The quest for a safe drinking water free from any form of contamination cannot be overemphasized. People around the world do not have safe drinking water and that has resulted into myriad of water borne diseases. A total of twenty-two (22) samples were collected from twelve (12) different brand and subjected to microbiological analysis. From the results obtained, pH of the analyzed samples had ranges from 6.5 to 7.5, which fall within the normal standard range of 6.5 to 8.5. The Fecal coliform count for all products gave 0cfu/ml, which indicates the absence of fecal contamination and the absence of Escherichia coli, the indicator organism under investigation. But other Bacterial isolated includes: Bacillus subtilis, Pseudomonas spp, Micrococcus luteus, Bacillus smithii, Citrobacter spp, Klebsiella oxytoca, Coagulase negative staphylococci. The above listed organisms are not considered pathogenic in healthy individuals, but in immunodeficient individuals, they are dangerous especially Pseudomonas which have been isolated in gastrointestinal infections, most package water products are expected to be free from fecal contamination, using the MPN testing method, it facilitates the identification of Escherichia coli which is one of the indicator organisms amongst others. The results of this study show that the packaged water products are safe for consumption. The total coliform count showed that the methods used for treatments eliminate coliforms and other heterotrophic organisms to a reasonable extent, since the count were above 10 cfu/ml. Risk of infection cannot be ascertained using colony count alone, rather, it is a measure for the effectiveness of filtration processes and serves as a measure for assessing the hygiene levels observed by each corporation.
Identification, Comparison, Microbial load, Water products, Ekiti state
Water is a clear, colorless, tasteless liquid composed of hydrogen and oxygen. According to the English dictionary water is defined as a clear liquid substance at room temperature and pressure. It is present naturally as rain, water can be gotten from rivers, lakes; seas. Water exists in three forms: solid (ice), liquid, and steam which is the gaseous form [1]. Water is a necessary requirement for all life forms. Air, water and food in the order of importance, are the main requirement for life. Humans can survive less than five minutes without air, about a week without water, and for about a month without food [2]. According to Igbeneghu and Lamikanra [3], water is used for drinking, bathing, medication, industrialization, processing of food, recreation etc. One third of intestinal infections globally, are caused by Water borne diseases [4]. The general idea is that drinking water should be totally free from microorganisms but is not especially processed packaged water being the area of concern is not free from microorganisms. Worldwide, about a billion people have no access to safe drinking water and 2.6 billion people lack proper sanitation, which results to 1.8 million people dying yearly from water related diseases, with children under the age of 5 years about 90% mostly in developing countries being infected [5]. Contaminated water supplies affect the growth and nutrition in young children adversely. Production of quality water product is increasingly difficult, because the demand for water is high. Implementing universal standard for drinking water is not being followed to the letter, due to differences in sociological conditions, varying climates, and other specific circumstances found all over the world. However, water treatments such as storage in open reservoirs, coagulation, filtration, and treatment with chemicals can be used to improve quality of the product from the original water (raw).
The parameters for drinking water quality are divided into physical, chemical and microbiological (which is the main area of concern to this study). Confirmation of coliforms and pathogenic organisms in treated drinking water is indicative of poor application of water treatment techniques [6]. Physical investigation involves product information, odor, and appearance which include examining for colour, turbidity, and presence of floating particles or extraneous materials [7]. Studies on sachet water products in Nigeria have shown that factors responsible for water contamination include sharp practices, poor hygiene of vendors, polluted environment, and non-adherence to WHO/NAFDAC regulations. Microbiological parameters investigate the microbes associated with waterborne disease, some of the microbes are coliform bacteria which require rapid detection and can be achieved using the MPN (Most Probable Number) method which determines the portability of water [8]. Some of the microorganisms regarded as contaminant include Escherichia coli, Vibrio cholerae, Salmonella species, viruses such as hepatitis A, and protozoan parasites such as Giardia lamblia. Nigeria, got to a point where it was believed that water contamination is more common with sachet than with bottled water which forced NAFDAC to declare a possible 'gradual' nationwide ban on sachet water to allow manufacturers of sachet water to start winding up or change to bottle packaging [9]. There are several rules and regulations for drinking water. In Nigeria, such regulations are monitored by National Agency for Food and Drug Administration and Control (NAFDAC), which was established as a parastatal of the Federal Ministry of Health by Decree No. 15 of 1993. This has resulted to some sachet water products being converted to bottled product without proper treatment. Waterborne disease cause about 3.4 million deaths every year making it the leading cause of morbidity and mortality all-over the world [10]. Based on WHO standard (from which Nigeria coined the quality of drinking water) heterotrophic bacteria present in drinking water should not exceed 10 cfu/ml and in 100 ml of water there should be no coliform present, showing that there is a limited amount of microorganism permitted to be present in drinking water. Heterotrophic bacteria when present in large amount in water products serves as an indication of poor manufacturing practices encountered during its processing. Diseases associated with contaminated water are hepatitis A, cholera, typhoid, amoebiasis, botulism, shigellosis, legionellosis, severe acute respiratory syndrome, etc. Water contamination not only occurs during processing but also due to how and where they were stored, the microbes in the water can proliferate to an amount that can become pathogenic. The aim of this study is to identify and compare the microbial load of different water products available in Ekiti State.
The study area of this work is Ado Ekiti, Ekiti State. Ado-Ekiti is a city in the Southwest Nigeria and lies on latitude 7° 35 and 7° 38 North of the equator and Longitude 5° 10 and 5° 15 East of the Greenwich Meridian [11]. It has population of 308,626 [12].
Twelve (12) sample brands were selected randomly, 5 sachets and 7 bottled samples. 22 samples were used in total and were designated A to L. Tap water was used as control [13].
Water products (sachet and bottled water) were gotten from places where people frequent regularly and products that are common to people was gotten 5 sachets and 7 bottled water of different brands were gotten and were subjected to microbiological analysis. All analysis was performed in the laboratory in ABUAD.
Method of analysis applied in this work includes plate count using the pour plate method and the presumptive coliform count using the multiple tube fermentation tests [14].
The top and neck of the bottles was cleaned with 75% alcohol and allowed to dry, the cap was opened and little of the water was poured out. The cap was closed and the water was mixed. The sachets of water were wiped with 75% alcohol, a portion of it was opened with a sterilized blade and a portion was poured out and the remaining was transferred to a sterile bottle and capped immediately.
The hydrogen ion concentration (pH) of water sample was measured using pH strips which were standardized by matching it to the producer's guide chart [15].
To test for the effectiveness of the treatment methods used by the water companies' coliform plate count is done. The method used in this work was the pour plate method [16].
For each dilution (1:10, 1:100) per sample, 9 ml of Ringer's solution was put into a sterile container except for the dilution of 1. To prepare 1:10 dilution, 1 ml of the water was added to the first tube and mixed and 1 ml was transferred from tube one to tube 2. Another pipette was used to mix the content of tube 2. 1 ml of each dilution was dispensed into clean petri dishes and molten agar of about 45 °C was poured. The petri dish was moved clockwise and anticlockwise 3x and allowed to gel and inverted. The plates were incubated at 37 °C for 24 to 48 hours. The colonies were counted where present.
Measured volumes of neat and diluted water are added to a series of tubes containing broth which serve as an indicator growth medium. A characteristics color change in any broth indicates the presence of indicator bacteria in the test sample. The most probable number or coliform forming units of the indicator organisms in the sample depends on the number and distribution of positive and negative reactions [14].
The presumptive coliform count is basically of two methods which could be used for the identification and counting of bacteria in water and they are the multiple tube method and the membrane filtration method. The most effective and sensitive amongst the two is the multiple tube method and is the method being applied in this study [14].
Sodium thiosulphate was mixed with the water sample, by inverting the bottle many times. Aseptically the broths were inoculated as follows; 1 × 50 ml of broth + 50 ml of water and 5 × 10 ml of broth + 10 ml of water. Content of each bottle was mixed and tubes were incubated at 37 °C for 24 hours with bottles loosely capped. All bottles showing acid and gas production after 24 hours were regarded as presumptive positive. Bottles giving negative results were reincubated further for 24 hours. Probability table was used to determine the most probable number of presumptive coliform per 100 ml of the sampled water.
This test is used for the confirmation of fecal contamination using indole test. Sterile wire loop was used to inoculate the Tryptone soya broth with a drop of the broth from the positive MPN cultures and observed for gas production at 44 °C and indole.
Escherichia coli are gram negative rod organism and belong to the family enterobacteria. They are naturally found in the intestinal tract, in soil and water. The organism is indole, motility, catalase positive and urea, citrate negative. E. coli belongs to the group of bacteria referred to as coliform bacteria and are used as indicators for fecal contamination and also referred to as thermotolerant coliform.
Tubes showing positive reactions from the presumptive test were subcultured on solid media such as SSA, MCA, and TCBA. Grams reaction was observed and biochemical test were done to identify them.
Gram staining reaction is used to identify pathogens in specimens and cultures by their Gram reaction either Gram positive or Gram negative and their morphology. Smear was made on a clean grease free slide, allowed to dry and heat fixed. Fixed smear was flooded with crystal violet stain for 60 seconds and later was washed off with clean water. It was cover with Lugol's iodine for 60 seconds and washed off with clean water. Acetone-alcohol was applied to decolorize rapidly (few seconds) and was washed off immediately with clean water. Counter stain was applied on the smear for 60 seconds and the stain was washed off with clean water. Slide was blotted out and place on a draining rack for the smear to air-dry. The smear was examined microscopically using 100x objectives. Gram positive organisms stained purple, while Gram negative organisms stained pink [14].
Indole production test was used for identification of enterobacteria of which most strains of Escherichia coli, Proteus vulgaris, Providencia rettgeri, Morganella morganii, and Providencia species released indole from the breakdown of the amino acid tryptophan. Bijou bottle containing 10 ml of sterile Tryptone water having inverted Durham's tube was inoculated with a drop of MacConkey broth containing the test organism and was positive for MPN. Broth was incubated at 44 °C for up to 24 h. 0.5 ml of Kovac's reagent was added to the overnight broth and was mixed gently to test for indole. It was examined for a red colour ring at the meniscus. Positive indole test had red meniscus at the top of the broth and negative indole test had no red meniscus at the top of the broth [14].
This test is used to identify those bacteria that produce the enzyme catalase and differentiate the form non-catalase producing bacteria. Into a clean test tube, 2-3 ml of the hydrogen peroxide solution was dispensed, sterile wooden stick was used to pick colonies of the test organism and immerse in the hydrogen peroxide solution and observed for immediate production of bubbles. For a catalase positive test, bubbles will be produced and for a catalase negative, there will be no production of bubbles [14].
This test helps to differentiate Staphylococcus aureus from other Staphylococcus species. Two separate drops of normal saline were placed on glass slide and distinct colonies were emulsified in them to make suspensions. Plasma was added to only one mixed and observed for agglutination within 10seconds. Clumping was reported as positive and no clumping seen was reported as negative [14].
The test was used to assist in the identification of enterobacteria. Method used was the Simmon's citrate agar. Citrate slopes was inoculated using sterile straight wire loop, first by streaking the slope with a distinct colony of the test organism and then stabbing the butt. The inoculated slants were incubated at 37 °C for 24 hours and observe for colour change in the medium. Positive citrate test resulted to a change in the slant from green to bright blue, whereas negative citrate test resulted to no change in the colour of the slant [14].
Testing for urease enzyme activity is important in differentiating enterobacteria. The test organism was inoculated heavily in a bijou bottle containing 3 ml sterile Christensen's modified urea slant and was incubated at 37 °C for 3-12 hours. The medium was observed for a pink colour. Positive urease test was indicated by the development of pink coloration and in negative urease test, the media remained unchanged.
Sterilized straight inoculating needle was used to pick distinct colony, TSI was first stabbed through the center of the medium to the bottom of the tube and the surface was streaked. Tubes were clogged with cotton wool and incubated at 37 °C in ambient air for 24 hours [14].
The data generated was analyzed using statistical package for social sciences version 17. Tables and charts were used to represent the frequencies.
Fluctuation in the pH of water has been reported to have effect on the toxicity of poisons in water, making the values of the pH extremely important [17]. pH of analyzed samples had ranges from 6.5 to 7.5, as shown in Table 1 and all fall within the normal range of 6.5 to 8.5.
Table 1: pH results of samples of water used for this study as indicated with the sample code. View Table 1
Fecal coliform count for all products gave 0 cfu/ml, indicating absence of fecal contamination and absence of Escherichia coli, the indicator organism being tested for. The result can be seen in Table 2. Products A to L are fit for consumption and fall within the WHO standard which indicates that treated packaged drinking water products should not contain any fecal coliform.
Table 2: The Total coliform counts (MPN/100 ml) for each water sample. View Table 2
Total coliform count was done using the pour plate method. The results obtained are as represented in Table 3. Low results ranged from 0 MPN/100 ml to 9MPN/ml which is within the acceptable permissible limit. This result indicates the presence of coliform bacteria in samples E, H, I, J.
Table 3: Plate count (CFU/ml) for the isolated organisms in the water samples. View Table 3
Plate count also referred to as Heterotrophic plate count examined for the presence of any bacteria. Products with code A, B,C, F,G and H as indicated in Table 4, are deem fit for consumption from the result above for both healthy and immunocompromised individuals, since the plate counts gave units within WHO stipulated standards, which is 1 × 102 cfu/ml. Results obtained that were high indicate malfunctioning in the treatment method used or handling during distribution.
Table 4: Bacteriological counts of packaged water samples. View Table 4
Table 5 and Table 6 contains list of isolated organisms which includes Bacillus subtilis, Pseudomonas spp, Micrococcus luteus, Bacillus smithii, Citrobacter spp, Klebsiella oxytoca, Coagulase negative staphylococci. The listed isolated organisms are not considered pathogenic in healthy individuals but immunodeficient individuals, they are dangerous especially Pseudomonas and have been isolated in gastrointestinal infections. According to Egbe and colleagues [18], the occurrence of pathogens in water resources indicates that such waters may result in the transmission of waterborne diseases. The occurrence of the isolated organisms is shown in Figure 1. The isolated organisms in relation to the samples of water used in this study are represented as a percentage of their occurrence in all the samples as shown in Figure 2, with Bacillus smithii 33% Bacillus subtilis 17%, Pseudomonas spp 5%, Micrococcus luteus 6%, Citrobacter spp 22%, Klebsiella oxytoca 11%, Coagulase negative staphylococci 6%.
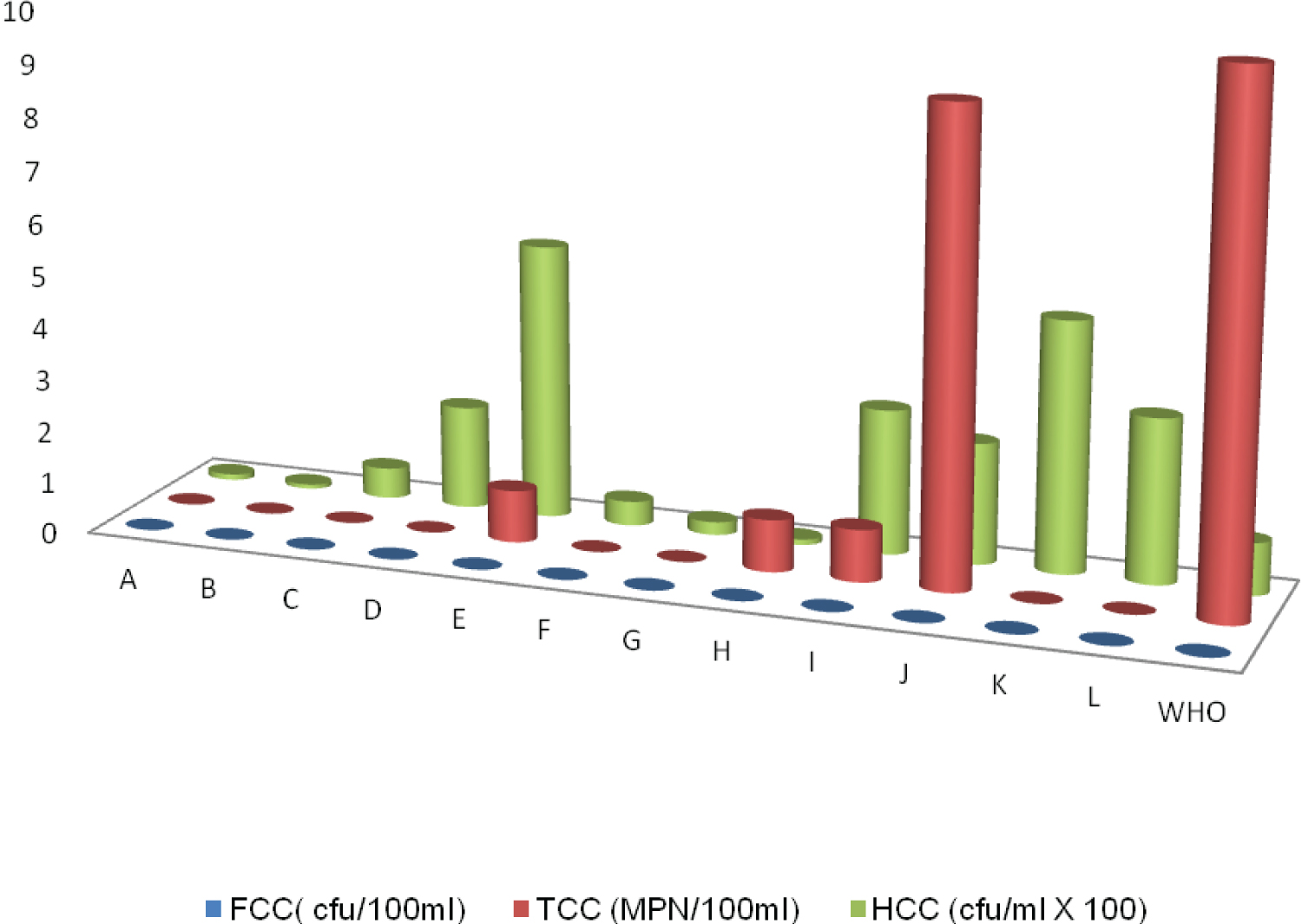 Figure 1: Comparison of CFU/ml of each product of sampled water.
Figure 1: Comparison of CFU/ml of each product of sampled water.
NB: Sample D, E, I, J, K, L as illustrated by the graph above had high plate counts.
View Figure 1
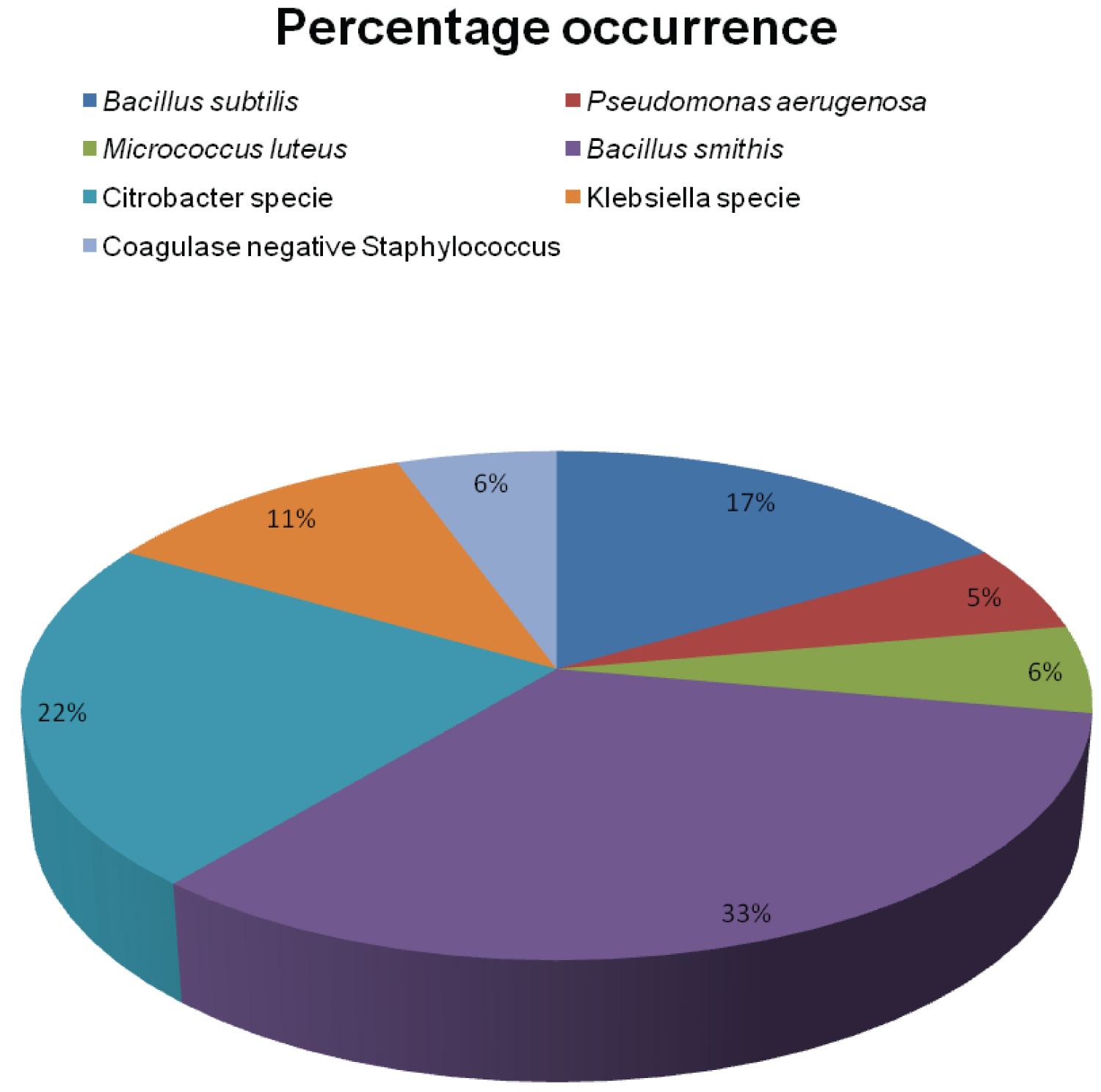 Figure 2: Percentage occurrence of isolated organisms of the water samples.
View Figure 2
Figure 2: Percentage occurrence of isolated organisms of the water samples.
View Figure 2
Table 5: Characterizations and identification of bacteria isolated from the water samples. View Table 5
Table 6: Occurrence of bacterial isolates in the packaged water samples. View Table 6
The microbial quality of drinking water is of concern to consumers and poses risk to public health [19]. According to Oyedeji and colleagues [20], packaged drinking water has been used as alternative drinking water source due to contamination of other sources of water such as wells, taps, tanks as a result of microbial contamination and other factors. The microbial populations are typically highest in surface water and rainwater, followed by ground water and least in sachet and bottled water. According to Mgbakor and colleagues [21], there is an increase in the demand, sale and indiscriminate consumption of packaged drinking water which includes sachet and bottle water in Nigeria and poses significant public health risks to the citizens especially individuals with compromised immune systems, which prompted this study. bottled water in Nigeria, is regarded as being safer than water dispensed and sold in sachets and is also more expensive which is why it is patronized mainly by people with a relatively large disposable income. For this study, Twenty-two water samples were collected representing twelve different brands of water were analyzed for fecal contamination, total coliform count, as well as biochemical analysis. Fluctuation in the pH of water has been reported to have effect on the toxicity of poisons in water, making the values of the pH extremely important [17]. pH of analyzed samples had ranges from 6.5 to 7.5, as shown in Table 1, which falls within the stipulated range of 6.5 to 8.0 and could be associated with the treatment methods of each of product corporation as observed in a study by Bikram and colleagues [22]. In a similar study, in Abeokuta metropolis, pH values of 6.0-7.54 were obtained from sachet water, according to Taiwo and colleagues [23]. Also, in a study carried out by Sule and colleagues [13], pH range of 6.5-8.0 were obtained. According to SON in Nigeria, thermo tolerant coliform (Escherichia coli); faecal streptococcus and Clostridium perfringens spore should be 0 cfu/100 ml in drinking water. The multiple tube fermentation study showed the presence of coliform bacteria other than Escherichia coli which is the indicator used for this study. All twenty-two samples showed no contamination with fecal coliform indicator, showing the absence of Escherichia coli. Other coliforms were present though, showing the production of acid in some and no production of gas, while others showed the production of gas and no production of acid, others showed both. In a study by Sule and colleagues [13], results between the ranges of 0 to 52 MPN/100 ml, whereas in this study total coliform confirmed were within the range of 0 to 9 MPN/100 ml. All products had values lower than the WHO standard which is 10MPN/100 ml. All brands used for the study conform to the required standard based on the result obtained. Adewoye and colleagues [24], obtained total coliform count which ranged from 0-20 MPN per 100 ml of sachet water. According to WHO, maximum permissible level of heterotrophic coliform count should not exceed 1 × 102 cfu/ml. The products used for this study had plate counts ranging from 0-5.30 × 102 cfu/ml based on the dilutions Growths were obtained from sample A, B, C and likewise other packaged products. It has been reported in other studies that bottled water is amongst water sources having high levels of HPC, which may be regrowth normally following treated drinking-water. Growth other than pathogenic organisms is an indication of poor performance of filtration or disinfection processes in the treatment process. Bottle waters have long expiry dates which would give room for microorganisms to proliferate and result to health issues. The organisms isolated in this study include Bacillus subtilis, Pseudomonas aeruginosa, Micrococcus luteus, Bacillus smithii, Citrobacter specie, Klebsiella specie, Coagulase negative Staphylococci. The presence of these organisms maybe as a result of post contamination. Some of these organisms are commensals in water and are present in the environment. The organisms isolated are not pathogenic in healthy individual, but some have been discovered to be of health concern in immunocompromised, individuals. Pseudomonas aeruginosa is an opportunistic bacterium and has been isolated in nosocomial infections, bacteremia and gastrointestinal infection. This organism also produces tissue damaging toxins. It should be noted that the risk of colonization from ingesting Pseudomonas aeruginosa is low and so possess no threat to healthy individuals, in the form of diarrheal disease [25]. In intensive care units in a hospital in Germany, according to Eckmanns and colleagues [26] an outbreak of hospital-acquired Pseudomonas aeruginosa infection caused by contaminated bottled water was reported. Pseudomonas aeruginosa was detected in Sample I and was the only product having contamination with the bacteria.
Micrococcus specie is present in the environments including water, dust and soil. It has also been isolated from animal and dairy products human skin. Micrococcus is believed to be saprophytic or commensal organism, since it sometimes involves in decomposition of plant. It is also an opportunistic pathogen [27].
A previous study in Ekiti state according to Falegan and colleagues [28], reported that sachet water samples collected from 5 different manufacturers yielded Pseudomonas aeruginosa 37.5%, Staphylococcus aureus 20.8%, Klebsiella spp. 25.02%, and Proteus sp. 16.68%. In this study on both sachet and bottle water the yields were Bacillus subtilis 17%, Pseudomonas aeruginosa 5%, Micrococcus luteus 6%, Bacillus smithii 33%, Citrobacter specie 22%, Klebsiella specie 17%, Coagulase negative Staphylococci 6% as shown in Figure 2. Medically important bacteria such as Escherichia coli, Vibrio cholerae, salmonella species, and Shigella species were not isolated in this study.
In conclusion, most package products are free from fecal contamination using the MPN method testing for Escherichia coli which is one of the indicator organisms amongst others. From the results it shows that the packaged products are safe for consumption. The total coliform count showed that the methods used for treatments were not able to eliminate coliforms and other heterotrophic organisms to a reasonable amount, since the count were above 10 cfu/ml. Risk of infection cannot be ascertained using colony count alone rather, it is a measure for the effectiveness of filtration processes and serves as a measure for assessing the hygiene levels observed by each company.
This study has revealed that though all samples were free from fecal indicator, they were not free from other organisms. Some of the isolated organisms are becoming resistant to antibiotic e.g. Pseudomonas spp. Immunocompromised individual also consume these products and maybe exposed to these organisms which would result to serious health issues.
Combined treatment methods should be enforced for all packaged water producing companies which should include heating and treatment with appropriate chemicals. Continuous assessment during production should be done. Awareness should be made to the public and individuals should check for both manufacturing and expiry date as well as batch number before consumption.
The authors profound gratitude goes to all the staff of the Department of Medical Laboratory Science, College of Medical and Health Sciences, Afe Babalola University, Ado-Ekiti, Ekiti State Nigeria for their kind gesture rendered to me during the course of this work.
The authors declare no conflict of interest.