International Archives of Urology and Complications
Repeated Intradetrusor Botulinum Toxin Type A Injections are Still Effective for Patients with Neurogenic Detrusor Overactivity Secondary to Spinal Cord Injury in China
Hui Chen1,3*, Xing Hua Yang2, Jing Wen Zeng3, Ma Ping Huang2, Qiu Ling Liu2, Jie Bing Huang2, Tian Hai Huang4, Ke Ji Xi4 and Chong He Jiang3
1Department of Urology, Guangdong Provincial Work Injury Rehabilitation Hospital and Jinan University, China
2Department of Spinal Cord Injury Recovery Center, Guangdong Provincial Work Injury Rehabilitation Hospital, China
3Departments of Urology, Qingyan City People's Hospital, Jinan University, China
4Department of Urology, Guangzhou First Municipal People's Hospital, China
*Corresponding author: Chen H, M.D, Department of Urology, Guangdong Provincial Work Injury Rehabilitation Hospital and Jinan University, Guangzhou 510440, China, E-mail: doc.chenhui@163.com
Int Arch Urol Complic, IAUC-1-005, (Volume 1, Issue 1), Research Article; ISSN: 2469-5742
Received: April 09, 2015 | Accepted: May 16, 2015 | Published: May 20, 2015
Citation: Chen H, Yang XH, Zeng JW, Huang MP, Liu QL, et al. (2015) Repeated Intradetrusor Botulinum Toxin Type A Injections are Still Effective for Patients with Neurogenic Detrusor Overactivity Secondary to Spinal Cord Injury in China. Int Arch Urol Complic 1:005. 10.23937/2469-5742/1510005
Copyright: © 2015 Chen H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: To assess effective outcomes following repeated treatment with intradetrusor botulinum toxin type an in patients with neurogenic detrusor overactivity (NDO).
Methods: Patients with NDO secondary to spinal cord injury (SCI) were enrolled. Botulinum toxin type A 200 U detrusor injections by a rigid cystoscope were repeated. Primary outcomes were urodynamic variables including maximum detrusor pressure during first involuntary detrusor contraction (PdetmaxIDC) filling cystometry, detrusor compliance (DC). Secondary outcomes were improvement of the patient's QoL measured by Incontinence-Specific Quality-of-Life Instrument (I-QoL), the validated short forms of Urogenital Distress Inventory (UDI-6) and the Incontinence Impact Questionnaire (IIQ-7). Related adverse events were recorded.
Results: From 2012 to 2014, 159 injections were performed in 52 patients (44 male, 8 female). The mean age was 36.67 years. The maximum number of repeated injections was five. BC increased from (4.03-7.45) to (6.96-10.86) ml/cm H2O, Pdetmax in bladder storage decreased from (42.80-79.52) to (26.40-43.33) cm H2O, respectively. The I-QoL, UDI-6 and IIQ-7 showed a consistent improvement after repeated injections.
Conclusions: Repeated intradetrusor botulinum toxin type A injections remain improve Quality of Life in patients with NDO secondary to spinal cord injury.
Keywords
Botulinum toxin type A, Detrusor overactivity, Neurogenic, Spinal cord injury, Quality of Life
Introduction
After spinal cord injury (SCI), the impaired bladder function can incur the risk of developing neurogenic lower urinary tract dysfunction (NLUTD) [1]. As one of the most common types of NLUTD, neurogenic detrusor overactivity (NDO) is characterized by spontaneous or provoked involuntary detrusor contractions during storage phase in urodynamic investigation [2,3]. NDO can cause urinary incontinence both day and night which dramatically impacted SCI patients' quality of life, such as avoidance and limiting behavior, psychosocial impact, and social embarrassment [4]. Therefore, improvement of the patient's Quality of Life (QoL) was one of the primary aims for treatment of NDO [5]. Botulinum toxin type A has been shown clinical efficacy for patients with NDO who experience side-effects or an inadequate response to antimuscarinic drugs [6,7].
This study describes the health-related quality of life (HRQL) of repeated detrusor injections of Botulinum toxin type A on patients with NDO, using the validated short forms of Urogenital Distress Inventory (UDI-6), the Incontinence Impact Questionnaire (IIQ-7), Incontinence-Specific Quality-of-Life Instrument (I- QoL).
Method
The study was performed at the urology and spinal cord injury recovery department in Guangdong provincial work injury rehabilitation hospital. The inclusion criteria [6] are SCI patients with: (1) Age >18 years ; (2) detrusor pressure >40cm H2O; (3) detrusor compliance < 20ml/cm H2O; (4) Patients regularly performed clean intermittent catheterization (CIC); (5) Patients who have an inadequate response to or are intolerant of an anticholinergic medication. Exclusion criteria included patients with: (1) Acute urinary tract infections; (2) Pregnancy or nursing mothers; (3) Bladder stone, bladder cancer, bladder tuberculosis; (4) Gastric retention and myasthenia gravis; (5) Bladder outlet obstruction; (6) Patients or caregivers who were unable to perform CIC. Previously, the protocol was approved by hospital ethics committee. All patients provided their written consent before undergoing treatment. Initial bladder diaries, cystometry, UDI-6, IIQ-7, and I-QoL were undertaken during the screening visit (approximately 2 weeks before injection).Urodynamic demonstration using a filling rate of 10-30mL/min of phasic or terminal detrusor overactivity was a requirement before treatment. Special attention was given to maximum detrusor pressure (Pdetmax), bladder compliance (BC). Changes in HRQL were assessed using the validated short forms of I-QoL ,IIQ-7, and UDI-6 .The I-QOL [8] is a validated, 22-item questionnaire assessing the impact of urinary incontinence on HRQOL. Responses are reported as a total scale score and three subscale scores: avoidance and limiting behavior, psychosocial impact, and social embarrassment. Lower scores indicate worse incontinence-related HRQOL. Total scores, as well as each of the subscale scores, range from 0 (worst) to 100 (best). The IIQ-7 [9] determines how much urine leakage affects activities, relationships and feelings. It includes seven questions with a four-point response scale (0, not at all; 1, slightly; 2, moderately; 3, greatly). The UDI-6 [9] evaluates the type of urinary symptoms and their degree of bothersomeness. It includes six questions with a four-point response scale (0, not at all; 1, somewhat; 2, moderately; 3, quite a bit).
After baseline evaluation, 200 U botulinum toxin type A (Botox®, Allergan Inc., Irvine, CA, USA) injection was performed under as following [6]: Using a rigid cystoscope, insert needle approximately 2mm into the detrusor (avoiding the trigone); space injections approximately 1cm apart; administer 30 injections of 1ml each (30ml total), evenly distributed across the detrusor; for the final injection, inject approximately 1ml of sterile normal saline so the full dose is delivered. The procedure and the dose were the same for the first and the subsequent injections in each patient. When patients reported a return of symptoms, DO on urodynamics was the indication for re-injection. Repeated injection was offered as soon as the symptoms and urodynamic outcome follow up study showed compliance and pressure were returning to levels as before the treatment. Patients were reassessed at 12 weeks after the first injection by cystometry and HRQL questionnaires.
Statistical analysis was performed using the SPSS 13.0 soft-ware package (SPSS, Inc., Chicago, IL). Statistical relationships between pre- and postoperative outcome parameters were sought by the Student's t-test for quantitative variables. Statistical significance was considered at P value < 0.05.
Result
From 2011 to 2014, 159 injections were performed in 52 patients (44 male, 8 female). The mean age was 36.67 years. The maximum number of repeated injections was five. The median duration of spinal cord injury was 8.41 month. All were performing regular CIC (Table 1).
![]()
Table 1: Patient disposition and demographics
View Table 1
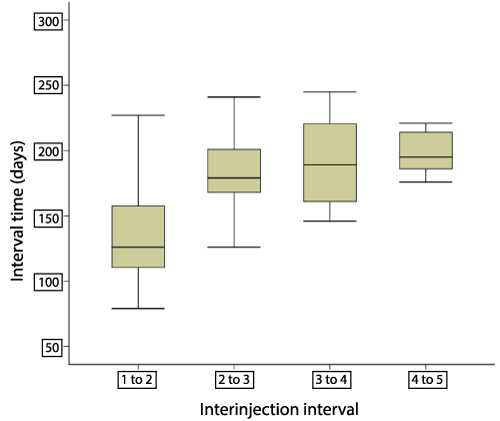
The introjections interval for all patients is shown in Figure 1. The mean interinjection interval was greater after the first treatment which levels out at an average of 133.09 days, but was the same thereafter.
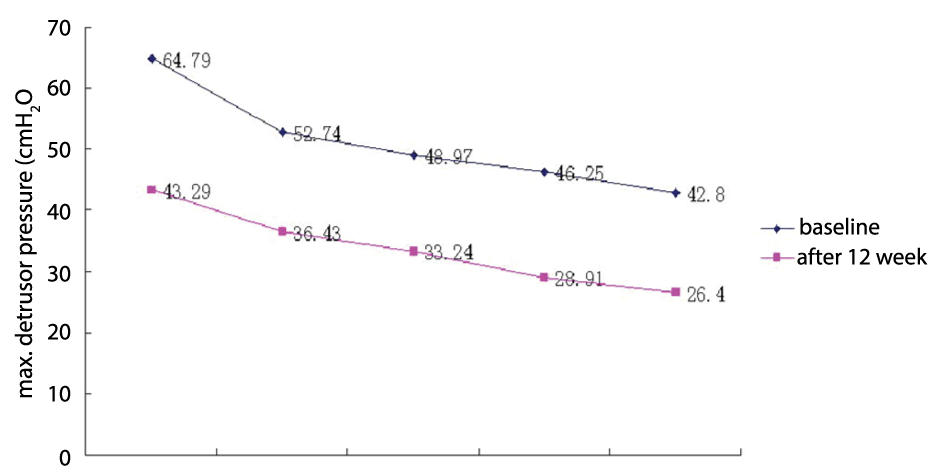
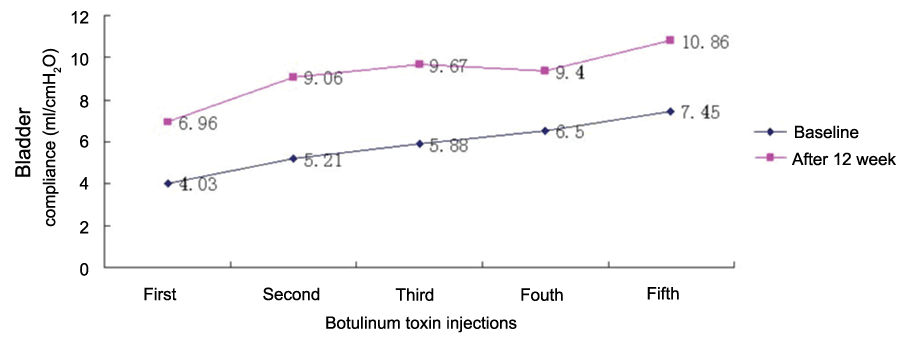
Three months after the first injection improvement of the urodynamic measures were listed in Figure 2,3. BC increased from (4.03-7.45) to (6.96-10.86) ml/cm H2O, respectively. Pdetmax in bladder storage decreased from (42.80-79.52) to (26.40-43.33) cm H2O.There were no reported side-effects or complications due to injection of botulinum toxin type A.
The results of I-QOL scores, IIQ-7 scores, and UDI-6 scores are summarized in Table 2. At baseline for each injection, median total I-QOL scores (34.31,38.83,39.14,41.00,34.66, respectively), IIQ-7 scores (14.02,12.38,11.53,11.88,11.60, respectively), UDI-6 scores (13.00,11.74, 11.00,11.50,11.00, respectively) were similar, indicating a relatively poor health-related quality of life relating to incontinence. At week 12 after each injection, all the median total I-QOL scores, IIQ-7 scores, UDI-6 scores improved: median total I-QOL scores ranging from 59.35 to 70.15; IIQ-7 scores ranging from 8.60 to 11.79; UDI-6scores ranging from 5.94 to 8.15.
![]()
Table 2: Comparison of the health related quality of life at baseline and after each intradetrusor injections of botulinum toxin in patients with NDO
View Table 2
In this study, no related adverse events were recorded.
Discussion
Neurogenic detrusor over activity (NDO) commonly occurs in patients with spinal cord injury (SCI). Botulinum toxin type A has been known to reduce signs and symptoms of neurogenic incontinence, and significantly improve health-related quality of life. However, the efficacy of a single botulinum toxin type A injection decreases with time and after a mean time of 3-10 [10-12] months repeated injection is necessary to maintain the clinical effects. Therefore, is the clinical improvement of continence still constant after repeated injections?
In our study, repeated intradetrusor botulinum toxin type A injections still achieved a low pressure bladder to preserve of the upper tract function for patients with NDO. These findings were supported by improvements in detrusor pressure and bladder compliance. A significant decrease in Pdetmax compared with baseline measurement was observed. Especially from the second to fifth injection, Pdetmax reduced to be below 40cm H2O at 12 weeks follow up. Wang SC [13] reported that for patients with bladder pressures above 40cm H2O, 81% were potentially dangerous to renal function.
The urodynamic reported changes with botulinum toxin type A monotherapy were translated into significant improvements in health-related quality of life throughout the repeated treatment period. These results imply that the effect of a botulinum toxin type A intradetrusor injection continued during the study period, even without concomitant use of anticholinergics. The mean injection interval was greater after the first treatment, but was the same thereafter. Compared to pre-treatment levels, the HRQL scores (I-QOL scores, IIQ-7 scores, and UDI-6 scores) show a consistent pattern of improvement at week 12 after each retreatments (Table 2). Improvements of HRQL scores show that patients less worried about not being able to get to the toilet on time. After treatment with botulinum toxin type A, patients were also less likely to feel depressed, frustrated, or helpless and less concerned about wetting them-selves or being embarrassed or humiliated. The results obtained in our study are similar to the previously report. Giannantoni [14] reported the sustained impact of botulinum toxin type A on HRQL in NDO patients with SCI who had been followed for 6 years, using I-QOL scores and reported findings similar to those of the present study. Kalsi [15] used a combined questionnaire based on the IIQ-7 scores, and UDI-6 scores and found that mean scores improved significantly at 16 week after treatment .The findings of the present study also show a consistent improvement in HRQL even after five treatments (mean of 3.06 injections).Therefore, the effect of repeated injections of Botulinum toxin type A on HRQL in NDO appears to be consistent.
Conclusions
Our results demonstrate that repeated intradetrusor botulinum toxin type A is an effective and well tolerated treatment for improving the health-related quality of life for SCI with NDO.
Acknowledgments
This study was supported by Medical Scientific Research Foundation of Guangdong Province, China (grant number A2013477).
References
-
Yezierski RP (2009) Spinal cord injury pain: spinal and supraspinal mechanisms. J Rehabil Res Dev 46: 95-107.
-
Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, et al. (2002) The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21: 167-178.
-
Schäfer W, Abrams P, Liao L, Mattiasson A, Pesce F, et al. (2002) Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn 21: 261-274.
-
Denys P, Corcos J, Everaert K, Chartier-Kastler E, Fowler C, et al. (2006) Improving the global management of the neurogenic bladder patient: part I. The complexity of patients. Curr Med Res Opin 22: 359-365.
-
Stöhrer M, Blok B, Castro-Diaz D, Chartier-Kastler E, Del Popolo G, et al. (2009) EAU guidelines on neurogenic lower urinary tract dysfunction. Eur Urol 56: 81-88.
-
Schurch B, de Sèze M, Denys P, Chartier-Kastler E, Haab F, et al. (2005) Botulinum toxin type A is a safe and effective treatment for neurogenic urinary incontinence: results of a single treatment, randomized, placebo controlled 6-month study. J Urol 174: 196-200.
-
Karsenty G, Reitz A, Lindemann G, Boy S, Schurch B (2006) Persistence of therapeutic effect after repeated injections of botulinum toxin type A to treat incontinence due to neurogenic detrusor overactivity. Urology 68: 1193-1197.
-
Wagner TH, Patrick DL, Bavendam TG, Martin ML, Buesching DP (1996) Quality of life of persons with urinary incontinence: development of a new measure. Urology 47: 67-71.
-
Uebersax JS, Wyman JF, Shumaker SA, McClish DK, Fantl JA (1995) Short forms to assess life quality and symptom distress for urinary incontinence in women: the Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Continence Program for Women Research Group. Neurourol Urodyn 14: 131-139.
-
Patel AK, Patterson JM, Chapple CR (2006) Botulinum toxin injections for neurogenic and idiopathic detrusor overactivity: A critical analysis of results. Eur Urol 50: 684-709.
-
Karsenty G, Denys P, Amarenco G, De Seze M, Game X, et al. (2008) Botulinum toxin A (Botox1) intradetrusor injections in adults with neurogenic detrusor overactivity/ neurogenic overactive bladder: a systematic literature review. Eur Urol 53: 275-287.
-
Apostolidis A, Dasgupta P, Denys P, Elneil S, Fowler CJ, et al. (2009) Recommendations on the use of botulinum toxin in the treatment of lower urinary tract disorders and pelvic floor dysfunctions: a European consensus report. Eur Urol 55: 100-120.
-
Wang SC, McGuire EJ, Bloom DA (1988) A bladder pressure management system for myelodysplasia--clinical outcome. J Urol 140: 1499-1502.
-
Giannantoni A, Mearini E, Del Zingaro M, Porena M (2009) Six-year follow-up of botulinum toxin A intradetrusorial injections in patients with refractory neurogenic detrusor overactivity: clinical and urodynamic results. European urology 55: 705-712.
-
Kalsi V, Apostolidis A, Popat R, Gonzales G, Fowler CJ, et al. (2006) Quality of life changes in patients with neurogenic versus idiopathic detrusor overactivity after intradetrusor injections of botulinum neurotoxin type A and correlations with lower urinary tract symptoms and urodynamic changes. Eur Urol 49: 528-535.