International Journal of Clinical Cardiology
Non-Invasive Assessment of Cardiac Autonomic Functions in Healthy Young Adults in Ile-Ife, South-Western Nigeria
Oluwadare Ogunlade1*, Abiodun O Ayoka1, Anthony Akintomide2, Rufus Ojo Akomolafe1, Olumide S Akinsomisoye1 and David Oyewusi Oyebola1
1Department of Physiological Sciences, Obafemi Awolowo University, Nigeria
2Department of Medicine, Obafemi Awolowo University, Nigeria
*Corresponding author: Oluwadare Ogunlade, Department of Physiological Sciences, Obafemi Awolowo University, Ile-Ife, Nigeria, Tel: +2348054106451, E-mail: ogunladeomotomilayo@gmail.com
Int J Clin Cardiol, IJCC-2-036, (Volume 2, Issue 3), Research Article; ISSN: 2378-2951
Received: May 01, 2015 | Accepted: June 08, 2015 | Published: June 10, 2015
Citation: Ogunlade O, Ayoka AO, Akintomide A, Akomolafe RO, Akinsomisoye OS, et al. (2015) Non-Invasive Assessment of Cardiac Autonomic Functions in Healthy Young Adults in Ile-Ife, South-Western Nigeria. Int J Clin Cardiol 2:036. 10.23937/2378-2951/1410036
Copyright: © 2015 Ogunlade O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: This study assessed the responses of Blood Pressure (BP) and Heart Rate (HR) to change of posture, Sustained Handgrip (SH),deep breathing(DB) and Valsalva manoeuvre (VM) in order to determining the normal limits and pattern of Autonomic Cardiovascular Indices (ACI) among healthy young adults.
Methods: Two hundred and four volunteer (98 men and 106 women) aged 18- 40 years (mean, 22.45 ± 4.86 years) participated in the study. After excluding systemic diseases by clinical evaluation, each participant performed five non-invasive cardiac autonomic function tests in series. Differences in supine and erect systolic BPs were assessed by sphygmomanometry, diastolic BP responses to SH were determined by dynamometry while HR variability in response to VM, DB and postural change were assessed by continuous electrocardiogram. Normal limits of ACI were determined at 5th and 95th percentiles.
Results: The mean (normal limits) for SBP response to change of posture (mmHg); DBP response to sustained handgrip (mmHg); Valsalva ratio; heart rate response to deep breathing (beats per minute); PTI; tachycardia ratio; bradycardia ratio and RHR(beats per minute) respectively were: -4.26 ± 9.17(-19.00-11.00); 20.14 ± 17.35(0.00-54.00); 1.55 ± 0.40(1.10-2.40); 31.82 ± 10.81(13.00-48.75); 1.41 ± 0.31(1.08-1.84); 0.80 ± 0.12(0.58-1.00); 1.21 ± 0.20(0.95-1.60) and 70.61 ± 12.23(53.00-93.00). During Valsalva maneouvre, Post-Strain Pause (PSP) which ranged from 1.28-12.76s was observed in 14(6.86%) of the participants. PSP occurred more frequently in women (9.43%) than men (4.08%). No significant gender difference in the duration of post-strain pause (t=-1.628, p=0.105). In majority of the participants (67.6%), an elevation of SBP occurred within 2minutes of rising from supine to erect position. Immediate Post-Standing Pause (IPSP) occurred among 139 (68.14%) participants.
Conclusion: Non-invasive assessment of cardiac autonomic function is feasible in Nigeria. Increase rather than decrease in systolic blood pressure response occurred more frequently upon a change from supine to erect posture. IPSP was a unique pattern of cardiac autonomic functions in study population.
Keywords
Assessment, Cardiac autonomic functions, Young adults
Background
The autonomic nervous system (ANS) is one of the main divisions of the nervous system. It is responsible for the regulation of visceral functions and maintenance of homeostasis of the internal milieu [1-3]. The portion of the ANS which influences the heart (cardiac autonomic nervous system) consists of a complex network of pre-ganglionic and postganglionic sympathetic and parasympathetic fibres that synapse on extrinsic and intrinsic cardiac ganglia and ultimately directly innervate cardiac myocytes [4]. The cardiac autonomic nervous system plays an integral role in the modulation of haemodynamics and cardiac electrophysiology [4]. An abnormality of cardiac autonomic nervous system is referred to as cardiac autonomic dysfunction (CAD) or neuropathy (CAN). It is a serious complication of many diseases. CAD carries approximately five-fold risk of mortality in patient with diseases affecting the cardiac autonomic nervous system especially diabetes mellitus [5-11]. The high mortality rate may be related to silent myocardial ischaemia or myocardial infarction, cardiac arrhythmias, cardiovascular or respiratory functional instability. CAD may occur in a diversity of diseases such as diabetes mellitus, alcoholism, Parkinsonism, malnutrition, vitamin deficiencies, heavy metal poisoning, rheumatic arthritis and kidney disorders [12,13].
The autonomic tests found to be useful in the assessment of cardiac autonomic functions include; blood pressure response to sustained handgrip and change of posture, heart rate variability test, heart rate and blood pressure response to Valsalva manoeuvre, heart rate response to deep breathing, head-up tilt, heart rate response to standing, cardiac meta-iodobenzyl-guanidine, muscle sympathetic nerve activity and plasma norepinephrine in supine and erect posture [14-17]. Commonly used indices of non-invasive cardiac autonomic function tests include: resting heart rate, Valsalva ratio, heart rate differences during deep breathing, E:I ratio, blood pressure difference during isometric exercise and change of posture, 30:15 ratio, tachycardia ratio and bradycardia ratio [18,19]. In Africa, the continent dominantly populated by the Black race, despite the rising trends in the prevalence of non-communicable diseases with proven significant impairment of cardiac autonomic function and consequential increasing mortality, data is sparse with regards to reference values for the standardized non-invasive cardiac autonomic function tests. In the few centres where patients are evaluated for cardiac autonomic function impairment, the Caucasian reference values are utilized for the assessment of the patients.
Methods
Participants
This was a cross-sectional descriptive study. The target population was apparently healthy adults (18-40 years) in Ile-Ife. Volunteers who consented to participate in the study were screened clinically. Inclusion criteria were; blood pressure (BP) <140/90mmHg and body mass index <30. Exclusion criteria were; age <18 years or >40 years, smokers, excess alcohol intake, pregnancy, lactation, hypertension, diabetes mellitus and other systemic diseases. The Ethics and Research Committee of the Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife approved the study protocol. A total of 204 volunteers (males and females) selected by purposive sampling technique participated in the study.
Cardiac autonomic function tests
Each participant performed a set of five non-invasive cardiac tests involving the use of sphygmomanometer, digital dynamometer and continuous recording of electrocardiogram (ECG) in a Research Laboratory. The first test was assessment of pulse rate (PR) and BP to determine the cardiovascular responses to change of posture. The second test was measurement of cardiovascular responses to sustained handgrip exercise. During the test, the maximum voluntary contraction (MVC) was determined by the use of dynamometer and the handgrip was thereafter maintained at 30% of MVC for a maximum period of 5minutes while cardiovascular parameters were recorded every minute. The third test involves evaluation of changes in cardiac electrical impulses in response to Valsalva maneouvre. The participant performed the manoeuvre by straining into a disposable mouth-piece attached to a manometer gauge in order to maintain an intraoral pressure of 40mmHg for 15s. ECG was recorded for 5s before the strain, 15s of the strain and 40s after the strain. Valsalva ratio and alterations in electrocardiographic intervals and waves were evaluated from the ECG strips. The fourth and fifth tests involved assessment of alteration in cardiac electrical impulses in response to deep breathing (6cycles of 5s inspiratory phase and 5s expiratory phase) and change of posture respectively.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation. Categorical variables were presented as numbers and percentages. Categorical variables were compared using chi square. P value <0.05 was considered as statistically significant. Lower and upper limits of normal were determined at 5th and 95th percentage respectively.
Results
Sociodemographic characteristics
The study group consisted of 204 young adults (98 men, 106 women) between the ages of 18 and 40 years (mean age 22.45 ± 4.86 years). The mean age for men and women were 23.72 ± 5.32 years and 21.26 ± 4.06 years respectively. The most frequent age group category was 21-30 years. Among the participants, 175 (85.80%) were Christians while 29(14.20%) were Muslims. The majority of the participants (90.20%) were students while the remaining 9.80% were staff of Obafemi Awolowo University, Ile-Ife. Most participants (85.30%) were of Yoruba ethnicity.
Systolic blood pressure response to change of posture
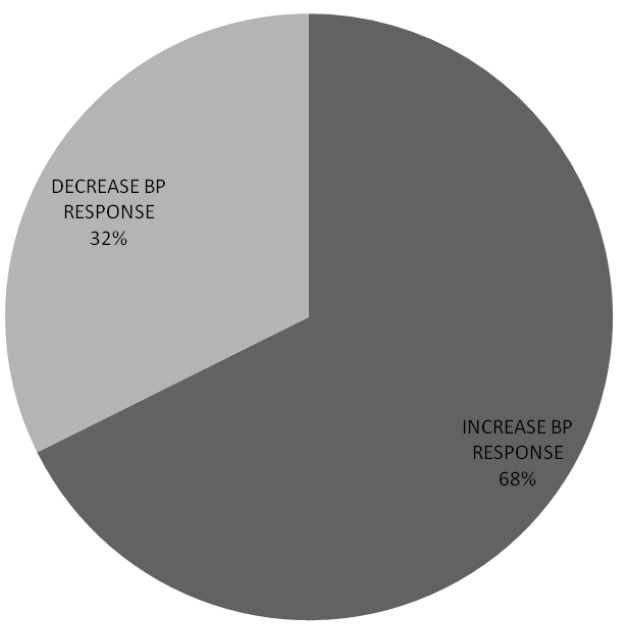
SBP response to change of posture was estimated from the difference between supine and erect SBP. Among sixty six (66) subjects (32.4%), the supine SBP was greater than erect SBP indicating a decrease in SBP within 1-2 minutes of standing while in 138 subjects (67.6%), supine SBP was less than erect SBP (Figure 1). No significant gender difference in SBP response to postural change (x2 =1.987, p value=0.159).
.
Figure 1: Systolic blood pressure response to postural change
Increase systolic blood pressure (BP) response was more frequent than decrease SBP response. Among sixty six (66) subjects (32.4%), the supine SBP was greater than erect SBP indicating a decrease in SBP within 1-2 minutes of standing while in 138 subjects (67.6%), supine SBP was less than erect SBP.
View Figure 1
Cardiovascular response to sustained handgrip
The mean of maximum SBP, DBP, MAP and PR recorded within 5 minutes of sustained handgrip at 30% MVC were; 137.41 ± 18.38mmHg, 88.90 ± 18.24mmHg, 105.07 ± 12.05mmHg and 90.10 ± 13.99 beats per minutes respectively (Table 1).
![]()
Table 1: Cardiovascular response to sustained handgrip
View Table 1
Cardiovascular response to valsalva maneouvre
The mean of the RR intervals 30s before the strain, shortest RR intervals during the 15s of the strain and longest RR intervals during the 20s after the strain of Valsalva maneouvre were: 0.81 ± 0.11s, 0.65 ± 0.12s and 0.98 ± 0.19s respectively. Post-strain pause (PSP) which ranged from 1.28-12.76s was observed in 14(6.86%) of the participants. PSP occurred more frequently in women (9.43%) than men (4.08%). No significant gender difference in the duration of post-strain pause (t=-1.628, p=0.105).
Cardiovascular responses to deep breathing
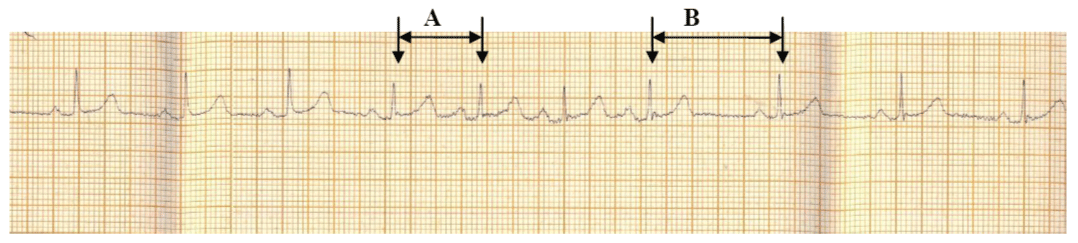
Heart rate response to deep breathing was illustrated in figure 2 which showed a reduction of RR interval (A) and increased heart rate during the phase of inspiration. During the phase of expiration, there was an increase in RR interval (B) and a decreased in heart rate. The mean minimum inspiratory RR interval, maximum inspiratory heart rate, maximum expiratory RR interval and minimum expiratory heart rate were: 0.66 ± 0.08s, 92.66 ± 11.04 beats per minute, 1.01 ± 0.17s and 61.19 ± 12.08s respectively. The mean amplitude of P wave during inspiration (1.54 ± 0.58mm) was higher than the mean P wave amplitude during expiration (1.03 ± 0.4mm).
.
Figure 2: Electrocardiographic strip showing changes in RR interval response to deep breathing
Heart rate response to deep breathing showed a reduction of RR interval (A) and increased heart rate during the phase of inspiration while during the phase of expiration, there was an increase in RR intervals (B) and a decreased in heart rate.
A: Inspiratory RR interval, B: Expiratory RR interval
View Figure 2
Cardiovascular responses to standing
Upon standing from supine position, RR intervals decreased and the heart rate increased towards 15th beat. There was an increase in RR intervals and decrease heart rate towards the 30th beat after standing erect without support or leaning on a couch. The mean shortest RR interval around 15th beat after standing, longest RR interval around 30th beat after standing, maximum HR around 15th beat after standing and minimum HR around 30th beat after standing were: 0.61 ± 0.09s, 0.85 ± 0.18s, 100.42 ± 14.59s and 73.29 ± 13.99s respectively. Immediate post-standing pause (IPSP) which ranged from 0.72-17.24s occurred in 139 (68.14%) of the participants. The mean and median of the IPSP were 4.87 ± 3.33s and 3.64s respectively. The upper limit of normal at 95th percentile was 10.76s. The mean IPSP in men and women were 3.68 ± 3.91 and 2.98 ± 3.20 respectively. IPSP occurred among 69 (70.41%) of men and 70 (66.67%) of women.
Normal limits of autonomic cardiovascular indices
Autonomic cardiovascular indices such as SBP response to change of posture, DBP response to sustained handgrip, Valsalva ratio, heart rate response to deep breathing (HDB) and 30:15 ratio or postural tachycardia index (PTI) were expressed in terms their means and normal limits (Table 2) (Figure 3-8).
.
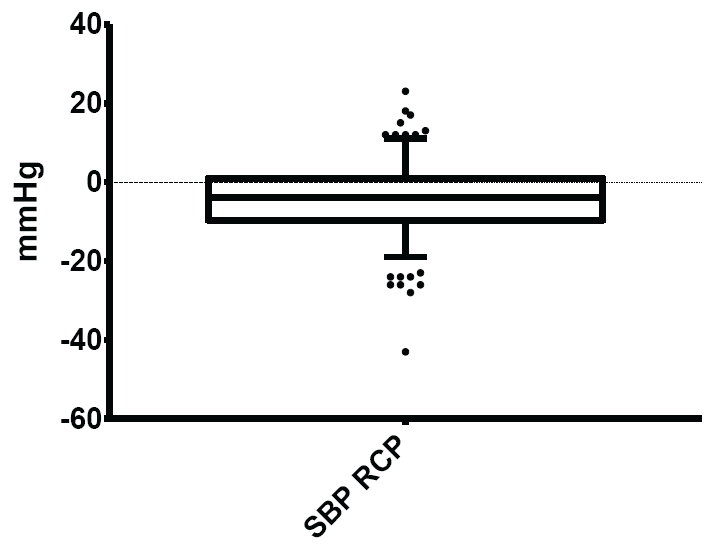
Figure 3: Normal limits of systolic blood pressure (SBP) response to change of posture
The mean and normal limits for systolic blood pressure response to change of posture were: - 4.26 ± 9.17mmHg and -19.00-11.00mmHg respectively.
View Figure 3
.
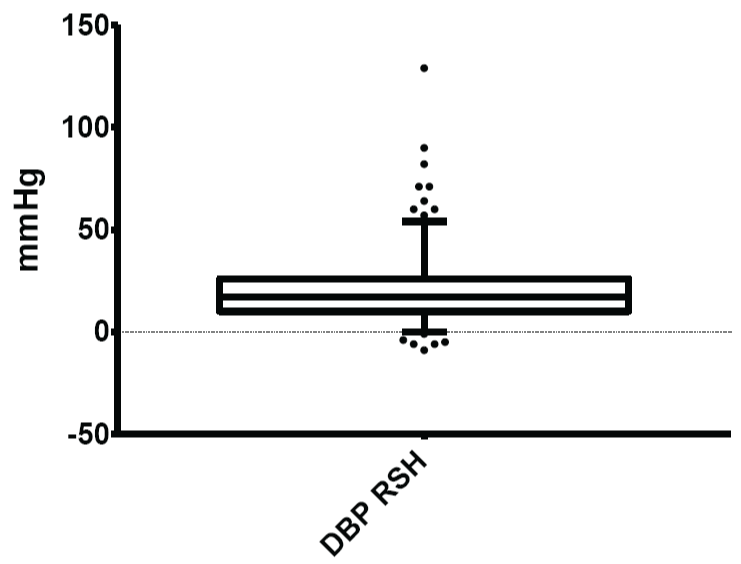
Figure 4: Normal limits of diastolic blood pressure (DBP) response to sustained handgrip
The mean and normal limits for diastolic blood pressure response to sustained handgrip were: 20.14 ± 9.17mmHg and 0.00 - 54.00mmHg respectively.
View Figure 4
.
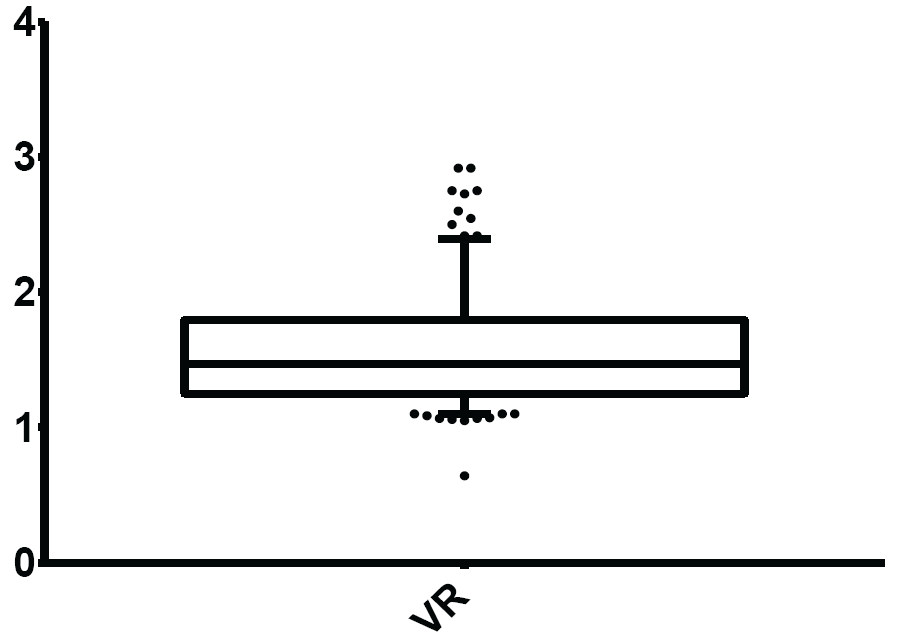
Figure 5: Normal limits of Valsalva Ratio (VR)
The mean and normal limits for Valsalva ratio were: 1.55 ± 0.40 and 1.10-2.40 respectively.
View Figure 5
.
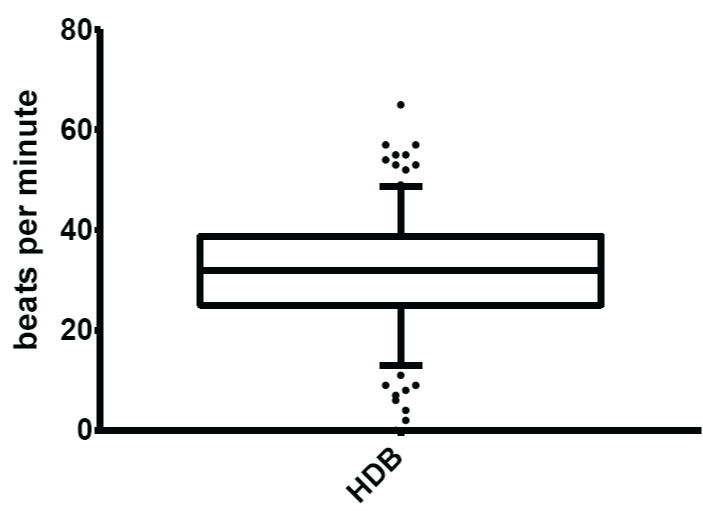
Figure 6: Normal limits of heart rate response to deep breathing (HDB)
The mean and normal limits for heart rate response to deep breathing were: 31.82 ± 10.81 beats per minute and 13.00 - 48.75 beats per minute respectively.
View Figure 6
.
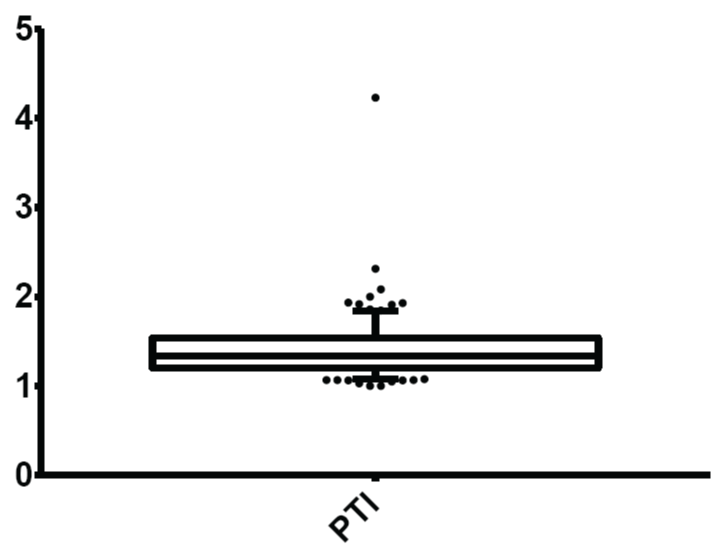
Figure 7: Normal limits of postural tachycardia index (PTI)/30:15 ratio
The mean and normal limits for postural tachycardia index were: 1.41± 0.31 and 1.08-1.84 respectively.
View Figure 7
.
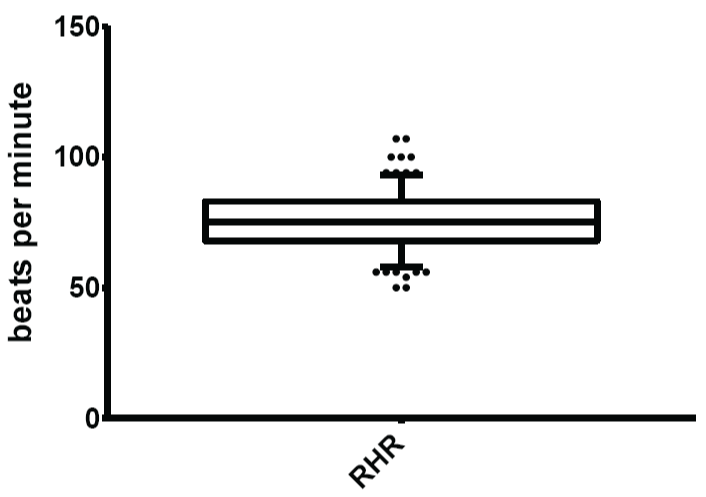
Figure 8: Normal limits of resting heart rate (RHR)
The mean and normal limits for resting heart rate were: 70.61 ± 12.23 beats per minute and 53.00-93.00 beats per minute respectively.
View Figure 8
![]()
Table 2: Normal limits of autonomic cardiovascular indices
View Table 2
Discussion
This present study utilized 5th and 95th percentiles for the estimation of cut-off values for assessment of autonomic cardiovascular indices. Previously established cut-off values for non-invasive battery of cardiac autonomic function tests were based on those defined by Ewing et al among the non-black population [14,18]. Postural change in systolic blood pressure had been established as a test of cardiac autonomic function mainly evaluating the sympathetic division of the autonomic nervous system [14,18]. In this study, the findings in majority of the subjects, 138(67.6%), revealed an elevation of SBP within 2 minutes of rising from supine to erect position while in 66 subjects (32.4%), there was a fall in SBP while rising from supine to erect posture. The systolic blood pressure response to postural change had been classified into two categories; increase SBP response (hypertensive response or orthostatic hypertension) and decrease SBP response (hypotensive response or orthostatic hypotension). In this study, the hypertensive response characterized by elevation of blood pressure upon assumption of erect posture occurred in majority of the subjects. Unlike the commonly described hypotensive response, hypertensive response is an underappreciated and understudied clinical phenomenon [20,21].
Autoregulatory mechanisms ensure relative fluctuations of blood pressure with postural change in healthy individuals [20]. Assumption of upright posture from supine position results in a small but measurable decrease in SBP due primarily to a redistribution of blood volume into the lower abdomen and extremities under the effect of gravity. In most people, this decrease in blood pressure is very slight and evanescent due to mobilization of neurohumoral and baroreflex mechanisms to maintain the blood pressure [22]. The initial physiological mechanisms bring about the hypotensive systolic blood pressure response while the compensatory mechanisms bring about restoration of blood pressure. Failure of the compensatory physiological responses bring about pathophysiological consequences resulting in orthostatic hypotension which is diagnosed when SBP falls more than 20mmHg within three minutes of standing from supine position [22]. On the other hand, the hypertensive response emanates from the pressor reflexes in response to change of posture. These consist of increase sympathetic drive and a decrease in the activity of the parasympathetic system. An increase in systolic blood pressure ≥ 20mmHg upon standing has been described as orthostatic hypertension [21]. The mechanism underlying development of orthostatic hypertension is not clearly understood but overcompensation of the pressor reflexes to postural change had been considered. Conditions associated with orthostatic hypertension include, essential hypertension, dysautonomias and type 2 diabetes mellitus. The finding of increase SBP response to change of posture among healthy young adults in this study suggests that a racial involvement might play a role.
Sustained handgrip is one of the standardized non-invasive tests of cardiac autonomic function. It was mainly designed for the evaluation of the changes mainly in the sympathetic division of the ANS [14,18]. During sustained handgrip, a sharp rise in blood pressure occurs, due to a heart-rate dependent increase in cardiac output with unchanging peripheral vascular resistance [18]. The rise in DBP was estimated by subtracting the sitting mean DBP before the handgrip from the mean of maximum DBP within 5 minutes of 30% of MVC. In this present study, the mean ± SD and normal limit of rise in DBP in response to sustained handgrip were 20.14 ± 17.35mmHg and 0.00-54.00mmHg respectively. In the previous study by Ewing and Clarke, 1982, a value ≥ 16mmHg was considered normal while a value ≤ 5mmHg was considered abnormal. It was observed that the available data on the study of autonomic function in African population conducted in Tanzania by Torsvik et al. 2008 lacked reference range on DBP response to sustained handgrip because of the unavailability of handgrip dynamometer for determination of maximum voluntary contraction.
Resting heart rate reflects the balance of parasympathetic and sympathetic influences at the sinoatrial node, with higher heart rate indicating a decreased parasympathetic influence or over activity of the sympathetic influence [24]. Increased resting heart rate is a predictor of cardiovascular mortality in subjects with and without diagnosed cardiovascular disease [25-28]. In this study, the mean resting heart rate (normal limits) was 70.61 ± 12.23 (53.00-93.00) beats per minute for both sexes. The resting heart rate is a good measure of balance in the autonomic nervous system as reduced parasympathetic activity causes resting tachycardia as an evidence of autonomic failure [29]. Evidence exists to show that the previously used reference limits of 60-100 beats per minutes for resting heart rate and the cut-off value >100 beats per minutes for sinus tachycardia or <60 beats per minutes for sinus bradycardia need to be revised in the light of emerging evidences that the reference limits may not be applicable universally as previously high-lighted by Palatini [30]. This concept was also supported by this present study.
The Valsalva maneouvre consists of four phases of acute short-lasting heart rate and blood pressure changes. The heart rate responses are the result of reflex mechanisms, predominantly of baroreceptor origin, which involve principally the parasympathetic autonomic nervous system [15,31]. Autonomic cardiovascular indices derived from the RR variability include: Valsalva, tachycardia and bradycardia ratios. Valsalva ratio was a reliable measure of cardiac autonomic function [32]. The normal limits of Valsalva ratio in this study was 1.10-2.40. In this study, Valsalva ratio <1.10 or >2.40 may be considered abnormal in the study population. The previously cited reference values defined by Ewing et al. 1982 described normal Valsalva ratio to be a value ≥1.21 and abnormal value to be a value ≤ 1.10. Data for comparison in African population is sparse. Available data on battery of cardiac autonomic function tests in Tanzania provided by Torsvik et al. 2008 omitted heart rate response to Valsalva manoeuvre due to lack of facilities for the procedure in the country. Therefore, the result of this present study gives landmark data in the quest of defining the pattern of cardiac autonomic function among Blacks. Tachycardia ratio and bradycardia ratio were described as indices of parasympathetic function during Valsalva manoeuvres [19]. In this study, mean ± SD (normal limits) of tachycardia ratio and bradycardia ratio were 0.80 ± 0.12 (0.58-1.00) and 1.21 ± 0.20 (0.95-1.60) respectively. However data is sparse on the reference limits and clinical relevance of the autonomic cardiovascular indices.
HDB is one of the most reliable indices of cardiac parasympathetic functions. The function of parasympathetic division of the ANS is reduced early in the development of cardiac autonomic neuropathy and heart rate variability induced by deep breathing is almost exclusively mediated by the parasympathetic fibres [14,33]. HDB was assessed as the difference between the maximum and minimum heart rate during deep breathing. In this present study, it was observed that the heart rate increased during inspiration and decreased during expiration in most participants. There was associated peaking and increased amplitude of P waves during inspiration. The mean inspiratory and expiratory P wave amplitudes were 1.54 ± 0.58mm and 1.03 ± 0.44mm respectively. The mean HDB estimated from the subtraction of maximum heart rate during inspiration from the minimum heart rate during expiration was 31.82 ± 10.81 beats per minute. The normal limits of the HDB ranged from 13.00-48.75 beats per minutes. This implied that HDB less than 13.00 beats per minute or greater than 48.75 beats per minute were considered abnormal. The lower cut-off value for the HDB in this study was slightly higher than 10 beats per minute defined by Ewing and Clarke, 1982. The upper cut-off value was not discussed by Ewing and Clarke in their early study on heart rate variation to deep breathing [18]. Immediate heart rate response to standing is an established non-invasive test of CAF. It was found to be very useful in the assessment of parasympathetic function. PTI was assessed by the aid of electrocardiograph and the print out electrocardiogram was assessed for the shortest RR interval at or around the 15th beat and the longest RR interval at or around the 30th beat after standing from a supine position. The characteristic heart rate response was expressed by the 30:15 ratio or postural tachycardia index. In the present study, the shortest RR interval indicating the maximum heart rate was not obtained at exactly 15th beat while the longest RR interval (indicating the minimum heart rate) was not obtained at 30th beat of standing. Immediate post-standing pause (IPSP), a phenomenon characterized by absence of P wave, QRS complex and T waves immediately after assumption of an erect posture from supine position was observed in majority of the participants. The IPSP ranged from 0.72-17.24s and was recordable in 139 participants (68.14% of the total number). The mean and median of the IPSP were 4.87 ± 3.33s and 3.64s respectively. The upper limit of normal of IPSP obtained in this study was 10.76s. The mean value of IPSP in men and women were 3.68 ± 3.91s and 2.98 ± 3.20s respectively. This probable physiological phenomenon was more frequently observed in men. Most of the participants with IPSP had no orthostatic symptom. IPSP was an observatory phenomenon in this study but no previous documentation could be found in literature and the physiological or clinical significance of IPSP is uncertain.
In this present study, the mean of 30:15ratio (PTI) was 1.41 ± 0.31 and the normal limit of PTI was 1.08-1.84. This implied that abnormal PTI occurred when PTI <1.08 or >1.84. According Ewing and Clarke, 1982, PTI ≤ 1.00 was considered abnormal whereas no upper limit for abnormality was mentioned. PTI which ranged from 1.01-1.03 was described as indicator of borderline autonomic function [14,15].
Conclusions
Non-invasive assessment of cardiac autonomic function is feasible in Nigeria. Increase rather than decrease in systolic blood pressure response occurred more frequently upon a change from supine to erect posture. IPSP was a unique pattern of cardiac autonomic functions in study population.
Acknowledgements
The authors would like to thank all the members of staff of the Department of Physiological Sciences, Obafemi Awolowo University for their technical and moral support.
References
-
Cannon WB (1929) Organization for physiological homeostasis. Physiol Rev 9: 399-431.
-
Hare K, Hinsey JC (1942) The autonomic nervous system. Ann Rev Physiol 4: 407-444.
-
Shields RW Jr (1993) Functional anatomy of the autonomic nervous system. J Clin Neurophysiol 10: 2-13.
-
Kapa S, Venkatachalam KL, Asirvatham SJ (2010) The autonomic nervous system in cardiac electrophysiology an elegant interaction and emerging concepts. Cardiol Rev 18: 275-284.
-
Ewing DJ, Campbell IW, Burt AA, Clarke BF (1973) Vascular reflexes in diabetic autonomic neuropathy. Lancet 2: 1354-1356.
-
Wheeler T, Watkins PJ (1973) Cardiac denervation in diabetes. Br Med J 4: 584-586.
-
Ziegler D (1994) Diabetic cardiovascular autonomic neuropathy: prognosis, diagnosis and treatment. Diabetes Metab Rev 10: 339-383.
-
Kempler P, Tesfaye S, Chaturvedi N, Stevens LK, Webb DJ, et al. (2002) Autonomic neuropathy is associated with increased cardiovascular risk factors: the EURODIAB IDDM Complications Study. Diabet Med 19: 900-909.
-
Kempler P (2003) Autonomic neuropathy: a marker of cardiovascular risk. Br J Diabetes Vasc Dis 3: 84-90.
-
Maser RE, Mitchell BD, Vinik AI, Freeman R (2003) The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care 26: 1895-1901.
-
Ieda M, Fukuda K (2009) Cardiac innervation and sudden cardiac death. Curr Cardiol Rev 5: 289-295.
-
Bannister RJ, Mathias CJ (1999) Introduction and classification of autonomic disorders. In: Mathias CJ, Bannister RJ (4th edn). Autonomic failure: A textbook of clinical disorders of the autonomic nervous system. London. Oxford Press: vii-xii.
-
Park J, Quyyumi AA, Middlekauff HR (2013) Exercise pressor response and arterial baroreflex unloading during exercise in chronic kidney disease. J Appl Physiol (1985) 114: 538-549.
-
Ewing DJ, Martyn CN, Young RJ, Clarke BF (1985) The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 8: 491-498.
-
Low PA (1993) Autonomic nervous system function. J Clin Neurophysiol 10: 14-27.
-
Low PA, Tomalia VA, Park KJ (2013) Autonomic function tests: some clinical applications. J Clin Neurol 9: 1-8.
-
Hering D, Kara T, Kucharska W, Somers VK, Narkiewicz K (2013) High-normal blood pressure is associated with increased resting sympathetic activity but normal responses to stress tests. Blood Press 22: 183-187.
-
Ewing DJ, Clarke BF (1982) Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed) 285: 916-918.
-
Pal GK, Velkumary S, Madanmohan (2004) Effect of short-term practice of breathing exercises on autonomic functions in normal human volunteers. Indian J Med Res 120: 115-121.
-
Benowitz NL, Zevin S, Carlsen S, Wright J, Schambelan M, et al. (1996) Orthostatic hypertension due to vascular adrenergic hypersensitivity. Hypertension 28: 42-46.
-
Fessel J, Robertson D (2006) Orthostatic hypertension: when pressor reflexes overcompensate. Nat Clin Pract Nephrol 2: 424-431.
-
Bradley JG, Davis KA (2003) Orthostatic hypotension. Am Fam Physician 68: 2393-2398.
-
Torsvik M, Häggblom A, Eide GE, Schmutzhard E, Vetvik K, et al. (2008) Cardiovascular autonomic function tests in an African population. BMC Endocr Disord 8: 19.
-
Malpas SC (2010) Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 90: 513-557.
-
Dyer AR, Persky V, Stamler J, Paul O, Shekelle RB, et al. (1980) Heart rate as a prognostic factor for coronary heart disease and mortality: findings in three Chicago epidemiologic studies. Am J Epidemiol 112: 736-749.
-
Kannel WB, Kannel C, Paffenbarger RS Jr, Cupples LA (1987) Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J 113: 1489-1494.
-
Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, et al. (2007) Resting heart rate in cardiovascular disease. J Am Coll Cardiol 50: 823-830.
-
Johansen CD, Olsen RH, Pedersen LR, Kumarathurai P, Mouridsen MR, et al. (2013) Resting, night-time, and 24 h heart rate as markers of cardiovascular risk in middle-aged and elderly men and women with no apparent heart disease. Eur Heart J 34: 1732-1739.
-
Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, et al. (2005) Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care 28: 956-962.
-
Palatini P (1999) Need for a revision of the normal limits of resting heart rate. Hypertension 33: 622-625.
-
Eckberg DL (1980) Parasympathetic cardiovascular control in human disease: a critical review of methods and results. Am J Physiol 239: H581-593.
-
Yale SH (2005) Antonio Maria Valsalva (1666 - 1723). Clin Med Res 3: 35-38.
-
May O, Arildsen H, Møller M (1999) Parasympathetic function during deep breathing in the general population: relation to coronary risk factors and normal range. J Intern Med 245: 287-294.





