This study aimed to estimate the prevalence and examine the characteristics of premenstrual syndrome (PMS) among female university students. It also aimed to determine the factors associated with the affective and somatic domains of PMS.
This study aimed to estimate the prevalence and examine the characteristics of premenstrual syndrome (PMS) among female university students. It also aimed to determine the factors associated with the affective and somatic domains of PMS.
Almost 63% of participants reported having PMS with 42.5% having severe PMS. The most common affective and somatic symptoms were "angry outbursts" and "abdominal bloating" respectively. Obesity, having unemployed fathers, caffeine consumption (coffee/tea), and pain medication use, and sleeping pill use were significantly associated with PMS. Among these factors, caffeine consumption, and medication use for pain were associated with PMS affective domain while BMI, caffeine consumption, and medication use for pain were related to PMS somatic domain.
The proportion of Lebanese females with PMS and affective and somatic symptoms are notably high. These findings highlight the importance of weight management to minimize PMS symptoms.
Premenstrual syndrome, Affective domains, Somatic domains, Lebanon, Prevalence, Obesity
Premenstrual syndrome (PMS) is characterized by affective and somatic symptoms appearing in the days preceding menses and interfering with women's daily life [1]. The American College of Obstetricians and Gynecologists (ACOG) criteria suggest the following PMS diagnosis: A clinical condition characterized by the cyclic presence of emotional and physical symptoms unrelated to any organic disease that appear during the luteal phase in each of the three prior menstrual cycles and spontaneously disappear within 4 days of the menstrual cycle onset, and do not relapse until at least cycle day 13 [2]. Women who experience PMS tend to report low health-related quality of life, frequent visits to health providers, decreased occupational productivity, and greater psychiatric comorbidities [3-5]. PMS prevalence varies worldwide, ranging from 12% (95% CI: 11-13) in France to 98% (95% CI: 97-100) in Iran. A meta-analysis showed an increasing trend in PMS prevalence. The pooled of PMS rate estimated from 12 countries located in Asia, Africa, South America and Europe's continents was found to be 48% (95% CI: 33-63) [6].
More than 20% of women have affective and somatic premenstrual symptoms that are clinically relevant [4]. The most common symptoms reported from developed countries such as Japan, Spain and France include lack of energy and anxiety [7], breast tenderness, headaches, muscular pain, and irritability [8], and anxiety [9] respectively. In developing countries such as Turkey, Lebanon and Nigeria, the most common reported symptoms were abdominal bloating and irritability [10], and breast tenderness [11,12]. The etiology of PMS has not yet been clearly elucidated [1,13]. Genetic, environmental, and psychological factors could impact hormonal fluctuations and thus lead to PMS symptoms [1,3,6,13]. Cyclical ovarian activity and the effect of estradiol and progesterone on the neurotransmitters like serotonin and gamma-amino butyric acid (GABA) appear to play key roles [3,6,13]. Absence of PMS before puberty, during pregnancy and after the menopause supports the theory that cyclical ovarian activity is important in PMS development.
Research describing PMS in Lebanon is limited to two brief communications aimed to assess menstrual cycle abnormalities and PMS prevalence respectively [12,14]. Both studies were conducted in a single university/medical center and among a small number of participants. The premenstrual syndrome (PMS) is particularly common in the younger age groups and therefore presents a significant public health problem among young girls. Therefore, assessing PMS, the factors associated with it as well as its symptoms in Lebanese females is needed. This study will estimate the proportion, severity and factors of PMS among Lebanese female university students. Moreover, it will explore the affective and somatic PMS domains as well as their respective associated factors.
This cross-sectional study was conducted during the Spring semester of 2015-2016 among female university students in Lebanon. The study was conducted at five large universities located within the Greater Beirut area, which includes more than 50% of university students in Lebanon, with a convenience sampling method being applied to enroll participants. Participants were recruited from four private universities and one public university, namely the Lebanese University, and from the following faculties: Sciences, medicine, humanities, business, and engineering. Participants were recruited throughout the semester up until 2 weeks prior to the final exam period. Flyers inviting participants to partake in this study were distributed in each of the campuses of the participating universities. Students who were interested in participating in the study were invited to meet in an auditorium where they were informed about the purpose of the study and were invited to participate in the survey. Participation in the study was voluntary, and did not involve financial or any other compensation. After being screened for inclusion/exclusion criteria, students were then asked to complete an anonymous questionnaire that was administered by a trained research assistant. The questionnaire required less than 15 minutes to be completed. In order to be included in the study, participants had to be female, Lebanese, and currently registered as full-time undergraduate students in the academic year 2015-2016 at the Lebanese and private universities. Ethics approval was obtained from the institutional review board at the Lebanese University. All participants signed a written informed consent form.
According to a previous study on PMS conducted in Lebanon, sample size was determined for a PMS prevalence of 7.1% [12]. Using the following formula for sample size calculation (N) = (Za2 × p × q)/d2 and with an α error of 5% and a margin of ± 1.1%, 2094 participants were needed for the study.
Participants were excluded if they had any medical condition interfering with the menstrual cycle such as endometriosis, pelvic inflammatory disease, uterine cancer and uterine fibroids. Students were asked about any of these medical conditions before completing the questionnaires. Overall, 2500 female students were found to be eligible and voluntarily participated. Among them, 50 were excluded as having the above mentioned medical conditions that interfere with the menstrual cycle. Moreover, 335 had incomplete information data for the following sociodemographic characteristics: Family profile, father or mother education, and father or mother income per month. After these exclusions, 2115 participants were included in the analysis.
A self-administered, structured, anonymous questionnaire was used and administered in English. The questionnaire consisted of four sections. The first section included questions on sociodemographic characteristics such as age, weight, height, age of menarche (>= 12 versus < 12-years-old), family profile (one or both parents dead versus parents alive), parents' occupations (employed versus not), educations (university level versus high school or below) and monthly incomes (>= US$ 2000 versus < US$ 2000). The second part consisted of items describing lifestyle characteristics such as living conditions (in university dormitory versus with a family or others), physical activity (>= 3 times versus < 3 times per week), smoking status (current smokers versus non-smokers and ex-smokers), caffeine and energy drink consumption (yes, no; if yes quantity per week). The third part included questions related to medication use in the past month such as calcium, vitamin D and contraceptive pills, as well as medications used for pain, nausea, vomiting, depression, weight gain, and sleeping pills. The fourth section consisted of 33 questions related to PMS affective and somatic symptoms as well as symptoms that accompany PMS, which are not included in PMS' definition, that were experienced by female university students five days prior to menses in each of the three prior menstrual cycles. These symptoms were abstracted based on those listed within the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) [15]. The participants rated the presence and severity of their symptoms based on a 4-point Likert scale from none (= 0) to mild (= 1) to moderate (= 2) to severe (= 3). Mild symptoms were defined as not limiting the daily activity, while moderate symptoms were considered if marked limitations with regard to daily activity were noted. Severe symptoms were considered if participants were unable to achieve the daily activities without discomfort [16]. Mild symptoms were merged with the category of no symptom in every variable which indicates the absence of the symptom and moderate and severe symptoms were merged together to indicate the presence of the symptom.
The prevalence of PMS, the main outcome variable in this study, was detected according to the diagnostic criteria proposed by the American College of Obstetricians and Gynecologists (ACOG) [2]. The presence of PMS was based on ACOG criteria as defined by females reporting at least 1 affective and 1 somatic symptom during the 5 days before menses over 3 consecutive menstrual cycles. The symptoms must be relieved within 4 days of the onset of menses, without any recurrence until at least cycle day 13 and be present in the absence of any pharmacologic therapy or alcohol use. Participants were asked to record the start date and end date of the symptoms in relation to the onset of menses. Participants who reported moderate or severe symptoms were considered as having PMS. PMS affective domain was defined as having at least one of the following symptoms: Depression, anxiety, angry outbursts, irritability, confusion or the thought to commit suicide during the five days preceding menses in each of the three prior menstrual cycles. PMS somatic domain was defined as having at least one of the following symptoms: Breast tenderness, swelling of extremities, abdominal bloating, fluid retention or headache during the five days preceding menses in each of the three prior menstrual cycles.
Descriptive statistics were performed to determine percentages of the participants' characteristics, PMS, severe PMS, and affective and somatic symptoms that accompany PMS. The associations between PMS versus non-PMS groups and PMS symptoms were assessed by the Chi square test. Then, individual relationships between 1) PMS and the sociodemographic, lifestyle factors, and medication use, and 2) Between PMS, PMS affective domain, and PMS somatic domain and symptoms that accompany PMS were evaluated. Multivariable regression analyses were then conducted in order to evaluate 1) The association between the outcome, PMS, and participants' characteristics while adjusting for potential confounders, 2) PMS/PMS affective domain/PMS somatic domain and symptoms that accompany PMS, which were not included in the PMS definition. All variables having a P-value < 0.05 in the bivariate analysis were included in the multivariable regression analysis. An alpha of 0.05 was used to determine statistical significance. All analyses were performed using SPSS version 22.0 (IBM, Inc, Chicago, IL).
Characteristics of the females who participated in this study (n = 2115) are shown in Table 1. Around 64% had normal BMI, 24% were current smokers, and 61% were attending the Lebanese university. Almost 63% of participants reported PMS. Among them, 42.5% (n = 562) reported severe PMS. Compared to the non-PMS group, PMS group included more obese participants, more females whose parents were dead (one or both), and more females with unemployed fathers. Moreover, PMS participants consumed more caffeine, and used more medications for pain, nausea, vomiting and sleeping pills. The most common affective symptom was angry outbursts (77.4%); while the most common somatic symptom was abdominal bloating (70.8%), as seen in Figure 1.
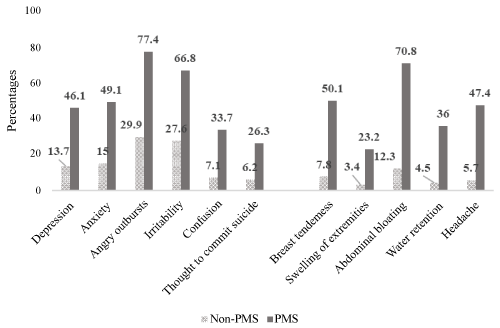 Figure 1: Differences in PMS affective and somatic symptoms (expressed as %) between non-PMS and PMS groups.
Figure 1: Differences in PMS affective and somatic symptoms (expressed as %) between non-PMS and PMS groups.
PMS affective symptoms: Depression, anxiety, anger, irritability, confusion, and the thought to commit suicide respectively.
PMS somatic symptoms: Breast tenderness, swelling of extremities, abdominal bloating, water retention and headache respectively.
P-value for each symptom compared between non-PMS and PMS groups is < 0.001.
View Figure 1
Table 1: Frequencies of sample characteristics for all participants and by PMS status. View Table 1
The following factors were related to having PMS: Obesity [adjusted (a) OR (95% CI) = 1.56 (1.10; 2.20) and 1.92 (1.20; 3.06) for overweight and obese participants respectively], having unemployed fathers [0.65 (0.42; 0.99)], and 0.65 (0.42; 0.99) for an income of US$ < 2000 and >= 2000 per month versus none respectively, caffeine consumption [1.39 (1.11; 1.73), 1.86 (1.48; 2.35) and 3.81 (2.04; 7.12) for 1 cup, 2-3 cups and >= 3 cups per day respectively], and medication use for pain [1.34 (1.11; 1.62)], and for sleeping pills [2.15 (1.23; 3.76)] (Table 2). The majority of participants reported experiencing mood swings, emotional lability and craving sweets as premenstrual symptoms not part of the PMS definition, regardless of PMS status (Table 3). The following factors were associated with PMS affective domains: studying in the humanities or business faculties [1.47 (1.16; 1.88)], caffeine consumption [1.50 (1.16; 1.93), 2.03 (1.55; 2.66) and 16.6 (3.89; 71.0) for 1 cup, 2-3 cups and >= 3 cups per day, respectively], and pain medication use [1.70 (1.36; 2.13)]. Factors associated with PMS somatic domain were BMI [1.43 (1.08; 1.90), 2.16 (1.49; 3.12) and 2.01 (1.22; 3.29) for normal, overweight and obese participants], family profile [2.61 (1.38; 4.94) for having one or both parents dead], caffeine consumption [1.33 (1.05; 1.68), 1.46 (1.14; 1.86) and 2.70 (1.43; 5.08) for 1 cup, 2-3 cups and >= 3 cups per day, respectively], and medication use for pain [1.24 (1.01; 1.51)] and for nausea [1.46 (1.03; 2.07)] (Table 4). Mood swings [(a) OR (95% CI) = 3.77 (2.98; 4.76)], weight gain, [3.08 (2.29; 4.14)], and dizziness [2.81 (1.84; 4.29)] showed the strongest associations with PMS; while mood swings [9.65 (7.13; 13.0)], forgetfulness [4.85 (2.61; 9.03)], and crying easily [3.22 (2.29; 4.52)] showed the strongest associations with PMS affective domains. On the other hand, PMS somatic domains were mostly associated with weight gain [3.83 (2.73; 5.38)], dizziness [2.42 (1.54; 3.78)] and constipation [2.04 (1.33; 3.14)], as outlined in Table 5.
Table 2: Unadjusted and adjusted associations between sociodemographic, health behavior and health related factors and PMS among premenopausal females in Lebanon. View Table 2
Table 3: Unadjusted and adjusted associations between sociodemographic, health behavior and health related factors and PMS among premenopausal females in Lebanon. View Table 3
Table 4: Unadjusted and adjusted associations between sociodemographic, health behavior and health related factors and PMS affective and somatic domains. View Table 4
Table 5: Unadjusted and adjusted associations between symptoms that accompany PMS, presence of PMS, affective and somatic domains. View Table 5
This study identified the proportion, severity and PMS associated factors among female university students in Lebanon and explored both the affective and somatic PMS domains, as well as the symptoms that accompany PMS. The findings showed that PMS is present in 62.5% of participants. Moreover, the factors positively related to PMS included obesity, having unemployed fathers, caffeine consumption, and medication use for pain and sleeping pills. The factors associated with PMS affective domains were majoring in humanities or business, caffeine consumption, and medication use for pain, while those associated with PMS somatic domain were obesity, having one or both parents dead, caffeine consumption, and medication use for pain and nausea.
A high proportion of participants reported having PMS (62.5%). Previously, a brief communication published in Lebanon showed a prevalence of 7.1% among medical students and medical health caregivers [12]. However, differences in sample size and study population prevent any direct between-study comparison. The variation in PMS prevalence within and between countries might be due to diagnostic criteria and assessment tools. Additional factors include cultural differences, types of studied populations, data collection methods used, and participants' honesty in reporting their symptoms [1,17,18]. Previous research have reported a wide range of PMS prevalence in various countries from 12% in France to 98% in Iran [6]. In the surrounding countries, PMS prevalence was found to be 35.6% in Saudi Arabia among medical students [19], 65% in Egypt among adolescents [20], and 72.1% in Turkey among Medical students [17]. Choi, et al. [21] indicated a PMS prevalence of 98.6%, 32.1% and 2.8% in a sample of 1000 women according to International Classification of Disease (ICD-10), ACOG, and DSM-IV criteria, respectively. Over 40% of the participants with PMS reported having severe symptoms. A previous study conducted among medical students found that severe symptoms were reported by 22.4% of the participants (with a PMS prevalence of 35.6%) [19] However, the diverse study groups and the different methods used in estimating the symptoms severity prevent the between-studies comparison. Nevertheless, we think that the high rate we obtained is mainly due to the students' good perceptions of their symptoms.
The most frequent affective symptoms were angry outbursts (77.4%) and irritability (66.8%) and the most common somatic symptoms were abdominal bloating (70.8%) and breast tenderness (50.1%). The most common symptoms reported here are consistent for diverse study groups such as medical students [10], medical students and medical health caregivers [12], or high school adolescents [16], which confirm our results. Other studies have reported that the most common symptoms among 15 to 19-years-old adolescents were difficulty concentrating, fatigue or lack of energy, and food cravings [7], poor individual work performance [16], and backaches [17]. Moreover, the present study found that symptoms that mostly accompany PMS affective domain were mood swings, forgetfulness, and easily crying. In addition, weight gain, dizziness and constipation were found to be mostly related to PMS somatic domain. To our knowledge, affective and somatic domains were not previously explored, thereby preventing comparison.
PMS etiology is multifactorial [17,22,23]. PMS was positively associated with increasing BMI in this study. A cross-sectional study conducted among 874 women aged 18 to 44 reported that obese women had almost a three-fold increased risk for PMS compare to underweight women [24]. Furthermore, another prospective study conducted among women free from PMS at baseline (n = 1057) and aged 27 to 44-years-old found that obese women at baseline had significantly higher risks of developing PMS over 10 years of follow-up [25]. It is thought that obesity modifies neurotransmitter function through its effect on estrogen and progesterone. Estrogen enhances serotonin action by increasing its synthesis, transport, reuptake and receptor expression, and postsynaptic responsiveness. Therefore, lower estradiol levels associated with adiposity may lead to impaired serotonin function and contribute to the occurrence of PMS [25].
A dose-response relationship between PMS and caffeine consumption was found. Our results are in concordance with previous research reporting that PMS is significantly high in students who consume 2 cups of coffee or above per day [17,26]. The reason behind this finding is that caffeine is a stimulant and it increases stress and emotional lability [27]. Moreover, this study found that students whose fathers have no income were more likely to have PMS. A similar result was reported by Balaha, et al. [28] who found that PMS was significantly higher in medical students with a rural residence, a proxy for a low socioeconomic status. Employment, education, and income level are indicators of perceived economic security as well as social and emotional stress. It has been suggested that psychological stress affects ovarian function and therefore, hormonal changes, via responses from the hypothalamic-pituitary-adrenal axis. Furthermore, our results indicated that pain medication and sleeping pill intake were positively associated with having PMS. These findings reflect a lack of professional medical consultation which might be replaced by self-medication mainly the use of pain and sleeping pills. The association between smoking status and PMS is inconsistent. This study did not find an association with smoking. Moreover, we did not find any association with exercise, similarly to Buddhabunyakan, et al. [16].
This study has several strengths. This is the first study in Lebanon to examine the factors related with PMS and both of its symptom domains. The questionnaire used included parts of validated instruments, thereby reducing the possibility of misclassification of symptoms. However, some limitations are present. The study is a cross-sectional one, therefore any temporal relationship between the factors examined and the outcome cannot be established. Moreover, affective and somatic symptoms were self-reported, which might include the possibility of information bias and a possible misclassification of symptoms severity. In addition, the sample collected was a convenient one, preventing generalization of our results to the female population in Lebanon.
In summary, the proportion of Lebanese female university students with PMS, and the affective and somatic symptoms experienced are notably high, and are in the continuum of rates reported from developed and developing countries. The findings of this study have important implications for PMS screening and management, as they highlight the importance of engaging in healthy behaviors such as weight management to minimize PMS symptoms and enhance quality of life. It is worthwhile to note that Lebanon has continuously been facing insecurity and political conflict. These factors are not present in countries where other studies have been conducted and such contextual factors are expected to have an adverse effect on mental health and so, any intervention that targets Lebanese females should be tailored to the environment and barriers present. More studies are needed to fully explore the factors related to PMS and its symptoms in Lebanon. Future studies that include objective measurement of dietary factors, exercise, sleep habits, and psychological factors as well as a calendar recording of symptoms for 90 consecutive days.
Assaad S contributed to hypothesis conception, study design, study logistics, and data collection. Akiki Z contributed to the analysis, interpretation, drafting and write up of the paper. Costanian C contributed towards study design, hypothesis conception, data analysis and interpretation, and manuscript drafting. Daou S and Rabah Z contributed to literature review, data collection, and study logistics. All authors provided critical insight, and revisions to the manuscript; all authors read and approved the final version of the manuscript submitted for publication.
The authors wish to thank the study participants.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
None to declare.
The study was reviewed and approved by the Lebanese University Institutional Review Board.