International Journal of Anesthetics and Anesthesiology
Fontan Physiology: Anaesthetic Implications for Non-Cardiac Surgery: A Case Report
Harikrishnan Kothandan1*, Lim Michelle Leanne2 and Shital Kumar Sharad Shah1
1Department of Anaesthesiology, Singapore General Hospital & National Heart Centre, Singapore
2Department of Anaesthesiology, Singapore General Hospital, Singapore
*Corresponding author: Harikrishnan Kothandan, Consultant Anaesthesiologist, Department of Anaesthesiology, Singapore General Hospital & National Heart Centre, Singapore, E-mail: harikrishnan.kothandan@sgh.com.sg
Int J Anesthetic Anesthesiol, IJAA-1-020, (Volume 2, Issue 1), Case Report; ISSN: 2377-4630
Received: January 20, 2015 | Accepted: February 04, 2015 | Published: February 06, 2015
Citation: Kothandan H, Leanne LM, Shah SKS (2015) Fontan Physiology: Anaesthetic Implications for Non-Cardiac Surgery: A Case Report. Int J Anesthetic Anesthesiol 2:020. 10.23937/2377-4630/2/1/1020
Copyright: © 2015 Kothandan H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Patients with congenital heart diseases are a growing population and noncardiac surgeries will become an important health care issue. Modification of surgical techniques and medical advances has improved the survival of patients with complex congenital cardiac abnormalities, resulting in more adult patients with fontan physiology presenting for non-cardiac surgery. The older fontan patient is at particular risk of thromboembolism, arrhythmias, progressive ventricular dysfunction, protein losing enteropathy and plastic bronchitis. Understanding the physiology of the fontan circulation is essential for the successful anaesthetic management of these patients. Anaesthesia should be administered only in a centre where the relevant cardiology and intensive care expertise are available. We report a case of fontan physiology in a 24 year old female patient who underwent sternal keloid revision under general anaesthesia.
Keywords
Congenital heart disease, Fontan, General anaesthesia
Introduction
As early as 1699, Chemineau described a heart composed of 2 atria’s but single ventricle [1]. In 1971, Francis Fontan and Eugene Baudet first described a procedure that diverted all systemic venous blood into the pulmonary arteries, without interposition of a ventricle, as a surgical palliation for tricuspid atresia [2]. The advancement of surgical techniques and medical management have resulted in greater than 90% survival rate 10 years after fontan palliation, allowing patients with complex congenital heart lesions to survive into adulthood [3-6]. As a result, patients with fontan palliation are increasingly presenting in the adult perioperative setting for obstetric, laparoscopic, orthopaedic and other noncardiac procedures [7-10] Understanding the fontan physiology circulation is essential for the successful anaesthetic management of these patients. It is recommended that adults with fontan physiology be managed at centres where the relevant cardiology and intensive care unit expertise are available. Anaesthesiologists who care for them must be familiar with the fontan physiology and perioperative issues to optimize outcomes because congenital heart disease is a risk factor for increased mortality for non-cardiac surgery.
Case Report
The hospital ethics committee granted approval for the publication of this case report and waiver of informed consent (CIRB Ref No: 2014/391/D). We report the anaesthetic management of a 24 year old female weighing 44 kg with a history of fontan’s procedure in childhood who underwent sternal keloid revision under general anaesthesia. She was previously seen by the plastic surgeon for sternal keloid with recurrent infection and pus discharge for one year duration, with inadequate response to medical therapy. She was born with Pulmonary Atresia with Intact Ventricular Septum (PAIVS), large Atrial Septal Defect (ASD) and a hypoplastic right ventricle. She underwent a left Blalock-Tausig (BT) shunt procedure as a neonate. At three years of age, a modified fontan’s procedure with BT shunt dissection, anastomosis between superior vena cava to the right pulmonary artery, lateral tunnel using gortex and over sewing of the Main Pulmonary Artery (MPA) was performed. Conversion to extra-cardiac fontan was done at 11 years of age. She was currently on Aspirin 100mg once a day, and on 6 monthly follow-up with her cardiologist. Functionally, she was independent in her activities of daily living, was able to tolerate physical activities up to a maximal effort tolerance of 4 (metabolic equivalents) METS, and did not have any symptoms of cardiovascular compromise.
On examination, she was alert but very anxious, afebrile with a blood pressure of 116/74mmHg and heart rate 95 beats per minute. She had a grade 2 systolic murmur while the rest of the systemic examination was unremarkable. A keloid was present extending from the suprasternal notch to the xiphisternum. Preoperative investigations were unremarkable. Electrocardiogram (ECG) showed sinus rhythm. She was reviewed in the Adult Congenital Heart Disease (ACHD) clinic prior to the operation. Her perioperative cardiac risk was estimated to be moderate, and a 2D-echo was arranged on the morning of the operation day as her last 2D-echo was performed more than 2 years ago. Her 2-D echo showed a large ASD with unrestricted flow, inferior vena cava (IVC) fontan pathway patent with low velocity flow, superior vena cava (SVC) connection to pulmonary artery (PA) patent with low velocity flow, hypoplastic right ventricle with right ventricular systolic pressure of (RVSP) 138mmHg. Her aspirin was discontinued for a week prior to the operation as requested by the surgeon after consultation with the cardiologist. On the day of surgery, the patient was reassessed and infective endocarditis prophylaxis was given with intravenous (IV) ampicillin 2g 1 hour prior to the procedure. She was premedicated with IV midazolam 1.5mg, and hydrated with 300ml of Ringer lactate solution in the induction room. The patient was taken to the operation theatre and standard monitoring was commenced comprising of a 5-lead Electrocardiogram (ECG), non-invasive blood pressure monitoring, pulse oximetry and the patient was preoxygenated for 3 minutes. Induction of anaesthesia was balanced and titrated so as to maintain haemodynamic stability. This was accomplished with the use of fentanyl 50mcg, ketamine 10mg and etomidate 10mg. Laryngoscopy was facilitated with 30mg of rocuronium and airway was safely secured with a size 7.0 endotracheal tube. An arterial line was inserted after induction with a 20g angiocath because the patient was very apprehensive prior to induction. Capnography and temperature monitoring was also established. Transesophageal echocardiography probe was inserted and a complete comprehensive examination was performed with attention to evaluate ventricular and valvular function (Figure1) Cardiac status was monitored throughout the procedure. Anaesthesia was maintained with desflurane 5-6% (MAC of 0.8%) in 50%oxygen: 50% air combination and low dose remifentanil 0.02-0.05 mcg/kg/min. The patient was ventilated with tidal volumes of 5ml/kg with a respiratory rate of 10 to 12 breaths/minute without any application of Positive End Expiratory Pressure (PEEP). The end-tidal carbon dioxide was maintained within the range of 30-35mmHg. Her blood pressure and heart rate was stable throughout the operation, and maintained 20% of baseline values. The operation lasted for 3 hours 15 minutes. Further analgesia given was 1g of paracetamol and 100mcg of fentanyl in divided doses towards the end of the surgery prior to discontinuation of the remifentanil infusion. No further neuromuscular paralysis was required after the intubating dose of rocuronium. IV dexamethasone 4mg and IV ondansetron 4mg were given to prevent Postoperative Nausea and Vomiting (PONV). At the end of the surgery, reversal was given with neostigmine 1.25mg, glycopyrrolate 0.2mg and extubation was performed successfully. A total of 1500ml of Hartmann’s solution was given intra-operatively. Urine output was 500ml throughout the procedure. A filtered drip was used and temperature was maintained using a fluid warmer, warm air blanket and under-body warmer. She was observed in the Post-Anaesthesia Care Unit (PACU) before being transferred to the high dependency unit for 24 hours. Patient controlled analgesia with morphine was prescribed for control of post-operative pain. She was discharged home on day 4 post-surgery.
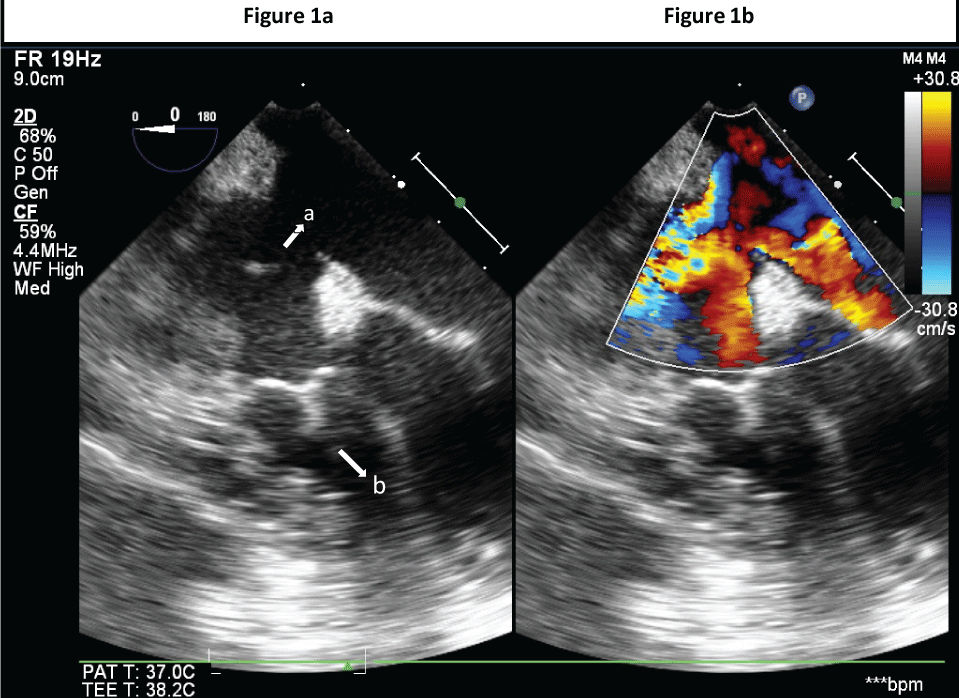
Figure 1a: TEE: Mid-Oesophageal 4-chamber 2D view showing: a) large ASD b) hypoplastic RV.
Figure 1b: TEE: Mid-Oesophageal 4-chamber 2D colour image view showing large ASD.
View Figure 1
Discussion
Before the development of fontan procedure, pulmonary blood flow in patients with single ventricle and pulmonary stenosis was surgically augmented by means of systemic to pulmonary artery shunts. These shunts improved life expectancy remarkably in the short term, but survival past the second decade remained unusual [11]. In 1971 Fontan and Baudet [2] and in 1973 Kreutzer et al. [12] independently described a right atrial to pulmonary artery shunt procedure for tricuspid atresia. It involved diverting systemic venous blood from the right atrium to the pulmonary arteries, thus bypassing the right ventricle. The Fontan procedure is often performed at 18 months - 4 years of age. This can be done in two ways:
a. Extra cardiac where the inferior vena cava is split from the heart and anastomosed using a conduit to the pulmonary artery. An opening is created between the right atrium and the conduit. This reduces the chance for pleural effusion to develop when the pulmonary vascular resistance (PVR) becomes significantly raised.
b. In Lateral tunnel Fontan where a baffle is placed inside the atrium so as to direct blood from the Inferior Vena Cava (IVC) to the lungs.
Patients with fontan physiology present a unique challenge to anaesthesiologists. A thorough understanding of fontan physiology is required for the successful management perioperatively. During the preoperative evaluation, it is important to consider the congenital pathology and degree of palliation completed at the time of assessment. The functional status and comorbidities found in these patients varies significantly, from the young patient who is well compensated to the adult with a failing ventricle. Evaluation of the adult with fontan physiology involves a thorough history and physical examination using a multisystem approach with attention to the unique characteristics of this patient population. A detailed medical history should focus on changes in health status, exercise capacity, hospital admissions, current medication, thorough physical examination, and baseline haematological and biochemical investigations are always necessary, even before minor surgery.
End-organ damage may be present, secondary to low Cardiac Output (CO) and chronically high venous pressure. A 12-lead ECG and echocardiography allow assessment of rhythm, ventricular and valvular function, pulmonary vascular resistance and ventricular end diastolic pressure. About 70% of the patients develop myocardial dysfunction and failure [13], and about 45% of patients develop atrial arrhythmias [14]. Such arrhythmias are extremely resistant to pharmacological therapy, and result in rapid haemodynamic deterioration and heart failure [2]. Protein losing enteropathy [15] presents with oedema, immunodeficiency, ascites, fat malabsorption and has a poor prognosis with a 60% five year survival after diagnosis [14]. Renal dysfunction has a higher prevalence in these patients due to systemic venous hypertension and reduced renal perfusion [14]. Patients with fontan physiology are at increased risk of thromboembolism [16], owing to low flow states, arrhythmias [17, and hypercoagulability [14]. Bridging therapy should be prescribed to patients on warfarin until treatment can be continued. Antiplatelet should be discontinued preoperatively and restarted as soon as possible after the operation. The waiting period depends on the specific agents: for ticlopidine it is 14 days; clopidogrel 7 days; abciximab 48 hours; and eptifibatide 8 hours. High-risk patients such as those with progressive right atrial dilatation, and atrial arrhythmias would more likely require prophylactic anticoagulation [18]. Perioperative antibiotic prophylaxis with broad spectrum cover is required for all procedures likely to produce a bacteraemia. The risk of air or fat emboli occurring during major surgery is relatively high in patients with a fenestration. Some cardiologists recommend closing a fenestration preoperatively for patients undergoing high-risk surgery.
In addition to standard monitoring, invasive arterial and Central Venous Pressure (CVP) monitoring is mandatory in fontan patients undergoing major surgery, particularly where significant volume shifts are expected. Monitoring the trend of the CVP can help in the assessment of vascular volume status, though it reflects only mean pulmonary artery pressure (mPAP) and not ventricular preload. Transoesophageal echocardiography can be used for intraoperative assessment of ventricular preload and function, and to monitor episodes of emboli.
The fontan circulation functions by passive flow of the systemic venous return to the pulmonary vasculature and then to the single ventricle. As such, pulmonary blood flow and cardiac output are the result of the pressure difference between the “upstream” component (consisting of the caval veins and the pulmonary artery) and the “downstream” component [19] (the pulmonary veins / atrium / single ventricle system).The systemic venous pressure of an ideal Fontan circulation is approximately 10-15mmHg and the pulmonary venous atrial (functional left atrium) pressure is approximately 5-10mmHg; this allows a transpulmonary gradient driving pressure of 5-8mmHg [19,20].Any factors affecting this gradient could possibly compromise the single ventricle filling and hence the CO [19,20].
Adequate flow in the upstream component of the circuit depends on a) an unobstructed venous return from IVC and Superior Vena Cava (SVC), b) preload c) patent anastomotic connections between the caval veins and pulmonary arteries, d) low intrathoracic pressure [21]. Downstream component requires: a) low PAP (< 15-20mmHg), b) low PVR, unobstructed pulmonary arterial / pulmonary venous flow. CO is maintained by adequate ventricular filling, normal AV valve, adequate diastolic and systolic function and normal sinus rhythm. Small alterations in ventricular function or the onset of arrhythmias can lead to decreased CO and symptomatic deterioration. Patients with fontan physiology often have baseline venoconstriction to maximally augment preload and therefore anaesthetics that cause venodilatation can be detrimental to CO and lead to cardiac instability [9].
Intraoperatively, the main focus is to maintain optimum CO. These patients have reduced compensatory mechanisms and hence even a slight compromise in CO can be hazardous. Every attempt should be made to ensure adequate preload, good ventricular filling and contractility while avoiding an increase in afterload. Since the blood flow from the systemic veins to the pulmonary circulation is passive, any increase in PVR can compromise ventricular filling and CO. Consequently, it is crucial to avoid any increase in PVR that might be precipitated by hypoxia, hypercarbia, acidosis, hypothermia, inadequate analgesia or anaesthesia, use of vasoactive drugs, excessive mean airway pressure, and compression of the lung by pleural effusion or PEEP.
Anaesthesiologists should choose induction agents with the least effect on myocardial contractility, cardiac output and pulmonary blood flow. Propofol and midazolam may cause profound decreases in venous return and myocardial depression. Etomidate is a good choice of induction agent for the preservation of myocardial contractility, PVR, and vascular tone [22,23]. Effects of ketamine in the adult, such as increased PVR and myocardial oxygen consumption, may limit its use in adult fontan patients [24]. It is advisable to avoid muscle relaxants with histamine releasing properties, which can result in hypotension and tachycardia [25]. Succinylcholine and rocuronium are recommended for rapid sequence induction because of their minimal vagolytic and minimal histamine release properties [25]. Cisatracurium and vecuronium are also useful for their stable haemodynamic properties. High concentrations of volatile agents should be avoided as they increase the chances of arrhythmias [14] and myocardial depression. Thus, a low concentration of an inhalational agent in combination with an infusion of a short acting opioid such as remifentanil which could provide a cardio-stable anaesthetic is recommended intraoperatively [14]. Inadequate pulmonary blood flow may occur either secondary to hypovolemia or increased pulmonary vascular resistance (PVR).For short procedures, spontaneous ventilation is better as long as severe hypercarbia is avoided [14]. Negative intrathoracic pressure increases the antegrade flow from the SVC, IVC, and hepatic venous circulation into the pulmonary arterial tree [26,27]. Conversely, positive intrathoracic pressure may limit antegrade flow or even reverse flow in these systemic venous beds [26]. Doppler and magnetic resonance imaging (MRI) studies have shown increased pulmonary blood flow with inspiration during spontaneous and negative-pressure ventilation [26,28]. For major surgery, or when prolonged anaesthesia is required, controlled ventilation with a ventilator strategy of low mean airway pressure, moderate alkalosis (PH=7.45, pCO2=35mmHg),tidal volume of 5-6 ml/kg, low respiratory rate, short inspiratory times, low PEEP usually allow adequate pulmonary blood flow with minimal haemodynamic effects. Regional anaesthesia can also be employed depending on the nature of surgery. Epidural anaesthesia has been successfully employed in such patients [29].For laparoscopic procedures, maintaining intra-abdominal pressures below 10mmHg decreases haemodynamic effects in this patient population. The presence of a fenestration increases the risk for paradoxical carbon dioxide or venous air emboli [9,30]. Most young patients with fontan circulation tolerate laparoscopic procedures very well as long as intra-abdominal pressure is kept less than 10mmHg, minimal surgical time, and adequate ventilation and intravascular volumes are maintained [9]. Lumbar epidural analgesia has been used successfully in fontan patients, particularly for vaginal or caesarean delivery [31,32]. Subarachnoid block is not recommended because of sympathetectomy, hypotension and bradycardia which are poorly tolerated in this patient population. Postoperatively, fontan patients should be admitted to a monitored unit. Maintaining adequate analgesia, ensuring adequate ventilation, fluid management and providing supplemental oxygen are essential.
Conclusion
The advancement of surgical techniques and medical management has improved the survival of patients with complex congenital cardiac abnormalities, resulting in more adult patients with fontan physiology presenting for non-cardiac surgery. A good understanding of fontan physiology and its complications is required for successful anaesthetic management. A thorough preoperative evaluation and planning, maintenance of normovolemia, avoidance of myocardial depression, avoidance of factors which will increase PVR, infective endocarditis prophylaxis, thromboembolism prophylaxis and good postoperative care are essential for successful outcome in this group of patients.
References
-
Peacock TB (1958) Malformations of the heart. In: Peacock TB, ed. On Malformations of the Human Heart: With Original Cases. London, UK: John Churchill: 10-102.
-
Fontan F, Baudet E (1971) Surgical repair of tricuspid atresia. Thorax 26: 240-248.
-
Khairy P, Fernandes SM, Mayer JE Jr, Triedman JK, Walsh EP, et al. (2008) Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 117: 85-92.
-
Brown JW, Ruzmetov M, Deschner BW, Rodefeld MD, Turrentine MW (2010) Lateral tunnel Fontan in the current era: is it still a good option? Ann Thorac Surg 89: 556-562.
-
d'Udekem Y, Cheung MM, Setyapranata S, Iyengar AJ, Kelly P, et al. (2009) How good is a good Fontan? Quality of life and exercise capacity of Fontans without arrhythmias. Ann Thorac Surg 88: 1961-1969.
-
Driscoll DJ (2007) Long-term results of the Fontan operation. Pediatr Cardiol 28: 438-442.
-
Hedequist DJ, Emans JB, Hall JE (2006) Operative treatment of scoliosis in patients with a Fontan circulation. Spine (Phila Pa 1976) 31: 202-205.
-
Cannesson M, Earing MG, Collange V, Kersten JR (2009) Anesthesia for noncardiac surgery in adults with congenital heart disease. Anesthesiology 111: 432-440.
-
McClain CD, McGowan FX, Kovatsis PG (2006) Laparoscopic surgery in a patient with Fontan physiology. Anesth Analg 103: 856-858.
-
Nitsche JF, Phillips SD, Rose CH, Brost BC, Watson WJ (2009) Pregnancy and delivery in patients with fontan circulation: a case report and review of obstetric management. Obstet Gynecol Surv 64: 607-614.
-
Dick M, Fyler DC, Nadas AS (1975) Tricuspid atresia: clinical course in 101 patients. Am J Cardiol 36: 327-337.
-
Kreutzer G, Galíndez E, Bono H, De Palma C, Laura JP (1973) An operation for the correction of tricuspid atresia. J Thorac Cardiovasc Surg 66: 613-621.
-
Piran S, Veldtman G, Siu S, Webb GD, Liu PP (2002) Heart failure and ventricular dysfunction in patients with single or systemic right ventricles. Circulation 105: 1189-1194.
-
Sandeep Nayak, Booker PD (2008) The Fontan Circulation. Continuing education in anaesthesia, critical care and pain 8.
-
Mertens L, Haler DJ, Sauer U, Somerville J, Gewillig M (1998) Protein-losing enteropathy after the Fontan operation: Aninternational multicenter study. J Thorac Cardiovasc Surg 115: 1063-1073.
-
Coon PD, Rychik J, Novello RT, Ro PS, Gaynor JW, et al. (2001) Thrombus formation after the Fontan operation. Ann Thorac Surg 71: 1990-1994.
-
Weipert J, Noebauer C, Schreiber C, Kostolny M, Zrenner B, et al. (2004) Occurrence and management of atrial arrhythmia after long-term Fontan circulation. J Thorac Cardiovasc Surg 127: 457-464.
-
Walker HA, Gatzoulis MA (2005) Prophylactic anticoagulation following the Fontan operation. Heart 91: 854-856.
-
MCGOWAN F (2005) Perioperative issues in patients with congenital heart disease. International Anesthesia Research Society 2005 Review course lectures: 53-61.
-
MOTOYAMA KE, DAVIS PJ (1996) Smith’s Anesthesia for infants and children (6th Edn): 445-539.
-
ZIPES, LIBBY, BORROW (2005) Braunwald’s Heart disease (27th Edn): 1489-1551.
-
Sprung J, Ogletree-Hughes ML, Moravec CS (2000) The effects of etomidate on the contractility of failing and nonfailing human heart muscle. Anesth Analg 91: 68-75.
-
Andropoulos DB, Stayer SA, Skjonsby BS, East DL, McKenzie ED, et al. (2002) Anesthetic and perioperative outcome of teenagers and adults with congenital heart disease. J Cardiothorac Vasc Anesth 16: 731-736.
-
Lovell AT (2004) Anaesthetic implications of grown-up congenital heart disease. Br J Anaesth 93: 129-139.
-
Naguib M, Samarkandi AH, Bakhamees HS, Magboul MA, el-Bakry AK (1995) Histamine-release haemodynamic changes produced by rocuronium, vecuronium, mivacurium, atracurium and tubocurarine. Br J Anaesth 75: 588-592.
-
Redington AN, Penny D, Shinebourne EA (1991) Pulmonary blood flow after total cavopulmonary shunt. Br Heart J 65: 213-217.
-
Shekerdemian LS, Bush A, Shore DF, Lincoln C, Redington AN (1997) Cardiopulmonary interactions after Fontan operations: augmentation of cardiac output using negative pressure ventilation. Circulation 96: 3934-3942.
-
28. tagging in patients having the Fontan operation. J Thorac Cardiovasc Surg 114: 1032-1041.
-
Arai M, Kanai A, Matsuzaki S, Takenaka T, Kato S (1997) [Thoracic epidural anesthesia for cholecystectomy in a patient after Fontan procedure]. Masui 46: 271-275.
-
Leyvi G, Wasnick JD (2010) Single-ventricle patient: pathophysiology and anesthetic management. J Cardiothorac Vasc Anesth 24: 121-130.
-
Carp H, Jayaram A, Vadhera R, Nichols M, Morton M (1994) Epidural anesthesia for cesarean delivery and vaginal birth after maternal Fontan repair: report of two cases. Anesth Analg 78: 1190-1192.
-
Ioscovich A, Briskin A, Fadeev A, Grisaru-Granovsky S, Halpern S (2006) Emergency cesarean section in a patient with Fontan circulation using an indwelling epidural catheter. J Clin Anesth 18: 631-634.





