International Journal of Anesthetics and Anesthesiology
A Case Report: Adaptive Servo Ventilation for a Patient with Acute Heart Failure and Central Sleep Apnea
Chol Kim1*, Yoshihiko Seino2 and Atsuhiro Sakamoto3
1Department of Anesthesiology, Nippon Medical School Chiba Hokusoh Hospital, Department of Anesthesiology and Pain Medicine, Nippon Medical School, Japan
2Department of Cardiovascular Medicine, Nippon Medical School Chiba Hokusoh Hospital, Department of Cardiovascular Medicine, Nippon Medical School, Japan
3Department of Anesthesiology and Pain Medicine, Nippon Medical School, Japan
*Corresponding author:
Chol Kim MD, PhD, Department of Anesthesiology, Nippon Medical School Chiba Hokusoh Hospital, 1715 Kamagari, Inzai, Chiba, 270-1694, Japan, Tel: 0476-99-1111, Fax: 0476-99-1931, E-mail: ckim@nms.ac.jp
Int J Anesthetic Anesthesiol, IJAA-4-055, (Volume 4, Issue 1), Case Report; ISSN: 2377-4630
Received: December 02, 2016 | Accepted: January 16, 2017 | Published: January 18, 2017
Citation: Kim C, Seino Y Sakamoto A (2017) A Case Report: Adaptive Servo Ventilation for a Patient with Acute Heart Failure and Central Sleep Apnea. Int J Anesthetic Anesthesiol 4:055. 10.23937/2377-4630/1410055
Copyright: © 2017 Kim C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Introduction
Sleep apnea is associated with cardiovascular diseases such as treatment-resistant hypertension, coronary artery disease, aortic dissection, arrhythmias, and chronic heart failure [1-5]. The prevalence of SAS, defined as an apnea-hypopnea index (AHI) of 5 or higher, has been estimated at 24% for men and 9% for women in the general population [6]. Sleep apnea is classified to obstructive sleep apnea (OSA), central sleep apnea (CSA), and mixed sleep apnea (Mix SA). OSA is caused by upper airway obstruction, and CSA is the result of a functional disorder of respiratory central nervous system. OSA is more often observed and related to the cardiovascular diseases. In contrast, CSA, which is uncommon in the normal population, is mainly observed in patients with chronic heart failure. Mix SA is a combination of both OSA and CSA, and sometimes develops after the treatment of OSA using CPAP (continuous positive air pressure).
The present manuscript is to report an obese patient hospitalized due to acute heart failure complicated with CSA, who was successfully treated with the guideline-based standard medical therapy and the use of ASV (Adaptive Servo Ventilation).
A Case Report
A 50-year-old male presented to the emergency room at night with complaints of paroxysmal nocturnal dyspnea and worsening shortness of breath on exertion. He was an active tireless estate agent till three months before, when he noticed shortness of breath on climbing the stairs at the station. Then dyspnea developed during sleep at night since one week before, but which was relieved soon by sitting up on the bed. However this night, the nocturnal dyspnea was hardly relieved by sitting and more worsened, and he was transferred by the ambulance car and admitted to this hospital.
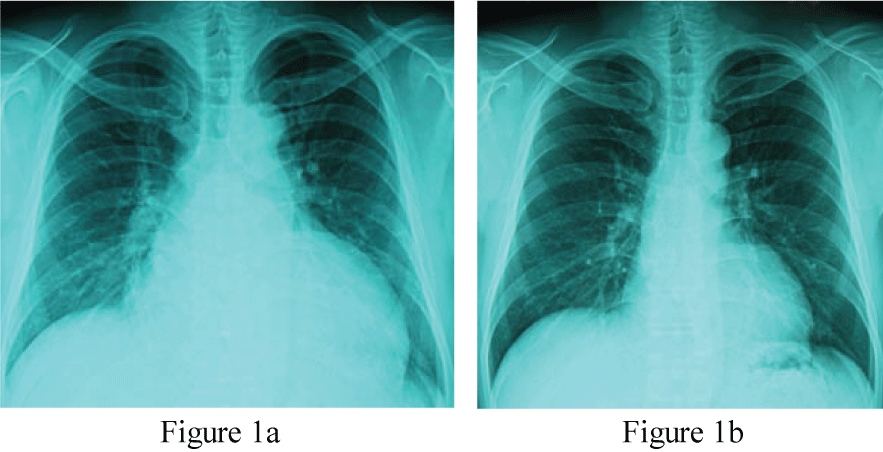
On examination, he was orthopnea condition. The temperature was 36.4 centigrade, and the pulse rate 104 beats per minute, regular, the blood pressure 170/100 mmHg, and the respiratory rate 25 per minute. The body height 179 cm, the body weight 102.2 kg, and the body mass index 31.9 kg/m2. Pulmonary course crackle was audible bilaterally, and the third heart sound gallop was manifest. The chest X-ray film taken after the emergency room treatment (Figure 1a) showed cardiothoracic ratio (CTR) 65% and the interstitial edema pattern. Electrocardiogram was regular sinus tachycardia with T wave inversion at leads I, aVL, V5 and V6, consistent with left ventricular hypertrophy.
.
Figure 1: Chest X-ray taken immediately after the emergency room care (Figure 1a, CTR 65%) and that taken 6 months after the therapy (Figure 1b, CTR 47%).
CTR: cardio thoracic ratio (%).
View Figure 1
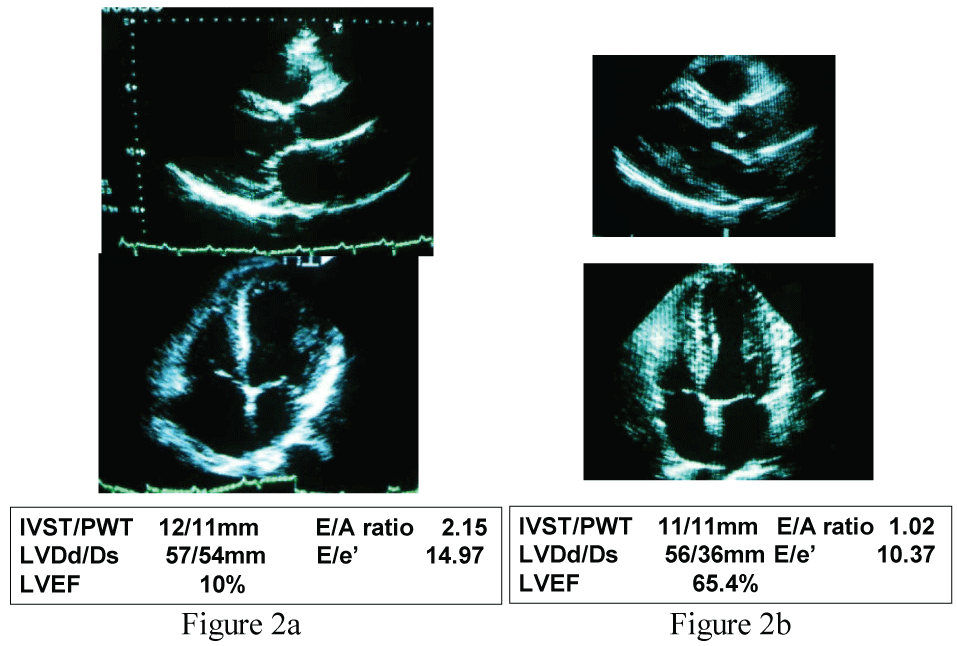
Point of care blood tests revealed elevated levels of B-type natriuretic peptide (BNP) 3,470pg/ml and troponin T 0.04 ng/ml consistent with minor myocardial injury associated with acute heart failure. The echocardiography displayed severe diffuse hypokinesia with left ventricular ejection fraction (LVEF) 10%, left ventricular end-systolic dimension (LVDs) 54 mm and left ventricular end-diastolic dimension (LVDd) 57 mm, interventricular septal thickness (IVST) 12 mm, posterior wall thickness (PWT) 11 mm, and E/A ratio 2.15 and E/e' 14.97 (Figure 2a). The technetium-99 m SESTAMIBI scintigraphy displayed no ischemic changes and acute coronary syndrome was ruled out.
.
Figure 2: Echocardiographic examinations performed immediately after the emergency room care (Figure 2a, LVEF 10%) and that done 6 months after the therapy (Figure 2b, LVEF 65.4%).
LVEF: left ventricular ejection fraction (%), IVST: interventricular septal thickness (mm), PWT: posterior wall thickness (mm), LVDd: left ventricular end-diastolic dimension (mm), LVDs: left ventricular end-systolic dimension (mm). E/A ratio and E/e' were obtained by tissue doppler echocardiography.
View Figure 2
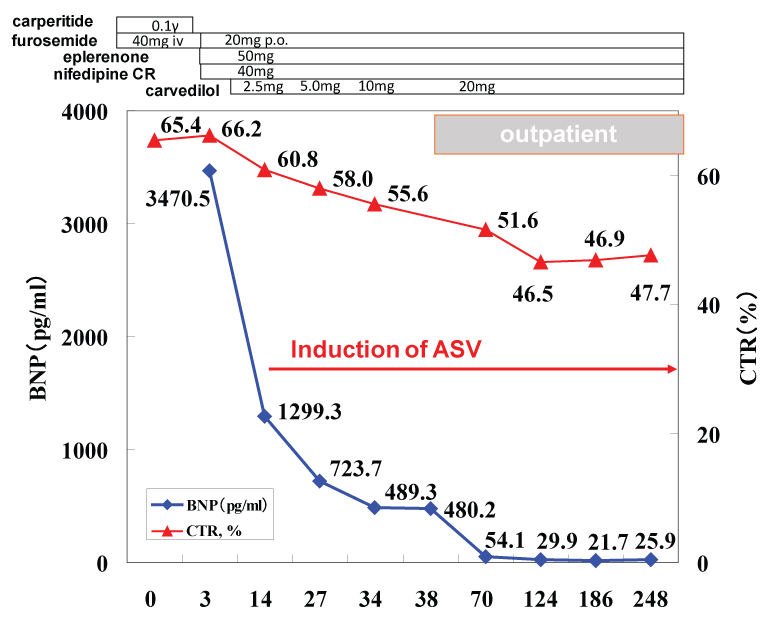
Clinical course and therapeutic interventions for the treatment of acute heart failure due to hypertensive heart disease and chronic kidney disease stage III (BUN 37.2 mg/ml and creatinine 1.79 mg/ml) were displayed in Figure 3. The acute phase treatments according to the guideline-based standard medical therapy using furosemide, alfa-natriuretic peptide (carperitide), vasodilators (eplerenone and long-acting nifedipine), and beta-blocker (carvedilol) brought good blood pressure control and improved congestive conditions. Because of the obesity and elevated blood pressure, presence of OSA was suspected initially, but the type-3 polygraphy revealed complication of CSA rather than OSA (Table 1). Thus ASV during night was induced. We used the ASV (AutoSet™ CS, ResMed, Sydney, Australia) in terms of the default settings (PEEP 5 cm H2O, minimum pressure support 3-5 cm H2O, maximum pressure support 8-12 cm H2O) for this patient's care. The dose of carvedilol was up-titrated to 20 mg/day, the standard dose for chronic heart failure in Japan, during outpatient care. The body weight was controlled to 79.3 kg (body mass index 24.7 kg/m2). The chest X-ray film (CTR 47.4%, Figure 1b), BNP level (29.9 pg/ml) and cardiac function (LVEF 65.4%, Figure 2b) were all normalized after 6 months.
.
Figure 3: Clinical course and therapeutic interventions during hospitalization and the outpatient are shown. BNP: B-type natriuretic peptide (pg/ml), CTR: cardio thoracic ratio (%), ASV: Adaptive Servo Ventilation.
View Figure 3
![]()
Table 1: Polygraphy examinations.
View Table 1
Heart Failure and Sleep Apnea
The characteristic symptoms of chronic heart failure are consistent with dyspnea on exertion, exercise hyperventilation, palpitation, easy fatigability, leg edema in the compensated condition, and then developing paroxysmal nocturnal dyspnea, orthopnea, and pulmonary edema in the decompensated condition. There is increasing evidence that sleep apnea contributes to the progression and prognosis in patients with chronic heart failure. Coexistence of sleep apnea, especially CSA, commonly referred as Cheyne-Stokes breathing, has been focused as one of the potent predictors for adverse prognosis in patients with chronic heart failure. The prevalence of CSA has been estimated at 20 to 40% in patients with chronic heart failure, and is related to severity of chronic heart failure, cardiac function, hemodynamic status, and neurohormonal derangement [7].
OSA is positioned as the upstream pathophysiology of heart failure, while CSA is in the downstream. As to the development of CSA in patients with chronic heart failure, principal factors leading to CSA are increased chemoreceptor sensitivity to carbon dioxide (PaCO2) and prolonged circulation time due to reduced cardiac output resulting delayed lung-chemoreceptor connection. Increased chemoreceptor sensitivity is closely related to arterial hypoxemia and pulmonary congestion accompanied with J-receptor stimulation during lying sleep at night. Furthermore recent investigations revealed excessive activation of sympathetic nervous system per se in severe heart failure brings about increased chemoreceptor sensitivity to PaCO2.
Increased chemoreceptor sensitivity to PaCO2 is assessed as enhanced ventilator response (VE-VCO2 slope) by cardiopulmonary exercise testing at the active condition. Dyspnea on exertion and exercise hyperventilation are common symptoms in chronic heart failure, and several studies revealed that both are related to VE-VCO2 slope and further AHI values [7-9]. The VE-VCO2 slope was significantly increased in patients with chronic heart failure and CSA compared with those without CSA. Patients with and without CSA did not differ with respect to exercise capacity assessed as peak VO2 nor left ventricular ejection fraction, while the significant positive correlation was demonstrated between VE-VCO2 slope and AHI [7]. These reports indicated the augmented chemoreceptor sensitivity to PaCO2 is as a common underlying pathophysiological mechanism developing exercise hyperventilation and dyspnea especially in patients with chronic heart failure and CSA [8-10].
ASV Therapy for Acute Heart Failure
Noninvasive positive pressure ventilation (NPPV) has been shown to improve pulmonary congestion in acute heart failure by unloading left ventricular preload through a reduction in venous return by adding positive end-expiratory pressure (PEEP) [11]. The ASV has been originally developed for the treatment of CSA, which is synchronized to the abnormal respiration patterns of individual patients and potentially allows in terms of home ASV therapy for the treatment of chronic heart failure patients.
In the present case, induction of ASV corrected disordered breathing and successfully improved symptoms and the representative parameters for heart failure such as CTR, BNP, and LVEF. ASV unloads preload condition through the reduction in excessive venous return by adding PEEP, which reduces pulmonary congestion and improves systemic oxygenation. ASV brings effective ventilation by supporting exhausted respiration muscles through the pressure support function according to the respiration patterns. ASV would suppress excessive activation of sympathetic nervous activity by unloading the excessive cardiac preload and after load condition and improvement in the arterial oxygenation. Via the mechanisms described above, ASV is considered to improve symptoms of heart failure and eventually to achieve the therapeutic goals for acute heart failure.
ASV Therapy for Chronic Heart Failure
On the background of significant influence of CSA on the cardiovascular and ventilator response in patients with chronic heart failure, therapeutic challenges for the improvement in quality of life (including quality of sleep) and improvement in the cardiovascular outcomes have been investigated. In the multicenter randomized trial (CHF-HOT study) [12-14], we have reported that nocturnal home oxygen therapy reduced AHI (21.0 ± 10.8 to 10.0 ± 11.6 events/h, mean ± SD, p < 0.001), and brought improvements in the NYHA class, the specific activity scale expressed as Mets (4.0 ± 1.2 to 5.0 ± 1.5 Mets, p < 0.001), and the LVEF (34.7 ± 10.4 to 38.2 ± 13.6%, p = 0.022) in patients with chronic heart failure and CSA, however improvement in the cardiovascular outcomes was not proved in the CHF-HOT study.
Recent reports regarding the ASV therapy for chronic heart failure patients complicated with CSA are notable. SERVE-HF (Treatment of Sleep-Disordered Breathing with Predominant Central Sleep Apnea by Adaptive Servo Ventilation in Patients with Heart Failure) trial [15] has shown that in contrast to their hypothesis, ASV therapy increased all-cause and cardiovascular mortality, and ASV therapy had no significant improvement in quality of life measures in patients with chronic heart failure complicated with CSA. These results contradict those of our SAVIOR-R and SAVIOR-C studies (Study of the Effects of Adaptive Servo-ventilation Therapy on Cardiac Function and Remodeling in Patients with Chronic Heart Failure) [16,17], which demonstrated improvements in quality of life and the New York Heart Association (NYHA) functional class(class III to class II), although improvement in LVEF and BNP levels and the improvement in the outcomes did not reach to the statistical significance.
The differences of the two studies are the subjects (chronic heart failure patients complicated with CSA versus those without the enrollment condition for sleep apnea, respectively) and the ASV pressure conditions (manually increase in the expiratory and inspiratory positive airway pressures to suppress the CSA versus maintain at or below the default levels to prevent lowering in cardiac output, respectively) [18].
Perioperative Management and Sleep Apnea
Sleep apnea would significantly influence on the perioperative management during systemic anesthesia because of possible occurrence of sleep apnea and the risk of cardiovascular complications. OSA is a serious complication in patients undergoing surgery. Loke, et al. has reported that OSA may be an independent risk factor for stroke and cardiovascular mortality [19]. Because OSA has linkage to cardiovascular diseases, it should influence perioperative and anesthetic management. In addition to cardiovascular diseases, other comorbidities such as pulmonary diseases and difficult airways are linked to OSA, influencing perioperative and anesthetic management.
In their systematic review and meta-analysis, Gaddam, et al. [20] noted many serious postoperative complications in adult patients with OSA undergoing non-upper airway surgery. They found that the incidence of postoperative hypoxemia is significantly higher in patients with OSA (odds ratio is 3.06); however, there was no significant difference in the incidence of re-intubation. Other respiratory complications such as aspiration, pneumonia, adult respiratory distress syndrome, and postoperative mechanical ventilation were also noted. Postoperative cardiac complications also occur significantly more frequently in patients with OSA than in those without it (odds ratio 1.76). These complications include dysrhythmias, abnormal heart rate, myocardial infarction and ischemia, hypotension, and congestive heart failure. The postoperative incidence of neurological complications such as delirium, agitation, confusion, and excessive drowsiness are also significantly higher in patients with OSA than in those without it (odds ratio 2.65). Prolonged postoperative oxygen therapy is significantly more often required in patients with OSA.
Fouladpour, et al. have reported that, in patients with OSA, cardiorespiratory arrest can occur in unmonitored settings and difficulty in airway management encountered in the operating room and/or post-anesthesia care unit, both potentially resulting in catastrophic outcomes, including death or permanent brain damage [21].
As shown above, OSA is a risk factor for postoperative complications. It is therefore important to identify possible OSA in surgical patients in the preoperative period. Even if there is not enough time to make an accurate diagnosis of OSA, screening for this condition should be performed. The guidelines provided by the Society of Anesthesia and Sleep Medicine cites four OSA screening tools; namely, the STOP-Bang questionnaire (Table 2), Berlin questionnaire, ASA Checklist, and P-SAP score [22,23]. Additionally, the DES-OSA score can be calculated [24]. Considering the linkage between OSA and cardiovascular diseases, we recommend identification of possible OSA preoperatively to avert serious postoperative complication.
![]()
Table 2: STOP-Bang questionnaire.
View Table 2
In the present case report, beneficial effects of ASV along with the standard medical therapy for a patient with acute heart failure complicated CSA were documented. Not only the acute heart failure symptoms but also the representative clinical parameters regarding the heart failure, CTR, BNP levels, cardiac function (LVEF) were all improved or normalized after the present therapies. In the present casa, ASV pressure settings were adjusted for maintain at or below the default levels to prevent lowering in cardiac output. The use of ASV for patients with chronic heart failure complicated with CSA are now controversial, though deliberate application and appropriate adjustment of the ASV pressure settings according to changes in heart failure condition should be important.
References
-
Lévy P, Ryan S, Oldenburg O, Parati G (2013) Sleep apnoea and the heart. Eur Respir Rev 22: 333-352.
-
Yanagi H, Imoto K, Suzuki S, Uchida K, Masuda M, et al. (2013) Acute aortic dissection associated with sleep apnea syndrome. Ann Thorac Cardiovasc Surg 19: 456-460.
-
Tobaldini E, Costantino G, Solbiati M, Cogliati C, Kara T, et al. (2016) Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci BiobehavRev S0149-7634: 30218-30224.
-
Inami T, Seino Y, Shimura T, Kurihara O, Kimata N, et al. (2016) Linkage of sleep-disordered breathing and acute aortic dissection with patent false lumen. Heart Vessels 31: 1069-1076.
-
Inami T, Seino Y, Otsuka T, Yamamoto M, Kimata N, et al. (2012) Links between sleep disordered breathing, coronary atherosclerotic burden, and cardiac biomarkers in patients with stable coronary artery disease. J Cardiol 60: 180-186.
-
Young T, Palta M, Dempsey J, Skatrud J, Weber S, et al. (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328: 1230-1235.
-
Javaheri S (1999) A mechanism of central sleep apnea in patients with heart failure. N Engl J Med 341: 949-954.
-
Arzt M, Harth M, Luchner A, Muders F, Holmer SR, et al. (2003) Enhanced ventilatory response to exercise in patients with chronic heart failure and central sleep apnea. Circulation 107: 1998-2003.
-
Mansfield D, Kaye DM, Brunner La Rocca H, Solin P, Esler MD, et al. (2003) Raised sympathetic nerve activity in heart failure and central sleep apnea is due to heart failure severity. Circulation 107: 1396-1400.
-
Meguro K, Adachi H, Oshima S, Taniguchi K, Nagai R (2005) Exercise tolerance, exercise hyperpnea and central chemosensitivity to carbon dioxide in sleep apnea syndrome in heart failure patients. Circ J 69: 695-699.
-
Bersten AD, Holt AW, Vedig AE, Skowronski GA, Baggoley CJ (1991) Treatment of severe cardiogenic pulmonary edema with continuous positive airway pressure delivered by face mask. N Engl J Med 325: 1825-1830.
-
Sasayama S, Izumi T, Seino Y, Ueshima K, Asanoi H, et al. (2006) Effects of nocturnal oxygen therapy on outcome measures in patients with chronic heart failure and cheyne-stokes respiration. Circ J 70: 1-7.
-
Seino Y, Imai H, Nakamoto T, Araki Y, Sasayama S, et al. (2007) Clinical efficacy and cost-benefit analysis of nocturnal home oxygen therapy in patients with central sleep apnea caused by chronic heart failure. Circ J 71: 1738-1743.
-
Sasayama S, Izumi T, Matsuzaki M, Matsumori A, Asanoi H, et al. (2009) Improvement of quality of life with nocturnal oxygen therapy in heart failure patients with central sleep apnea. Circ J 73: 1255-1262.
-
Cowie MR, Woehrle H, Wegscheider K, Angermann C, d'Ortho MP, et al. (2015) Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. N Engl J Med 373: 1095-1105.
-
Momomura S, Seino Y, Kihara Y, Adachi H, Yasumura Y, et al. (2015) Adaptive servo-ventilation therapy using an innovative ventilator for patients with chronic heart failure: a real-world, multicenter, retrospective, observational study (SAVIOR-R). Heart Vessels 30: 805-817.
-
Momomura S, Seino Y, Kihara Y, Adachi H, Yasumura Y, et al. (2015) Adaptive servo-ventilation therapy for patients with chronic heart failure in a confirmatory, multicenter, randomized, controlled study. Circ J 79: 981-990.
-
Kihara Y, Seino Y, Momomura S, SAVIOR-C investigators (2016) Adaptive Servo-Ventilation for Central Sleep Apnea in Heart Failure. N Engl J Med 374: 687-691.
-
Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK (2012) Association of obstructive sleep apnea with risk of serious cardiovascular events: a systematic review and meta-analysis. Circulation Cardiovascular quality and outcomes 5: 720-728.
-
Gaddam S, Gunukula SK, Mador MJ (2014) Post-operative outcomes in adult obstructive sleep apnea patients undergoing non-upper airway surgery: a systematic review and meta-analysis. Sleep Breath 18: 615-633.
-
Fouladpour N, Jesudoss R, Bolden N, Shaman Z, Auckley D (2016) Perioperative Complications in Obstructive Sleep Apnea Patients Undergoing Surgery: A Review of the Legal Literature. Anesth Analg 122: 145-151.
-
Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, et al. (2008) STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 108: 812-821.
-
Chung F, Memtsoudis SG, Ramachandran SK, Nagappa M, Opperer M, et al. (2016) Society of Anesthesia and Sleep Medicine Guidelines on Preoperative Screening and Assessment of Adult Patients With Obstructive Sleep Apnea. Anesthesia and Analgesia 123: 452-473.
-
Deflandre E, Degey S, Brichant JF, Donneau AF, Frognier R, et al. (2016) Pre-Operative Ability of Clinical Scores to Predict Obstructive Sleep Apnea (OSA) Severity in Susceptible Surgical Patients. Obes Surg.





