International Journal of Anesthetics and Anesthesiology
Effect of Sevoflurane and Desflurane on Erythrocyte Deformability during Ischaemia-Reperfusion Injury of Lower Extremity in Diabetic Rats
Muhammed Enes Aydin1, Meral Erdal Erbatur1, Faruk Metin Çomu2 and Mustafa Arslan1*
1Department of Anaesthesiology and Reanimation, Gazi University Medical Faculty, Turkey
2Department of Physiology, Kirikkale University Medical Faculty, Turkey
*Corresponding author: Mustafa Arslan, Gazi University Medical Faculty, Department of Anesthesiology and Reanimation, 06510 Ankara Turkey, Tel: 90-312-202-67-39, E-mail: marslan36@yahoo.com/ mustarslan@gmail.com
Int J Anesthetic Anesthesiol, IJAA-1-026, (Volume 2, Issue 2), Original Article; ISSN: 2377-4630
Received: February 12, 2015 | Accepted: March 26, 2015 | Published: March 28, 2015
Citation: Aydin ME, Erbatur ME, Çomu FM, Arslan M (2015) Effect of Sevoflurane and Desflurane on Erythrocyte Deformability during Ischaemia-Reperfusion Injury of Lower Extremity in Diabetic Rats. Int J Anesthetic Anesthesiol 2:026. 10.23937/2377-4630/2/2/1026
Copyright: © 2015 Aydin ME, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: It is known that blood viscosity and erythrocyte aggregation are increased and erythrocyte deformability is decreased in diabetic patients. Ischemia reperfusion injury (I/R) in lower extremity is a frequent and important clinical phenomenon. Blood rheology is known to be affected by numerous factor including anaesthetic drugs. Accordingly, we aimed to investigate the effects of sevoflurane and desflurane on erythrocyte deformability in infrarenal aorta of diabetic rats undergoing I/R.
Materials and methods: In this study, 30 male Wistar albino rats. After the effects of chronic diabetes encountered diabetic rats were randomly assigned into diabetic control (group DC), diabetic I/R group (group D-I/R), diabetic I/R group with desflurane (group D-I/R-D), and diabetic I/R group with sevoflurane (group D-I/R-S) groups. Another 6 rats without diabetes were assigned as control group (group C). 4 weeks after the injection of streptozotocin diabetic rats were anaesthetized by desflurane 6% or sevoflurane 2% at a dose by which minimal alveolar concentration (MAC) for rats would be one. The drugs were given for 4 hours within 100% oxygen at a rate of 4L.min1. Erythrocyte samples were obtained from heparinized whole blood samples. Measurements for deformability were conducted on erythrocyte suspensions within serum physiologic tamponized with phosphate.
Results: Deformability index was significantly increased in diabetic rats (p< 0.001), however, it was similar in GroupD-I/R, GroupD-I/R-D and GroupD-I/R-S (p=0.570, p=0.951 respectively). It was significantly increased in GroupD-I/R,D-I/R-D and D-I/R-S when compared to GroupDC (p=0.001, p=0.004, p=0.001 respectively). Relative resistance was increased in diabeticand I/R models.
Conclusion: Neither sevoflurane nor desflurane caused a negative effect on erythrocyte deformability in infrarenal aorta of diabetic rats undergoing I/R diabetic rats. However these findings should be further investigated in larger and more detailed studies.
Keywords
Diabetes mellitus, Ischemia-reperfusion, Sevoflurane, Desflurane, Erythrocyte deformability, Rat
Introduction
In the recent two or three decades, the prevalence of diabetes mellitus (DM) has rapidly increased throughout the world, the estimation being that it will increase by 200% in the next few decades [1-5]. As a result, physicians will be faced with an increasing population of diabetic patients undergoing anesthesia and surgery. These patients are carrying high risk for serious cardiovascular complications eventually leading to significant increases in mortality and length of stay rates in hospital [1-3].
In-vitro and in-vivo studies suggest that lipid peroxidation plays an important role in the pathogenesis of diabetic complications [6,7].
Hemorheological parameters which include hematocrit, plasma proteins, erythrocyte aggregation, and erythrocyte deformability in DM, are often disturbed [8].
Ischemia reperfusion injury (I/R) in lower extremity is a frequent and important clinical phenomenon. Reperfusion period following an ischemic insult may paradoxically cause increased rates of mortality and morbidity due to systemic complications. Local edema and muscle tissue necrosis are likely to be followed by systemic inflamatory response syndrome and multiple organ failure (kidney, respiratory and circulatory system etc.) as reperfusion advances [9-11].
General anesthesia agents are known to affect cardiovascular functions and microcirculation dynamics [12]. However, whether these agents change plasma rheology and/or may result in deterioration of tissue perfusion remains controversial. Changes in plasma viscosity has been listed among the factors associated with anesthesia procedures responsible for deterioration of tissue and organ perfusion [13,14]. After surgical procedures performed under general anesthesia, erythrocyte deformability and increased aggregation may be observed [14]. Isoflurane, sevoflurane and desflurane may decrease erythrocyte immune function and desflurane may alter erythrocyte deformability during anaesthesia and surgery [15].
The capillary filtration coefficient is lowered by sevoflurane. It has been demonstrated that sevoflurane, when compared with intravenous anesthetics such as propofol, could have beneficial effects on the microcirculation by decreasing extravasation of plasma into the interstitial space, and thus limiting tissue edema [16]. Sevoflurane could also have a protective effect on endothelial cells against ischemia-reperfusion injury [17].
Volatile anaesthetic administered during general anaesthesia increase peripheral perfusion. This correlation has been demonstrated for sevoflurane [18] and desflurane [19] anaesthesia on the peripheraltissue flow.
In vitro, volatile anesthetics such as halothane,enflurane and isoflurane inhibit insulin responsesto glucose in a reversible and dose-dependentmanner [20-24]. A clinical study by Diltoer and Camu [22] showed that glucose tolerance was impairedby isoflurane. In an experimental study [23], halogenated anesthetic agents, such as halothaneor sevoflurane, produced greater negativeinotropic effects in diabetic patients comparedwith normal myocardium, possibly because diabetesexacerbates anesthetic-induced alterationsin troponin-tropomyosin complex activity.
The aim of this study was to investigate erythrocyte deformability indices of desflurane and sevoflurane on erythrocyte deformability in in infrarenal aorta of diabetic rats undergoing I/R.
Material and Method
Animals and experimental protocol
The study was performed upon the approval of Gazi University Experimental Animals Ethics Committee in Gazi University Experimental and Clinical Research Center (GUDAM). All of the procedures were performed according to the accepted standards of the Guide for the Care and Use of Laboratory Animals.
In this study, 30 male Wistar albino rats weighing between 200 and 250g, raised under the same environmental conditions, were used. The rats were kept under 20-21°C at cycles of 12-hour daylight and 12-hour darkness and had free access to food until 2 hours before the anesthesia procedure.
The animals were randomly separated into five groups, each containing 6 rats: diabetic control (group DC), diabetic I/R group (group D-I/R), diabetic I/R group with desflurane (group D-I/R-D), and diabetic I/R group with sevoflurane (group D-I/R-S) groups. Another 6 rats without diabetes were assigned as control group (group C)
STZ (Sigma Chemicals, St. Louis, MO, USA) were prepared by dissolving in saline solution (0.9% NaCl). STZ was freshly prepared just before the treatment at a dose of 55mg.kg-1 body weight. Blood glucose levels of diabetic rats were checked by glucometer (mg/dl) 3 days after administration of STZ. Rats were classified as diabetic if their fasting blood glucose (FBG) levels exceeded 250mg.dl-1, and only animals with FBGs of >250mg.dl-1 were included in the diabetic groups (diabetes only, diabetes I/R, diabetes I/R plus sevoflurane and diabetes I/R plus desflurane). The rats were kept alive 4 weeks after streptozotocin injection to allow development of chronic diabetes before they were exposed to sevoflurane and desflurane [24].
I/R: The abdomen was shaved and each animal was fixed in a supine position on the operating table. The abdomen was cleaned with 1% polyvinyliodine and when dry, the operating field was covered with a sterile drape and median laparotomy was performed. Infrarenal segment of the aorta was clamped for 2 hours. After removing the clamp, reperfusion was established for another 2 hours.
Before the study was started anaesthetic gas vaporisers were calibrated. Anaesthetic gases were set at a minimum alveolar concentration (MAC) of 1 and desflurane 6% and sevoflurane 2% were administered. The anaesthesia procedure was conducted with the rats in a transparent plastic container of 40x40x70 cm. in size. The container, which allowed for observations of the rats, was connected to a half open anaesthesia machine with static hoses. The anaesthetic gases were released into the container in 100% O2.
The rats were divided into five groups (n=6). The control, DC and D-I/R groups were not subjected to any application. Desflurane (Suprane, Eczacibasi, Istanbul, Türkiye) was administered at 6% inspiratory concentration, 6L.min-1 in 100% O2 for 2 hours, and sevoflurane (Sevorane, Abbot, Istanbul, Türkiye) was administered at 2% inspiratory concentration, 6L.min-1 in 100% O2 for 4 hours.
After anaesthesia procedure, all the rats were given ketamine 100mg.kg-1 intraperitoneally. Heparinized total blood samples were used to prepare erythrocyte packs. Deformability measurements were done by erythrocyte suspensions with 5% HCT in phosphate buffered saline buffer.
Deformability measurements
Blood samples were taken very carefully and measurement process was as fast (the first 5 minutes) as possible to avoid hemolysis of erythrocytes. The collected blood was centrifuged at 1000 rpm for ten minutes. Serum and buffy coat on erythrocytes were removed. The isotonic PBS buffer was added to collapsing erythrocytes and this was centrifuged at 1000 rpm for ten minutes. Liquid on the upper surface was removed. Finally pure red cell packs were obtained from the washing process which was repeated three times. Erythrocytes packs were mixed with PBS buffer to generate a suspension with the value of 5% HCT. Those erythrocyte suspensions were used for the measurement of deformability. Collection and deformability measurements of erythrocytes were done at 22°C.
The constant-current filtrometer system was used for measurement of erythrocytes deformability. Samples to be measured were prepared as 10ml of erythrocytes suspension and PBS buffer. The flow rate was held constant at 1.5ml/min with an infusion pump. A 28mm nucleoporin polycarbonate filter with a 5 µm pore diameter was used. Consisting pressure changes while the erythrocytes passing through from the filter were detected by the pressure transducer and the data was transferred to computer with the help of MP 30 data equation systems (Biopac Systems Inc, Commat, USA). The necessary calculations were performed with related computer programs by measuring the pressure changes at various times. Pressure calibration of the system was performed each time before measuring the samples. Firstly buffer (PT) and then erythrocytes (PE) were passed through from the filtration system and the changes in pressure were measured. The relative refractory period value (Rrel) was calculated by relating the pressure value of erythrocytes suspension to pressure value of buffer. Increasing in Rrel as the deformability index was interpreted as the adversely affected the ability of erythrocytes deformability [25,26].
Statistical analysis
Statistical Package for the Social Sciences (SPSS, Chicago, IL, USA) 17.0 program was used for statistical analysis. The importance of the difference of the mean erythrocyte deformability values were assessed by using Kruskal-Wallis test. Bonferroni adjusted Mann-Whitney U test was used after significant Kruskal-Wallis to determine which group differs from the other. Results were expressed as mean ± Standard deviation (Mean ± SD). Statistical significance was set at a p value< 0.05.
Results
Deformability index was significantly increased in diabetic rats (p< 0.001), however, it was similar in Group D-I/R, Group D-I/R-D and GroupD-I/R-S (p=0.570, p=0.951, respectively). It was significantly increased in Group D-I/R, D-I/R-D and D-I/R-S when compared to Group DC (p=0.001, p=0.004, p=0.001, respectively) (Figure 1). Relative resistance was increased in diabeticand I/R models.
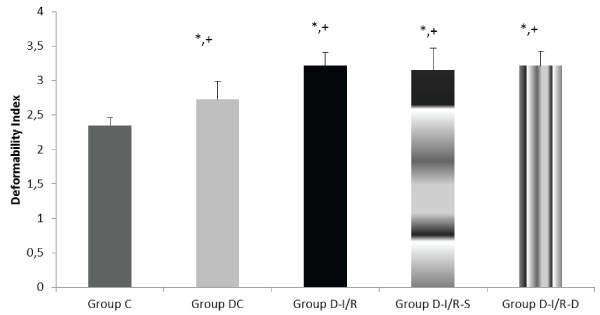
Erythrocyte deformability values of the groups. Each bar represents the mean ± sd
*p< 0.05 compared to the Group C; + p< 0.05 compared to the Group DC
View Figure 1
Discussion
For the maintenance of tissue perfusion effective blood flow is required in the microcirculation. The rheologic measurements conducted on patients who were subjected to major elective surgery revealed that, blood viscosity, fibrinogen and erythrocyte aggregation were increased, due to decreased erythrocyte deformability, blood flow in microcirculation and thus oxygen delivery to tissues were attenuated. It is well known that anesthetic agents are among the numerous factors affecting blood rheology [27].
Erythrocytes deform while passing through the capillaries which are smaller than their diameters. The erythrocyte deformability is determined by numerous factors including the ratio of surface area to volume, the phospholipid composition of erythrocyte cell membrane and viscosity of the intracellular fluid. The decrease in erythrocyte deformability results in impairment of tissue perfusion in peripheric tissues [28].
It is known that blood viscosity and erythrocyte aggregation are increased and erythrocyte deformability is decreased in diabetic patients. The derangement in the blood rheology impairs microvascular blood flow and thus leads to aggravation of microangiopathy and increase in the development of diabetic microvascular complications [28].
Erythrocyte membranes are vulnerable to lipid peroxidation because of the lipid components of their membranes. Lipid peroxidation has adverse effects on the deformability of erythrocytes [29]. A decrease in erythrocyte deformability impairs tissue oxygenation and causes complications at the microvascular level [30].
Even a small decrease in erythrocyte deformability causes problems at microvessel level [30]. Some studies reported that erythrocyte deformability levels were found to be decreased in DM [31,32].
In the literature, there are only limited findings about the effects of DM on erythrocytes. Allen et al. [33] indicated that the erythrocyte membrane lipid profile is destroyed in DM. Yang et al. [34] reported the decreased erythrocyte deformability levels in DM.
Hemorheologic factors may be directly or indirectly affected by anesthetic agents and their metabolites. Anaesthetics alter the diameters of arterioles and venules and the response of these structures to stress. The effects of anaesthetic agents on microcirculation are specific and dose dependent. The mechanisms that cause this interaction may be associated with oxidative disorders that occur during or after various anaeasthesia applications [35-37].
Alterations in the erythrocyte deformability may result in poor perfusion that can contribute to vascular complications of post-anaesthetic period that may arise in addition to other well-known mechanisms. This may lead to inadequate recovery [36].
Yerer et al. [38] investigated the effects of desflurane on deformability and found that it impaired the deformability in young and old rats. Aydogan et al. [39] showed the negative effects of sevoflurane on the deformability in the old rats.
Comu et al. [40] found that neither desflurane nor sevoflurane had negative effect on erythrocyte deformability in diabetic male rats.
Conclusion
In conclusion, the results of this study clearly demonstrated that erythrocyte deformability is significantly altered in experimental infrarenal aorta IR injury in the diabetic rat. This might lead to further problems in microcirculation. Thus, measurement of erythrocyte deformability might have an important impact on the follow-up for IR injury. Additionally, we found that neither desflurane nor sevoflurane had negative effect on erythrocyte deformability in infrarenal aorta of diabetic rats undergoing I/R. Other aspects of these findings, including clinical significance and practical applications, merit further experimental and clinical investigation.
References
-
Robertshaw HJ, Hall GM (2006) Diabetes mellitus: anaesthetic management. Anaesthesia 61: 1187-1190.
-
McAnulty GR, Robertshaw HJ, Hall GM (2000) Anaesthetic management of patients with diabetes mellitus. Br J Anaesth 85: 80-90.
-
McAnulty GR, Hall GM (2003) Anaesthesia for the diabetic patient. Br J Anaesth 90: 428-429.
-
Gu W, Pagel PS, Warltier DC, Kersten JR (2003) Modifying cardiovascular risk in diabetes mellitus. Anesthesiology 98: 774-779.
-
Kadoi Y (2010) Anesthetic considerations in diabetic patients. Part I: preoperative considerations of patients with diabetes mellitus. J Anesth 24: 739-747.
-
Mishra N, Singh N (2013) Blood viscosity, lipid profile, and lipid peroxidation in type-1 diabetic patients with good and poor glycemic control. N Am J Med Sci 5: 562-566.
-
Singh R, Bhardwaj P, Sharma P (2013) Antioxidant and toxicological evaluation of Cassia sopherain streptozotocin-induced diabetic Wistar rats. Pharmacognosy Res 5: 225-232.
-
Cho YI, Mooney MP, Cho DJ (2008) Hemorheological disorders in diabetes mellitus. J Diabetes Sci Technol 2: 1130-1138.
-
Duru S, Koca U, Oztekin S, Olguner C, Kar A, et al. (2005) Antithrombin III pretreatment reduces neutrophil recruitment into the lung and skeletal muscle tissues in the rat model of bilateral lower limb ischemia and reperfusion: a pilot study. Acta Anaesthesiol Scand 49: 1142-1148.
-
Turchányi B, Tóth B, Rácz I, Vendégh Z, Furész J, et al. (2005) Ischemia reperfusion injury of the skeletal muscle after selective deafferentation. Physiol Res 54: 25-32.
-
Lin B, Ginsberg MD, Busto R, Li L (2000) Hyperglycemia triggers massive neutrophil deposition in brain following transient ischemia in rats. Neurosci Lett 278: 1-4.
-
Erdogan C, Erdem A, Akinci SB, Dikmenoglu N, Basgül E, et al (2005) The effects of midazolam on erythrocyte deformability and plasma viscosity in rats. Anestezi Dergisi 13: 205-208.
-
Muller R, Musikic P (1987) Hemorheology in surgery--a review. Angiology 38: 581-592.
-
Dormandy JA (1984) Effects of anaesthesia and surgery on the flow properties of blood. Microcirc Endothelium Lymphatics 1: 151-168.
-
Huang ZB (2008) Effects of different inhalation anesthetic agents on erythrocyte immune function and deformability in the elder patients. Journal of the Fourth Military Medical University 22.
-
Bruegger D, Bauer A, Finsterer U, Bernasconi P, Kreimeier U, et al. (2002) Microvascular changes during anesthesia: sevoflurane compared with propofol. Acta Anaesthesiol Scand 46: 481-487.
-
Annecke T, Chappell D, Chen C, Jacob M, Welsch U, et al. (2010) Sevoflurane preserves the endothelial glycocalyx against ischaemia-reperfusion injury. Br J Anaesth 104: 414-421.
-
Hager H, Reddy D, Kurz A (2003) Perfusion Index-a valuable tool to assess changes in peripheral perfusion caused by sevoflurane? Anesthesiology 99: A593.
-
Fijalkowska A, Kowalczyk M (2010) Peripheral blood perfusion during desflurane anaesthesia. Anaesthesiol Intensive Ther 42: 11-14.
-
Desborough JP, Jones PM, Persaud SJ, Landon MJ, Howell SL (1993) Isoflurane inhibits insulin secretion from isolated rat pancreatic islets of Langerhans. Br J Anaesth 71: 873-876.
-
Gingerich R, Wright PH, Paradise RR (1974) Inhibition by halothane of glucose-stimulated insulin secretion in isolated pieces of rat pancreas. Anesthesiology 40: 449-452.
-
Diltoer M, Camu F (1988) Glucose homeostasis and insulin secretion during isoflurane anesthesia in humans. Anesthesiology 68: 880-886.
-
David JS, Tavernier B, Amour J, Vivien B, Coriat P, et al. (2004) Myocardial effects of halothane and sevoflurane in diabetic rats. Anesthesiology 100: 1179-1187.
-
Kadoi Y (2012) Blood glucose control in the perioperative period. Minerva Anestesiol 78: 574-595.
-
Arslan M, Comu FM, Isik B, Unal Y, Cekmen N, et al. (2010) Effects of the general anaesthetic agent, propofol, on erythrocyte deformability. Bratisl Lek Listy 111: 126-128.
-
Arslan M, Comu FM, Isik B, Ozturk L, Kesimci E (2012) Effect of dexmedetomidine on erythrocyte deformability during ischemia-reperfusion injury of liver in diabetic rats. Bratisl Lek Listy 113: 687-691.
-
Ramakrishnan S, Grebe R, Singh M, Schmid-Schönbein H (1999) Influence of local anaesthetics on the aggregation and deformability of erythrocytes. Clin Hemorheol Microcirc 20: 21-26.
-
Unal FA, Erolcay H (2012) Effect of magnesium sulphate infusion on blood rheology during gynecologic oncology surgery. Göztepe Tip Dergisi 27: 174-181.
-
Tavazzi B, Di Pierro D, Amorini AM, Fazzina G, Tuttobene M, et al. (2000) Energy metabolism and lipid peroxidation of human erythrocytes as a function of increased oxidative stress. Eur J Biochem 267: 684-689.
-
Yapislar H, Aydogan S (2012) Effect of carnosine on erythrocyte deformability in diabetic rats. Arch Physiol Biochem 118: 265-272.
-
Brown CD, Ghali HS, Zhao Z, Thomas LL, Friedman EA (2005) Association of reduced red blood cell deformability and diabetic nephropathy. Kidney Int 67: 295-300.
-
Testa I, Mantrini S, Gregorio A, Refe A, Bonfigli AR, et al. (2003) Red blood cell deformability in diabetic retinopahty. Biorheology 32: 389.
-
Allen HG, Allen JC, Boyd LC, Alston-Mills BP, Fenner GP (2006) Determination of membrane lipid differences in insulin resistant diabetes mellitus type 2 in whites and blacks. Nutrition 22: 1096-1102.
-
Yang ZC, Xia K, Wang L, Jia SJ, Li D, et al. (2007) Asymmetric dimethylarginine reduced erythrocyte deformability in streptozotocin-induced diabetic rats. Microvasc Res 73: 131-136.
-
Tsuchiya M, Asada A, Maeda K, Ueda Y, Sato EF, et al. (2001) Propofol versus midazolam regarding their antioxidant activities. Am J Respir Crit Care Med 163: 26-31.
-
Eger EI 2nd1, Johnson BH, Strum DP, Ferrell LD (1987) Studies of the toxicity of I-653, halothane, and isoflurane in enzyme-induced, hypoxic rats. Anesth Analg 66: 1227-1229.
-
de Bruijne AW, van Steveninck J (1977) The influence of anesthetics on glycerol and K+ translocation across the membrane of red blood cells. Biochem Pharmacol 26: 779-781.
-
Yerer MB, Aydogan S, Comu FM, Arslan M, Gunes-Ekinci I, et al. (2006) The red blood cell deformability alterations under desfluran anesthesia in rats. Clin Hemorheol Microcirc 35: 213-216.
- Aydogan S, Yerer MB, Comu FM, Arslan M, Gunes-Ekinci I, et al. (2006) The influence of sevoflurane anesthesia on the rat red blood cell deformability. Clin Hemorheol Microcirc 35: 297-300.
-
Comu FM, Sivgin V, Özköse Z, Arslan M (2014) Comparative effects of sevoflurane and desflurane on erythrocyte deformability in streptozotocin-induced diabetic rats. British Journal of Medicine & Medical Research 4 : 3954-3962.





