Epinephrine is one of the major hormones orchestrating numerous physiological responses of the organism exposed to acute or chronic stress. In those cases the plasma level of the hormone exceeds manifold the physiological values present in healthy resting individuals. Higher epinephrine concentrations affect the immune system and modulate macrophages immune responses. The purpose of the present work was to detect if physiological concentrations of epinephrine may alter the capacity of human peripheral blood mononuclear cells (PBMC) for cytokine production, without and following co-incubation with HT-29 cells from a human colon cancer cell line.
PBMC were incubated for 24 hours with the following concentrations of epinephrine-0.025 ng/ml, 0.05 ng/ml and 0.1 ng/ml added at 10 µl/ml culture medium. The secretion of TNFα, IL-1β, IL-6, IFNγ, IL-10, and IL-2 was detected using the ELISA method. In another set of experiments the production of the same cytokines was assessed following co-incubation of PBMC with HT-29 cells.
24 hrs of incubation with epinephrine had no effect on PBMC proliferation rate, whereas that of HT-29 colon cancer cells was significantly reduced. While epinephrine caused increased secretion of IL-6 by non-stimulated PBMC, the production of TNFα, IL-1β, IFNγ, and IL-10 was not affected. Epinephrine exerted no effect on TNFα, IL-1β, IL-6 and IL-10 production induced by LPS and on the release of IL-2 by PBMC activated with PMA. However the production of IFNγ by PBMC stimulated with PMA was increased. The secretion of pro-inflammatory cytokines hereby tested by HT-29-stimulated PBMC was not affected upon incubation with epinephrine. On the other hand, IL-10 secretion by HT-29 stimulated PBMC was slightly reduced.
While small, physiological doses of epinephrine slightly stimulated PBMC for IL-6 and IL-10 production, it appears that the capacity of the mononuclear cells for massive cytokine release could be stirred only with extensive amounts of hormone, even higher than those freed during acute and chronic stress as studies have indicated. Small doses of epinephrine do not alter the immune cross talk between PBMC and HT-29 colon cancer cells.
Epinephrine, Mononuclear cells, Macrophages, Cytokines, HT-29 cells, Immune cells, Colon cancer, Cross talk
Epinephrine, a member of the catecholamine group, is the main hormone and neurotransmitter that acts in situations eliciting short-term stress response by targeting α- and β-adrenoreceptors on various cells. The adrenoreceptors are designated as α2 and β2 subtypes, β2 being the most expressed on immune cells, and further subdivide to additional brands located on a variety of cells [1,2]. Ağac et al. [3] have observed that β2-adrenoreceptors on the immune cells not only block secretion of pro-inflammatory cytokines in response to norepinephrine, but they are able to induce a marked secretion of the anti-inflammatory cytokine IL-10. As a therapeutic agent epinephrine is the drug of choice in cases of acute anaphylaxis [4,5]. However, in addition to its participation in cases linked with acute and chronic stress reactions, studies have shown that epinephrine plays an important role as immunomodulator [6]. In that sense its effect on the morphological and functional activity of the macrophages merits attention. It has been reported that epinephrine regulates the size, morphology, motility and the capacity for phagocytosis of rat peritoneal macrophages and modulates their immune responses by affecting their capability for cytokine production [6-8]. Notably, the kind of cytokines produced by immune cells is related to the type of receptors activated by catecholamines. Stimulation of α-adrenoreceptors induces TNFα and IL-1β production, while incitement of β-adrenoreceptors results in inhibition of TNFα, IL-1β, IL-2, IL-4, IL-6, IL-10 and IL-12 secretion [7,9,10]. It has been shown that stimulation of α2-adrenoreceptor expressed on lamina propria mononuclear cells accelerated the progress of experimental colitis in mice, while their blockade restrained the inflammatory response and ameliorated the progress of the disease [11]. It is evident therefore that increased epinephrine secretion during stress, as in cases of infection and inflammation, will activate the adrenoreceptors on the immune cells and enable them to respond to invading pathogens [12]. Accordingly, exposure of whole blood cells to epinephrine modulated pro-inflammatory cytokine production [13]. In these cases, innate cells, such as specific CD4+CD25− regulatory lymphocytes expressing β2-adrenoceptors, might play a crucial role in inflammation and particularly in sepsis, since when exposed to epinephrine they may restrain the production of pro-inflammatory cytokines [2]. Remarkably, malignant cells also possess adrenoreceptors that belong mainly to the β subfamily. Thus, β3 expression has been detected in cells of patients with leukemia, melanoma, vascular tumors and colon carcinoma. It has been shown that these receptors are closely connected to the transformation of tumor associated macrophages from anti-tumor subtype to pro-inflammatory M1 one, suggesting a mechanism by which β blockers may exert an anti-tumor effect [14]. It has been reported that epinephrine stimulates HT-29 colon cancer cells' proliferation via both β1- and β2 adrenoceptors arising the possibility to include β-blockers to the therapeutic armament of colon cancer [15-17]. Previous studies have shown the existence of an immune dialogue between mononuclear cells and carcinoma cells expressed by a modified cytokine production by the immune cells [18]. Since epinephrine is one of the major players in the creation of either acute or chronic stress, researchers have carried out studies applying relatively huge doses of the drug. The question hereby posed was if relatively small doses of epinephrine, similar to the physiological ones, would affect the capacity for cytokine production by human peripheral blood mononuclear cells (PMBC) and to assess its activity as a mediator of the cross talk between PBMC and cells from HT-29 human carcinoma line.
Epinephrine was purchased from S.A.L.F.S.p.A. (Laboratorio Farmacologico, Cenate Sotto, Bergamo, IT), diluted with 0.9% NaCl solution and added at the onset of cultures at final concentrations of 0.025 ng/ml, 0.05 ng/ml and 0.1 ng/ml, in a volume of 10 µl/ml.
Peripheral blood mononuclear cells (PBMC) were obtained from adult donors' venous blood after signing an informed consent and expressing their agreement to use components of their blood for research as long as they are not required for therapeutic purposes. The cells were separated by Lymphoprep-1077 (Axis-Shield PoC AS, Oslo, Norway) gradient centrifugation, washed twice in phosphate buffered saline (PBS) and suspended in RPMI-1640 medium (Biological Industries, Beith Haemek, Israel) containing 10% heat inactivated fetal bovine serum (FBS), and designated as complete medium (CM).
HT-29 human colon cancer cells were obtained from American Type Cultural Collection, Rockville, MD. The cells were maintained in CM containing Mc-Coy's 5A medium (Biological Industries Co, Beth-Haemek, Israel), supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine and antibiotics (penicillin, streptomycin and nystatin-Biological Industries Co, Beth-Haemek, Israel). The cells were grown in T-75 culture flasks at 37℃ in a humidified atmosphere containing 5% CO2.
0.5 ml of PBMC (4 × 106/ml of CM) was incubated with an equal volume of CM without or with 40 ng/ml lipopolysaccharide (LPS, E. coli, Sigma) or with 1 µg/ml of Phorbol meristate acetate (PMA) and 0.5 µg/ml of ionomycin (Sigma, Israel) and in another set of experiments with 0.5 ml of HT-29 colon cancer cells (4 × 105/ml of CM) suspended in appropriate CM. Epinephrine was added at the onset of cultures at concentrations as indicated. The cultures were incubated for 24 hrs at 37℃ in a humidified atmosphere containing 5% CO2. At the end of the incubation period the cells were removed by centrifugation at 250 g for 10 min., the supernatants were collected and kept at -70℃ until assayed for cytokines content.
The effect of epinephrine on PBMC and HT-29 cell proliferation was examined applying XTT proliferation assay kit (Biological Industries, Beith Haemek, Israel). In short: 0.1 ml aliquots of PBMC or HT-29 cells obtained after trypsinization and suspended at 105/ml in appropriate CM were added to each one of 96 well plates and incubated for 24 hrs in the absence or presence of epinephrine added at the onset of cultures at concentrations as indicated. At the end of the incubation period the cells were stained according to the manufacturer's instructions. The plates were incubated for additional 3 hrs at 37℃ in a humidified incubator containing 5% CO2 and the absorbance was measured at 450 nm using an ELISA reader.
The concentration of the following cytokines: TNFα, IL-1β, IL-6, IFNγ, IL-10, and IL-2 in the supernatants was tested using ELISA kits specific for these cytokines (Biosource International, Camarillo, CA) as detailed in the guide-line provided by the manufacturer. The detection levels of these kits were: 15 pg/ml for IL-6 and 30 pg/ml for the remaining cytokines.
A linear mixed model with repeated measures and the assumption of compound symmetry (CS) was used to assess the effects of epinephrine on cytokine production by PBMC and cancer cell line. SAS/Base and SAS/Stat vs. 9.4 for Windows PC were used for this analysis. Paired t-test was applied to compare between the levels of cytokine produced with various concentrations of epinephrine and that found in control cultures (incubated without epinephrine). The results are expressed as mean ± SEM.
Incubation of PBMC with the above mentioned concentrations of epinephrine for 24 hrs did not affect the proliferation rate examined by XTT test (F = 0.94, p = 0). However, the proliferation of HT-29 cells incubated at the same culture conditions was significantly reduced (F = 4.95, p = 0.014) and was lowered by 20% at 0.025 and 0.05 ng/ml of epinephrine (p = 0.066).
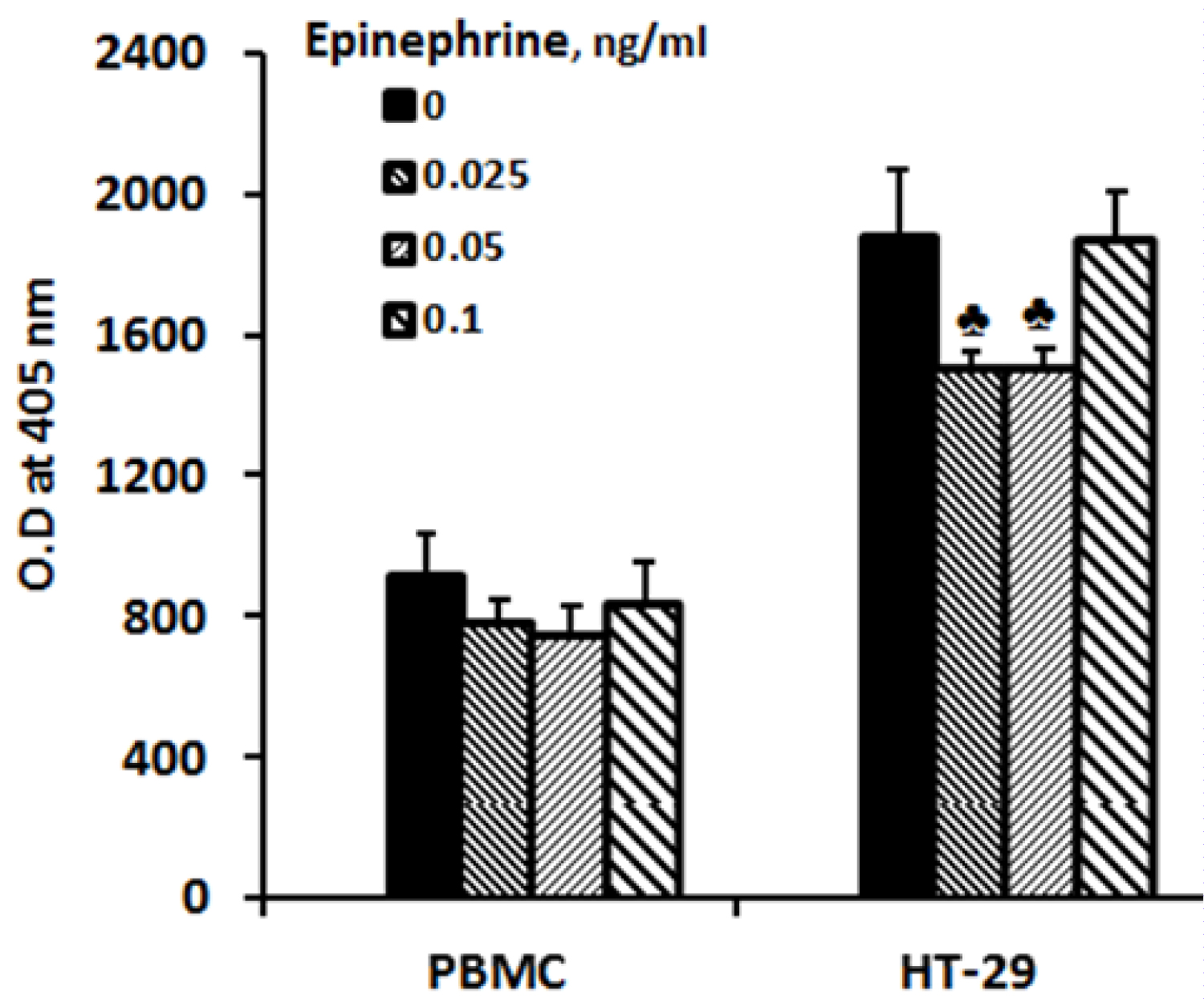 Figure 1: Effect of epinephrine on cell proliferation.
Figure 1: Effect of epinephrine on cell proliferation.
PBMC or HT-29 cells were incubated for 24 hrs at 37℃ in a humidified incubator containing 5% CO2 in the absence (0) or presence of epinephrine added at the onset of cultures at concentrations as indicated. The proliferation rate was examined applying XTT assay as described in Materials and Methods. At the end of the incubation period the cells were stained according to the manufacturer's instructions, and the absorbance was measured at 450 nm using an ELISA reader. Each column represents the mean of 6 experiments. Bars represent SEM. ♣-represents statistically significant difference from cells incubated without epinephrine (0) (♣p = 0.06).
View Figure 1
HT-29 cells: No detectable concentrations of any of the cytokines examined could be found in supernatants collected from HT-29 cells (2 × 105/ml) incubated for 24 hrs without or with epinephrine.
Non-stimulated PBMC (Table 1 and Table 2): Incubation of non-stimulated PBMC with epinephrine revealed no significant effect on the secretion of TNFα, IL-1β, IFNγ and IL-10. However the production of IL-6 was increased (F = 3.62, p = 0.058) and was higher by 18% (p < 0.05) when PBMC were incubated for 24 hrs with 0.1 ng/ml of epinephrine.
Table 1: Effect of epinephrine on pro-inflammatory cytokine production by PBMC. View Table 1
Table 2: Effect of epinephrine on anti-inflammatory cytokine production by PBMC. View Table 2
LPS or PMA-induced cytokine production (Table 2 and Table 3): Epinephrine did not exert any effect on the secretion of TNFα, IL-1β, IL-6 and IL-10 induced by LPS, or the release of IL-2 following stimulation with PMA. However the production of IFNγ by PBMC stimulated with PMA was increased (F = 3.55, p = 0.06) and was higher by 18% at epinephrine concentration of 0.05 ng/ml (p < 0.05).
Table 3: Effect of epinephrine on IL-2 and IFNγ production by PBMC. View Table 3
HT-29-induced cytokine production: The production of the tested pro-inflammatory cytokines by PBMC induced by HT-29 cells was not affected upon incubation with epinephrine at the concentrations as described (Table 1 and Table 3). However IL-10 secretion by HT-29 stimulated PBMC was slightly reduced (F = 2.89, p = 0.094), and was lower by 10% at epinephrine concentration of 0.05 ng/ml (p < 0.05) (Table 2).
The interpretation of the present results should be based on the fact that the problem to be clarified was if small doses of epinephrine may modulate the immune activity of PBMC. Therefore, we have used concentrations of epinephrine comparable to the mean plasma value of 0.034 ± 0.002 ng/ml obtained from 60 healthy individuals [19]. In our study epinephrine did not affect PBMC proliferation, but inhibited the proliferation of HT-29 cells with the lower doses applied. In a study working with A549 lung cell line Huang et al. [20] could achieve a decrease in cell proliferation of less than 10 percent applying 0.01 ng/ml of epinephrine. Increasing the dose up to 10 ng/ml did not markedly affect the proliferation of the cancer cells. β-adrenergic signaling to tumor cells and tumor associated macrophages has been shown to promote tumor development and dissemination [16-21]. However, these events were observed when doses larger than the physiological ones were applied. In animals with colorectal carcinoma higher level of epinephrine achieved after their exposure to chronic stress did stimulate tumor growth. Moreover, treatment of the animals with adrenoreceptor antagonists inhibited the process [22]. In a study carried out with HT-29 colon cancer cells, applying epinephrine at a huge concentration of 10 µM (1830 ng/ml!) caused stimulated cell proliferation by 35% and has been explained by inciting β-adrenoreceptors' signaling via a COX-2 dependent pathway [15]. Likewise in this study the dose of epinephrine markedly exceeded not only the physiological ones used in the present work, but also the higher levels of epinephrine reported in humans following chronic and acute stress (up to 1.37 ng/ml and 35.9 ng/ml respectively) [19]. The doses of epinephrine used in the present study increased the secretion of IL-6 by non-stimulated PBMC and that of PMA/ionomycin-induced IFNγ production, but did not exert any effect as for their capacity to generate the remaining cytokines examined, whether the PBMC have been stimulated or not. The secretion of the anti-inflammatory cytokine IL-10 induced by HT-29 cells was slightly reduced at 0.05 ng/ml. Similarly to these observations Piaza et al. [23] did not detect any effect of α2 agonists or their antagonists on the capacity of human lung macrophages for TNF-α, IL-6 and IL-8 production. Wahle et al. [24] have suggested that β2 adrenoreceptors are the principal factors that mediate cytokine production by PBMC. According to Margaryan et al. [13] exposure of non-stimulated whole blood cells to epinephrine showed a dose-dependent increase in IL-8 secretion, whereas LPS stimulated cells expressed a markedly decreased IL-8 and IL-1β production. However, it should be clarified that these results were observed applying vast doses of epinephrine (1.83 µg/ml-183 µg/ml) being 104-106 times higher than those used in our study. Reports have shown that combination of LPS with epinephrine markedly stimulated inflammatory responses in comparison to the effect of each one of them separately applied [25]. The question arises why the effect of epinephrine on LPS stimulated PBMC for cytokine production in our study was not expressed. One possible explanation is that we worked with a relatively high dose of LPS (40 ng/ml), whereas in another study increased production of IL-6, IL-8 and TNFα by LPS stimulated human monocytes under the effect of epinephrine was obtained using much smaller doses of LPS (0.2 ng/ml, 1 ng/ml and 10 ng/ml). Moreover, lower LPS concentrations exerted higher cytokine expression [26].
The capacity of epinephrine to stimulate tumor cells for cytokine production merits consideration. Bearing in mind that one way to promote this activity proceeds through expression of adrenoreceptors it is conceivable to assume that tumor cells indeed possess such receptors as has been demonstrated for HT-29 colon cancer cells [15,16,27]. Catecholamine stimulation could enhance melanoma cells to release IL-6, IL-8, VEGF-A and FGF-2, cytokines expressing inflammatory, angiogenic and motility properties. It is of interest that these cytokines act on melanoma cells to enhance the expression of β2- and β3 adrenoreceptors [14].
Tumor associated macrophages (TAM) maintain an immune dialogue with the tumor cells leading to modulation of interleukin productionas it has been shown previously by incubation of PBMC with colon cancer cells [18]. Notably, the immune balance between PBMC and cancer cells may be affected by a number of chemical and nutrition mediators as it has been formerly described [28,29]. In the present study epinephrine applied at small doses did not affect the immune equilibrium between PBMC and HT-29 cells providing an additional indication that epinephrine, only at higher quantities, may activate macrophages' and cancer cells' adrenoreceptors, as it has been shown on mice with breast tumor exposed to chronic stress in which the epinephrine level was 124 ± 12 ng/ml compared with 18 ng/ml in the controls [30].
In conclusion, while high quantities of epinephrine delivered in cases of acute and chronic stress affect macrophages' immune functions, the results hereby presented indicate that the hormone, applied in vitro at physiological doses, exert a minimal effect on PBMC immune responses and the immune dialogue between PBMC and HT-29 cells from a human colon carcinoma line.
Our sincere thanks are given to Ms. Tzippy Shochat, M.Sc., Statistical Consultant, Rabin Medical Center, Beilinson Hospital, for her prodigious support in performing the statistical computations.
None.
None (The research has been carried out on a voluntary basis).
All authors have contributed equally to the work.