Inflammation has an important role in chronic obstructive pulmonary diseases (COPD) and statins are believed to have anti-inflammatory effects beyond low-density lipoprotein cholesterol reduction. This study aimed to assess the effect of statin on the function of the right ventricle and inflammatory markers in COPD patients.
A total of 76 clinically stable COPD patients were included in this randomized, double-blind study. Patients were randomly assigned to receive atorvastatin (40 mg/d) or a placebo over a period of three months. All the patients underwent spirometry, echocardiography, and high-sensitivity C-reactive protein (hsCRP) measurement at baseline and at the end of the study. Right ventricular (RV) systolic function was assessed by echocardiography using the systolic velocity of tricuspid annulus (Sm) and longitudinal strain in the base and mid segments of RV free wall (SRB, SRM). Pulmonary artery pressure (PAP) was estimated by the tricuspid regurgitation gradient.
Fifty-five patients (31 in atorvastatin and 24 in the placebo group) completed the course of intervention. Atorvastatin resulted in improvement in RVSB (p = 0.03) and decrease in hsCRP (p = 0.03) compared to placebo group while RV SRM was significantly improved in atorvastatin group (p = 0.01) but this change was not significant between groups (p = 0.44).
Statins could improve the prognosis in COPD patients by improving right ventricular hemodynamic.
Chronic obstructive pulmonary disease, C-reactive protein, Right ventricular function, Pulmonary artery pressure
Chronic obstructive pulmonary disease (COPD) is a preventable, progressive and incurable inflammatory disease affecting many organs and causing irreversible airflow limitation [1,2]. Chronic obstructive pulmonary disease is predicted to become the fourth leading cause of death in developed countries till 2030 [3]. Activation of inflammatory cells including neutrophils and high levels of several circulating systemic inflammatory markers including C-reactive protein (CRP) is considered the etiology [4]. Several studies suggest a close inverse relationship between CRP and lung function in COPD [5,6]. Treatment of COPD is extremely difficult because of its different side effects and multi organ involvement [1,2].
One of the worst side effects of COPD is pulmonary hypertension, which increases mortality and morbidity [7,8]. COPD can cause pulmonary hypertension due to vasoconstriction, chronic hypoxia, and decreasing vasodilators like nitric oxide. Increased pulmonary vascular resistance can lead to right ventricular (RV) failure and death [7,8]. The incidence of heart failure (HF) is 4.5 times higher in COPD patients and presently there is no way to prevent pulmonary hypertension in COPD patients [9].
The 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA), also known as statins, reductase inhibitors (statins) have numerous effects on vascular wall function. Statins have been found to have various effects including anti-inflammatory, immunosuppressive, and cardiovascular effects. Statins may also improve the endothelial function indirectly by lowering cholesterol levels and peripheral vascular perfusion [10,11].
Majority of evidence endorsing the potential therapeutic benefit of the statin drug class comes from animal studies [12-14]. Few studies indicate that statins have an independent protective effect in decreasing all-cause mortality and prevention of exacerbation in COPD patients, prevention of cancer and lung function decline [15,16]. Statins were found to be capable of decreasing the production of inflammatory mediators, which is regarded as potential treatment for the pathophysiology of COPD [17,18]. Previous studies found that statins might be beneficial in prevention or reduction of pulmonary hypertension, right ventricular failure, and coronary endothelial dysfunction in patients with COPD regardless of blood cholesterol level or presence of ischemic heart disease [19-21]. There is scarcity of human studies that assess the effectiveness of statins in COPD patients. The aim of this study was to study the effects of atorvastatin on cardiac function and inflammatory markers in COPD patients.
This single-blind randomized controlled trial was performed on COPD patients who referred to the pulmonary clinic in the Mashhad University of Medical Sciences from 2013 to 2015. The trial was approved by the institutional ethical review board at the Mashhad University of Medical Sciences and registered at the Iranian Registry of Clinical Trials (registration number: IRCT201108177356N1). Written informed consent was obtained from all patients prior to participation in the study.
Patients were included based on the criteria proposed by the American Thoracic Society standards [22]. Exclusion criteria were diagnosis of concomitant coronary artery disease, atrial fibrillation, positive drug history for administration of any lipid lowering medication including atorvastatin, pregnancy, and pulmonary artery hypertension (PAH) due to other etiologies as well as active liver disease, alanine transaminase (ALT) or aspartate transaminase (AST) greater than threefold of the upper normal limit. Subjects were randomly assigned into two groups: the atorvastatin and the placebo group. Randomization was performed using a random block permutation method according to a computer generated randomization list, with randomly varied block lengths [23]. The random allocation sequence was performed by a biostatistician. Details of the series were known to the investigators. The patients and research analyzer were blinded until the study was completed. The flowchart of the study is presented in Figure 1.
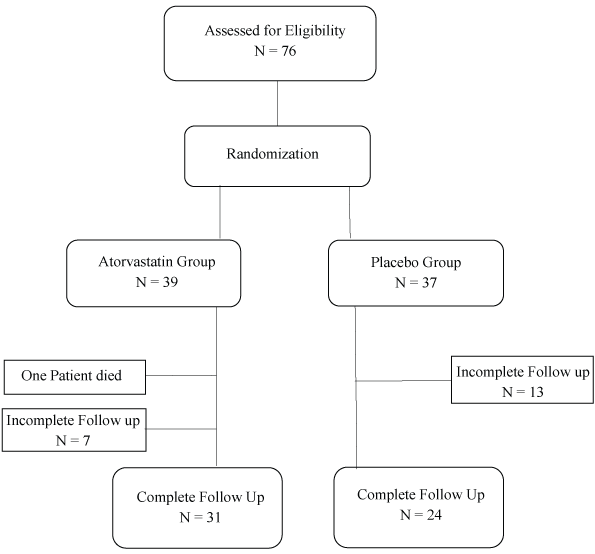 Figure 1: Schematic overview of the trial.
View Figure 1
Figure 1: Schematic overview of the trial.
View Figure 1
After randomization, the intervention group received atorvastatin 40 mg once a day and the control group received a placebo. Primary outcomes were pulmonary artery pressure (PAP) and RV function and size, at three months after medication consumption. The secondary outcome was hsCRP level.
Subjects in both groups were evaluated at baseline and three months after the initiation of the intervention. Assessments included measuring the serum level of hsCRP and transthoracic echocardiography. Echocardiography was performed for both groups using a Vivid 7 Dimension ultrasound scanner (GE Vingmed, Horten, Norway) with a 4-MHz transducer S' wave of the tricuspid annulus, and the longitudinal systolic strain in the base and mid segment of the RV free wall were used to assess RV systolic function (Figure 2). In addition, the E wave velocity of the tricuspid inflow, E' peak velocity in the lateral annulus of the tricuspid valve, and E/E' were measured as echocardiographic parameters to evaluate RV diastolic function. The PAP was calculated through the tricuspid regurgitation peak pressure gradient. Global left ventricular longitudinal strain (GLS) was assessed with the automated functional imaging (AFI) method using three apical views (apical long-axis, 4- and 2- chamber views) in the grayscale. The RV and right atrium (RA) size and left ventricular ejection fraction (LVEF) were also measured.
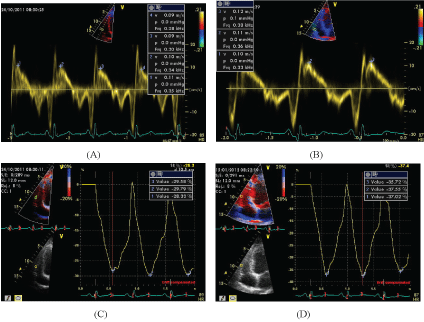 Figure 2: Echocardiographic Assessment of right ventricle function using Sm (A,B) and Longitudinal Strain in RV free wall (C,D) which was done before (A,C) and three months after Atorvastatin Therapy (B,D). Note the improvement in Sm and RV strain in this case.
View Figure 2
Figure 2: Echocardiographic Assessment of right ventricle function using Sm (A,B) and Longitudinal Strain in RV free wall (C,D) which was done before (A,C) and three months after Atorvastatin Therapy (B,D). Note the improvement in Sm and RV strain in this case.
View Figure 2
The serum level of FBS, cholesterol, triglyceride, HDL, LDL, AST, ALT, and CPK was measured in all participants. Spirometry was also performed to measure FEV1, FVC, and FEV1/FVC.
Data were analyzed using the statistical package for social sciences (SPSS) software version 22 (IBM Inc, Chicago, Il, USA). Normality of continuous variables was assessed using the Shapiro-Wilk test. Normally distributed variables were presented as mean ± standard deviation (SD) while non-normally distributed variables were presented as median and interquartile range (IQR). Categorical variables were presented as frequency and percentage. The student t-test and Mann-Whitney tests were used for comparison of parametric and non-parametric variables between study groups. The chi-square test was used to compare the distribution pattern of categorical between groups. Response to atorvastatin versus placebo was assessed by analysis of covariance (ANCOVA) considering age, baseline echocardiographic data and baseline FEV1/FVC as covariates. Since no significant difference was observed between groups at baseline, no adjustment was made for other study variables in the repeated measures ANOVA. The P value less than 0.05 was considered statistically significant.
Seventy-six eligible subjects were included initially and were randomized into atorvastatin (n = 39) and placebo (n = 37) groups. During the study process one subject from the atorvastatin group died due to the deterioration of the disease and 20 subjects (7 in atorvastatin and 13 in placebo group) were excluded due to loss of follow up resulting in the final 55 subjects (31 in atorvastatin group and 24 in the placebo group) (Figure 1).
Mean age of the subjects (38, 69.1% male and 17, 30.9% female) was 63.98 ± 10.56 years. Mean age of subjects in the atorvastatin group was 65.35 ± 10.07 years while the mean age of the subjects in the placebo group was 62.21 ± 11.11 years. The characteristics of study subjects at baseline are described in Table 1. There was no significant difference between groups at baseline (Table 1).
Table 1: Baseline characteristics of the patients (demographic data, biochemistry analysis, Pulmonary function tests and echocardiographic parameters). View Table 1
There was significant correlation between PAP and Sm (r: 0.49; p = 0.001) and longitudinal strain (r = 0.41; p = 0.01).
There was a significant change in RV SRB in atorvastatin group from baseline (p < 0.001) resulting in a significant difference in RV SRB at the end of the study between atorvastatin and placebo group (p = 0.01) (Table 2). RV SRM and PAP changed significantly in the atorvastatin group from baseline (p < 0.001 and p = 0.001 respectively) Atorvastatin administration resulted in a significant reduction in hsCRP at the end of the study compared to the placebo group (p = 0.04) (Table 2).
Table 2: Changes in echocardiographic parameters during the intervention. View Table 2
No significant difference was observed in spirometry parameters between atorvastatin and placebo groups neither at baseline nor at the end of the intervention (Table 3).
Table 3: Changes in spirometry parameters during the intervention. View Table 3
The role of statins in decreasing PAP in COPD patients is still controversial and data from studies done on the effects of statins in preventing pulmonary hypertension in COPD patients are scarce. There is a high interest in the use of statins for the treatment of pulmonary arterial hypertension (PAH) and so recently, statins are being evaluated for the treatment of PAH [19,21,24]. Although the potential role of statins in treating COPD is controversial [25,26], current evidence shows that statins have an effective influence on outcomes in patients with COPD [19,21,24]. Despite these hopeful results, in all researches, up to this time, there are many limitations that should be considered. The effective dose, interval and duration of administration, and effect of various statins need to be accurately determined in the target population. At present there is insufficient evidence, although new, to vindicate a clinical indication for statin therapy in patients with COPD regardless of its function in protecting the cardiovascular system.
This study found that statin administration can significantly decrease PAP. In another study on 16 patients with pulmonary hypertension administration of simvastatin was found to improve cardiac output [27]. The therapeutic value of 80 mg/d simvastatin in patients with pulmonary arterial hypertension (PAH) was assessed in another study for 12 months which found RV mass and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels decreased in the stain users at 6th month and increase in both RV mass and NT-proBNP thereafter in a number of patients [28]. In another study on 112 patients with severe COPD statin use was found to be associated with a 4.2 mmHg (95% CI: 2 to 6.4, P = < 0.001) lower PAWP and a 2.6 mmHg (95% CI: 0.3 to 4.9, P = 0.03) reduction in mean PAP independent of PAWP [29]. In the contrary to the results of the current study, some of the previous studies could not find any effect for statin administration on systolic pulmonary arterial pressure and cardiac output [30] or PAP [31].
A few studies found a beneficial effect for statins in reducing the exacerbation of COPD and duration of hospitalization [32-34]. By reducing hospitalization, governments and patients will save significant amounts of money. Another study found that statin use in COPD patients reduced short-term (30-day) and long-term mortality (10-year) especially when statins were combined with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers, suggesting a possible cardiopulmonary protection induced by those drugs [35,36].
Some studies have suggested CRP as an inflammatory marker on cardiac risk in COPD patients [37,38]. In our study, CRP decreased significantly after three months in patients in atorvastatin group. This finding was in line with the findings of a previous study [39]. In contrast to the findings of this study, Kaczmarek found no differences in the level of circulating inflammatory markers after a 3-month treatment with statin. The difference between the findings of the mentioned studies are because of non-comparable sample size and genetic differences in study populations or the inclusion of COPD patients with pulmonary hypertension [30,40].
This study that patients with higher hsCRP had higher PAP. Furthermore, statins reduced hsCRP and PAP. Hence, we have to pay more attention to patient with higher CRP levels. By administrating such patient's statin, PAP will decrease and we can prevent pulmonary hypertension. This study failed to identify any effect for statins on ejection fraction because the patients had normal ejection fraction before and after the usage of statins. Future studies are needed to evaluate the role of statins in COPD patients with systolic heart failure.
This study has few limitations. This study was not designed to evaluate survival advantage and we did not follow our patients for a long duration, so future studies are needed with a longer follow up period. This study only assessed the effects of atorvastatin due to its availability and reasonable price.
Statins have been shown to have anti-inflammatory properties in different diseases. The findings of this study proposed possible benefits for atorvastatin administration in COPD patients.
The authors wish to thank Vice Chancellor for Education and the Research Committee of University for their support.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.