, n = 9,
3) First to third degree relatives of those with the V205M mutation but testing negative for the V205M mutation, n = 16 and
4) First to third degree relatives of those with SLE and testing negative for the V205M mutation, n = 23.
Exclusions
Individuals below the age of sixteen years were excluded. All men were also excluded due to low prevalence of SLE among males (n = 2), and the confounding difference in QTc between men and women with the V205M mutation [10]. Furthermore, those with medical co-morbidities that might affect the QTc interval, such as known cardiovascular disease, diabetes and other autoimmune diseases (rheumatoid or other inflammatory arthritis) were excluded. Those with arthritis of unknown etiology were not excluded. Those homozygous for the V205M mutation (n = 4) were also excluded since that group has significantly increased QTc compared to heterozygous carriers [25]. One case that had SLE and the V205M mutation was excluded and 4 cases with a possible or probable diagnosis of SLE that could not be confirmed were also excluded. All relatives greater than 3 degrees of relationship from V205M-positive or SLE individuals were excluded from the control groups.
QTc measurements
Corrected QT (QTc) measurements, blinded to mutation and disease status, were made on all available ECGs (12-lead) for each participant in the study by HJ, under the supervision of AT, an electro physiologist. QTc values were determined using the tangent method [26], Bazett correction [27] and an average RR interval. For each individual, the longest QTc in any lead from all available ECGs was used to determine the study QTc for each participant.
Statistical analysis
The software program Graphpad Prism was used for all statistical analyses. Two-tailed t-test was performed to compare mean QTc values of subgroups. Means with 95% confidence intervals were reported and p values equal to or less than 0.05 was considered statistically significant. One-way ANOVA with Tukey post-test and test for linear trend was used to compare means of multiple groups.
Results
Statistical analysis
The mean ages of participants in each of the groups were: (1) Mutation positive - 49 years (SD ± 15), (2) Affected with SLE - 51 years (SD ± 10), (3) First to third degree relatives of those with the mutation, but testing negative for the mutation - 43 years (SD ± 19) (4) First to third degree relatives of those with SLE, but testing negative for the mutation - 48 years (SD ± 15).
The mean QTc of each of the four groups showed a descending trend (Figure 1): mutation positive individuals had the highest mean QTc (473.3 ± 26 ms) followed by SLE patients (466.4 ± 34 ms), relatives of SLE patients (451 ± 20 ms) and mutation negative individuals related to a mutation positive person (434.8 ± 24 ms). Through ANOVA, the mean QTc values of the groups were significantly different (p < 0.0001). Tukey's post-test showed significant difference between: 1) SLE vs. V205M-negative group (p < 0.05), 2) V205M-positive vs. V205M negative group (p < 0.001), and 3) V205M negative related to SLE vs. V205M positive group (p < 0.05).
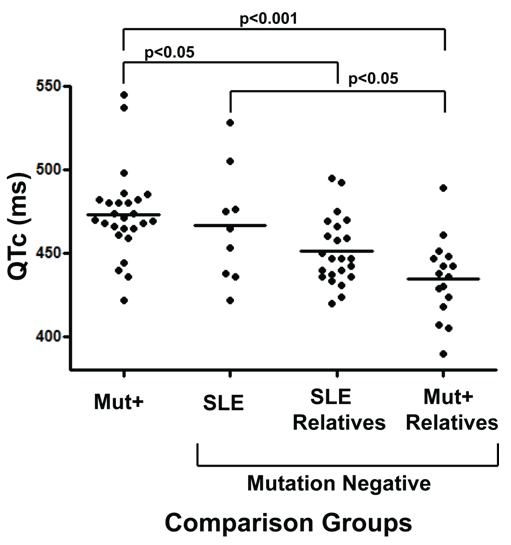
.
Figure 1: QTc intervals of SLE patients compared to LQTS mutation positive cases and their relatives. From left to right 1). KCNQ1 V205M mutation positive (mut+) persons without SLE (n = 26), 2). SLE cases (n = 9), 3). Relatives of those with SLE (n = 23) and 4). Relatives of those with the KCNQ1 V205M mutation (n = 16). All persons are female and have been tested for the LQTS causing mutation, and only those in group 1 are KCNQ1 V205M mutation positive. SLE, relatives of SLE and relatives of mutation positive are all negative for the KCNQ1 mutation. Each point represents the highest QTc determination of all ECGs from one individual. The short horizontal bars represent each group's mean QTc. Mean QTc values ± standard deviation of each group, from left to right on x-axis, are: 473.3 ± 26 ms, 466.4 ± 34 ms, 451.0 ± 20 ms and 434.8 ± 24 ms. (QTc: corrected QT interval; SLE: systemic lupus erythematosus).
View Figure 1
Discussion
Systemic lupus erythematosus has been previously reported to occur with increased prevalence in First Nations in Canada and in Native Americans. For example, Atkins et al. [16] reported the prevalence of SLE in the Nuu-Chah-Nulth to be 0.5% [16]. Two subsequent reports have corroborated increased rates – Peschken et al. [17] reported a 42.3/100,000 rate in First Nations and Native Americans, compared to 20.6/100,000 in Canadians Caucasians [17] and in a more recent study, Ferucci et al. [28] from the United States suggest a prevalence of 178/100,000 (0.12%), three times the previous estimation in Native Americans and Alaska Natives [28]. Although the prevalence of SLE is said to be high in the Gitxsan, the overall prevalence has not been determined. Therefore, in our study ascertaining participants based on presumption of LQTS we were surprised that the over-all study population rate of SLE was 4% which is 8 times higher than previous estimates of SLE in First Nations of BC. Furthermore, at the time of the analysis 11% of those with a QTc greater than 470 ms had a diagnosis of SLE. Ascertainment bias based on eligibility of a possible diagnosis of LQTS in our study may have resulted in a disproportionate number of those with SLE entering our study. Considering previous reports suggesting that QTc may be elevated in those with SLE, we set out to determine if that was the case in our study population.
Since the QTc may be influenced by numerous factors [29] we chose our comparison groups as close family based controls of mutation positive LQTS (group 1), and with SLE (group 2), and excluded those with other potential reasons for a prolonged QTc such as the presence of other chronic disease from all case and control groups. Our results support that those participants diagnosed with SLE in the Gitxsan community exhibit an elevated QTc, consistent with previous reports [6,21,30]. Of note, a gradient was observed in the mean QTc of four comparison groups of participants, with the lowest being the mutation negative relatives of those mutation positive and the highest being those with the V205M mutation. Those with SLE had a mean QTc lower than those with the mutation, but not significantly so. In turn, their relatives had a mean QTc lower but not significantly different than those with SLE.
The mechanism for QT interval prolongation in SLE has been the focus of previous research and may be related to the presence of anti-Ro/SSA antibodies. Lazzerini et al. [6] observed a correlation between prolonged QTc and the presence of anti-Ro/SSA antibodies in patients with connective tissue diseases including SLE. They hypothesized an etiological role of anti-Ro/SSA antibodies in increasing risk of arrhythmia [23]. Their hypothesis is supported by the fact that children born to anti-Ro/SSA positive mothers exhibit transient QT interval prolongation [31]. Bourré-Tessier et al. [30] found that anti-Ro/SSA positive SLE patient's exhibit prolonged QTc when compared to anti-Ro/SSA negative SLE patients. It has been hypothesized that cross-reaction between anti-Ro/SSA antibodies and the KCNH2 delayed rectifier potassium channels (IKr) in the heart could contribute to a prolonged QT interval by inhibiting IKr [30,32] much in the same way that KCNH2 is affected by numerous drugs. LQTS induced by anti-KCNH2 antibody has been reported in at least one patient [32] when researchers sought to determine the basis of a new diagnosis of LQTS with no known LQTS mutations but the presence of positive anti-SSA/Ro antibodies. A more recent study has supported the hypothesis further by immunizing guinea pigs with the Ro antigen, and demonstrating QT prolongation on ECG after the development of high titers of the anti-Ro antibody [33]. Further in vitro studies in that same study, demonstrated Anti-Ro antibodies inhibited IKr, explaining the resultant prolonged QT interval [33]. There are other possibilities to consider in that certain drugs used in the treatment of SLE (hydroxychloroquine) may prolong the QTc [23,34,35], and some have suggested the prolonged QTc in SLE could be related to autonomic dysfunction known in SLE. This hypothesis deserves further exploration [36].
The lack of significant difference between the QTc of SLE patients and their relatives is a novel finding and may be due to the presence of autoantibodies and pre-clinical autoimmune disease. Important to also note is that SLE is caused by a poorly understood combination of environmental and genetic factors. Although over 100 candidate genes have been identified in genome wide association and linkage studies [37], the nature of transmission appears to be complex, involving interactions between several genes and their variants [38,39]. Independent of SLE antibodies, other yet to be defined genetic factors predisposing to SLE could also unknowingly affect the QT interval and be present in undiagnosed family members.
The finding that those with SLE are at risk for an increased QTc has management implications for those affected. A prolonged QTc is an independent risk factor for ventricular arrhythmias and sudden cardiac death [1,12,13,40,41] and avoidance of QT prolonging drugs [42] has been proposed to reduce risk [30,33].
Conclusion
Our study supports previous evidence that those with SLE are at risk for a prolonged QTc. This finding has management implications for those affected, especially in First Nations populations where SLE is common. A prolonged QTc is a risk factor for arrhythmia and sudden cardiac death, avoidance of QT prolonging drugs need to be considered. In cases where QT prolonging drugs are necessary for treatment of chronic diseases, such as SLE, regular cardiac monitoring should be considered.
Limitations
Several aspects remain to be addressed by future research. Due to low sample size, men and mutation positive SLE patients were excluded, including one patient with a diagnosis of SLE and LQTS based on the V205M mutation status. History of QT prolonging drugs was reported in each subgroup, however this study did not assess temporal association between QTc and drug use. Furthermore, other genetic variants influencing the QTc may be present in families with SLE. It is possible that SLE and the V205M mutation, and/or other variants could have an additive or synergistic effect on prolongation of the QTc. Future work could examine the influence of anti-Ro/SSA, other autoantibodies, and genetic varaints on the background QTc in this population.
Acknowledgements
We would like to thank the participants of the LQTS project, and the Gitxsan Health Society for their governance and support of this on-going project. This work was partially funded by the Canadian Institutes of Health Research grant number 81197 to L. Arbour period.
Ethics Statement
This study was conducted with the approval of the University of British Columbia and Northern Health Authority ethics boards and adhered to ethical standards. This study was carried out in a participatory manner in collaboration with the Gitxsan Health Society Board.
References
-
Goldenberg I, Moss AJ (2008) Long QT syndrome. J Am Coll Cardiol 51: 2291-2300.
-
Mahida S, Hogarth AJ, Cowan C, Tayebjee MH, Graham LN, et al. (2013) Genetics of congenital and drug-induced long QT syndromes: current evidence and future research perspectives. J Interv Card Electrophysiol 37: 9-19.
-
Kannankeril PJ, Roden DM (2007) Drug-induced long QT and torsade de pointes: recent advances. Curr Opin Cardiol 22: 39-43.
-
Camm AJ, Janse MJ, Roden DM, Rosen MR, Cinca J, et al. (2000) Congenital and acquired long QT syndrome. Eur Heart J 21: 1232-1237.
-
Pourmoghaddas A, Hekmatnia A (2003) The relationship between QTc interval and cardiac autonomic neuropathy in diabetes mellitus. Mol Cell Biochem 249: 125-128.
-
Lazzerini PE, Acampa M, Guideri F, Capecchi PL, Campanella V, et al. (2004) Prolongation of the corrected QT interval in adult patients with anti-Ro/SSA-positive connective tissue diseases. Arthritis Rheum 50: 1248-1252.
-
Chauhan K, Ackerman MJ, Crowson CS, Matteson EL, Gabriel SE (2015) Population-based study of QT interval prolongation in patients with rheumatoid arthritis. Clin Exp Rheumatol 33: 84-89.
-
Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M, Besana A, et al. (2009) Prevalence of the congenital long-QT syndrome. Circulation 120: 1761-1767.
-
Bokil NJ, Baisden JM, Radford DJ, Summers KM (2010) Molecular genetics of long QT syndrome. Mol Genet Metab 101: 1-8.
-
Arbour L, Rezazadeh S, Eldstrom J, Weget-Simms G, Rupps R, et al. (2008) A KCNQ1 V205M missense mutation causes a high rate of long QT syndrome in a First Nations community of northern British Columbia: a community-based approach to understanding the impact. Genetics in medicine 10: 545-550.
-
Hofman N, Wilde AA, Tan HL, Kääb S, van Langen IM, et al. (2007) Diagnostic criteria for congenital long QT syndrome in the era of molecular genetics: do we need a scoring system? Eur Heart J 28: 575-580.
-
Chiang CE (2004) Congenital and acquired long QT syndrome. Current concepts and management. Cardiol Rev 12: 222-234.
-
Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J (1991) QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation 83: 1888-1894.
-
Jackson H, Huisman LA, Sanatani S, Arbour LT (2011) Long QT syndrome. CMAJ 183: 1272-1275.
-
Ziegler D, Zentai C, Perz S, Rathmann W, Haastert B, et al. (2006) Selective contribution of diabetes and other cardiovascular risk factors to cardiac autonomic dysfunction in the general population. Exp Clin Endocrinol Diabetes 114: 153-159.
-
Atkins C, Reuffel L, Roddy J, Platts M, Robinson H, et al. (1988) Rheumatic disease in the Nuu-Chah-Nulth native Indians of the Pacific Northwest. J Rheumatol 15: 684-690.
-
Peschken CA, Esdaile JM (2000) Systemic lupus erythematosus in North American Indians: a population based study. J Rheumatol 27: 1884-1891.
-
Barnabe C, Joseph L, Belisle P, Labrecque J, Edworthy S, et al. (2012) Prevalence of systemic lupus erythematosus and systemic sclerosis in the First Nations population of Alberta, Canada. Arthritis Care Res (Hoboken) 64: 138-143.
-
Doria A, Iaccarino L, Sarzi-Puttini P, Atzeni F, Turriel M, et al. (2005) Cardiac involvement in systemic lupus erythematosus. Lupus 14: 683-686.
-
Rahman A, Isenberg DA (2008) Systemic lupus erythematosus. N Engl J Med 358: 929-939.
-
Cardoso CR, Sales MA, Papi JA, Salles GF (2005) QT-interval parameters are increased in systemic lupus erythematosus patients. Lupus 14: 846-852.
-
Bourre-Tessier J, Urowitz MB, Clarke AE, Bernatsky S, Krantz MJ, et al. (2015) Electrocardiographic findings in systemic lupus erythematosus: data from an international inception cohort. Arthritis Care Res (Hoboken) 67: 128-135.
-
Lazzerini PE, Capecchi PL, Laghi-Pasini F (2015) Long QT Syndrome: An Emerging Role for Inflammation and Immunity. Front Cardiovasc Med 2: 26.
-
Arbour L, Cook D (2006) DNA on loan: issues to consider when carrying out genetic research with aboriginal families and communities. Community Genet 9: 153-160.
-
Jackson HA, McIntosh S, Whittome B, Asuri S, Casey B, et al. (2014) LQTS in Northern BC: homozygosity for KCNQ1 V205M presents with a more severe cardiac phenotype but with minimal impact on auditory function. Clin Genet 86: 85-90.
-
Postema PG, De Jong JS, Van der Bilt IA, Wilde AA (2008) Accurate electrocardiographic assessment of the QT interval: teach the tangent. Heart rhythm 5: 1015-1018.
-
Bazett H (1920) An analysis of the time relations of electrocardiograms. Heart 7: 353-370.
-
Ferucci ED, Johnston JM, Gaddy JR, Sumner L, Posever JO, et al. (2014) Prevalence and incidence of systemic lupus erythematosus in a population-based registry of American Indian and Alaska Native people, 2007-2009. Arthritis Rheumatol 66: 2494-2502.
-
Amin AS, Pinto YM, Wilde AA (2013) Long QT syndrome: beyond the causal mutation. J Physiol 591: 4125-4139.
-
Bourré-Tessier J, Clarke AE, Huynh T, Bernatsky S, Joseph L, et al. (2011) Prolonged corrected QT interval in anti-Ro/SSA-positive adults with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 63: 1031-1037.
-
Cimaz R, Stramba-Badiale M, Brucato A, Catelli L, Panzeri P, et al. (2000) QT interval prolongation in asymptomatic anti-SSA/Ro-positive infants without congenital heart block. Arthritis Rheum 43: 1049-1053.
-
Nakamura K, Katayama Y, Kusano KF, Haraoka K, Tani Y, et al. (2007) Anti-KCNH2 antibody-induced long QT syndrome: novel acquired form of long QT syndrome. J Am Coll Cardiol 50: 1808-1809.
-
Yue Y, Castrichini M, Srivastava U, Fabris F, Shah K, et al. (2015) Pathogenesis of the Novel Autoimmune-Associated Long-QT Syndrome. Circulation 132: 230-240.
-
Nord JE, Shah PK, Rinaldi RZ, Weisman MH (2004) Hydroxychloroquine cardiotoxicity in systemic lupus erythematosus: a report of 2 cases and review of the literature. Semin Arthritis Rheum 33: 336-351.
-
Teixeira RA, Borba EF, Pedrosa A, Nishioka S, Viana VS, et al. (2014) Evidence for cardiac safety and antiarrhythmic potential of chloroquine in systemic lupus erythematosus. Europace 16: 887-892.
-
Nomura A, Kishimoto M, Takahashi O, Deshpande GA, Yamaguchi K, et al. (2014) Prolongation of heart rate-corrected QT interval is a predictor of cardiac autonomic dysfunction in patients with systemic lupus erythematosus. Rheumatol Int 34: 643-647.
-
Sestak AL, Nath SK, Sawalha AH, Harley JB (2007) Current status of lupus genetics. Arthritis Res Ther 9: 210.
-
Kelly JA, Moser KL, Harley JB (2002) The genetics of systemic lupus erythematosus: putting the pieces together. Genes Immun 3 Suppl 1: S71-85.
-
Niewold TB (2015) Advances in lupus genetics. Curr Opin Rheumatol 27: 440-447.
-
Straus SM, Kors JA, De Bruin ML, van der Hooft CS, Hofman A, et al. (2006) Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol 47: 362-367.
-
Goldenberg I, Horr S, Moss AJ, Lopes CM, Barsheshet A, et al. (2011) Risk for life-threatening cardiac events in patients with genotype-confirmed long-QT syndrome and normal-range corrected QT intervals. J Am Coll Cardiol 57: 51-59.
-
Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, et al. (2013) HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 10: 1932-1963.





