Dynamic conditions stimulate the bone remodeling process by improving the nutrients transport and increasing the expression of osteogenic cells. The purpose of this study was to evaluate the effect of mechanical stimulation on the osteogenic differentiation of human adipose-derived stromal cells.
Cells were cultured under static and dynamic conditions in collagen scaffolds for 14 days. The mechanical stimulation was performed using a biaxial rotating bioreactor (5 × rpm and perfusion flow rate of 10 × rpm). Cell viability was analyzed with a living cell count and a MTT assay. Changes in expression of specific stem cell marker, osteogenic marker and endothelial markers were analyzed on gene (RT-qPCR) and protein (IHC) level. Data were statistically analyzed by one-way ANOVA (p = 0.05).
Cell viability was higher under dynamic condition and cells migrated deeper in the collagen matrix. Expression of stem cell marker (ANPEP/CD13, CD44, THY1/CD90) was significant higher under dynamic condition. This was also observed for osteogenic markers (collagen 1, osteopontin, osteonectin).
The mechanical stimulation increased significantly cell viability and differentiation potential of human adipose-derived stromal cells.
hADSC, Dynamic stress, Expression profile, Biaxial bioreactor
Bone fracture defects require an interventional treatment which is dependent from the size of the injured site: Whereas smaller bone defects may be spontaneously solved and comprise minimal intervention approaches, larger bone defects usually require the use of replacement material in order to facilitate the healing process [1,2]. Although autologous bone grafting is the gold-standard to facilitate bone repair, the approach is limited due to the low availability of bone material and morbidity of the donor-site. In order to overcome these limitations, tissue engineering approaches have been developed and have been shown a rapidly growth on the field of regenerative medicine. The technique simulates the physiological process of bone repair by using a combination of osteoprogenitors, cytokines and bioactive carriers. In this sense, the use of stem cells is the start point to stimulate osteogenesis [3-8].
Mesenchymal stem cells play an important role in tissue engineering approaches due to their capacity to differentiate in multiple cell lineages [2,9]. These cells may be acquired from different sources, and their differentiation potential depends on the donation site [10-12]. For the treatment of bone defects, mesenchymal cells are typically harvested from the bone marrow [13-15], which is considered a painful and time-consuming procedure. Furthermore, it is associated with a risk of cell contamination and graft loss [16].
In this regard, human adipose-derived stem cells (hADSCs) present some advantages due to their abundant source of osteoprogenitors, easy accessibility and the large quantity of donor tissue available [16-19]. Although the osteogenic capacity of hADSCs seems to be lower in comparison to bone marrow-derived stem cells [20], intrinsic and extrinsic inducing factors, as an osteogenic medium or mechanical vibration, are used to stimulate the osteogenesis [21-26].
Mechanical vibration by means of bioreactors is able to simulate the physiological movements occurred in vivo during the bone remodeling by mimic in vitro physiological mechanostimulation [27]. Under dynamic culture, there is an improvement on the nutrients transport, associated with an increase on the expression of osteogenic cells, favoring the bone remodeling processes [28]. Although this method seems to be effective to enhance proliferation and differentiation of hADSCs [29], the protocol usually comprises the use of an osteogenic medium [30,31], and a possible synergistic effect cannot be excluded. Thus, the mechanism by which the mechanical vibration alone stimulates the bone regeneration is not yet fully understood. The present study aimed to evaluate whether the mechanical vibration would be able to stimulate bone formation without using an osteogenic medium.
In order to evaluate the effect of dynamic stimulation on hADSCs, cells were cultured during 14 days in static or dynamic conditions without using osteogenic inducing factors. Cell proliferation and differentiation analysis was performed after 7 and 14 days of culture.
Human subcutaneous fat tissue was collected under sterile conditions from the leftover tissue of patients submitted to elective abdominal surgery on the General and Visceral Surgery, University Hospital Muenster, Germany. Isolation and cell growth were described previously [11].
The constant conditions (37 ℃, 5% CO2 and 95% humidity) were kept during the whole experimental period. Cell culture medium was alpha eagle minimal medium (α-MEM, Lonza, Switzerland) containing 10% fetal bovine serum (10%), amphotericin B, glutamine, and penicillin/streptomycin (each 1%; all Biochrom Merck, Germany).
Collagen punches with a diameter of 10 mm were cut from a collagen membrane ("PARASORB® Fleece HD", Resorba Medical GmbH, Germany) under sterile conditions and used as scaffolds. Each scaffold was placed into a 24-well culture plate (TPP, Switzerland) and fixed on the well bottom with 400 µl low melt agarose (4% in α-MEM, Plaque Agarose, Biozym, Germany) during 24 h. hADSC were seeded with 1 × 105 cells onto each collagen scaffold, and incubated for initial incubation for 4 days.
After the initial culture, the hADSC were randomly divided into two experimental groups based on static and dynamic conditions. For the static condition, the cells remained in 24-well culture plates for the following 10 days. For the dynamic condition, after the initial incubation, the cells were submitted to mechanical vibration during the same period (10 days).
The dynamic condition was simulated by a biaxial rotation bioreactor ("TisXell Regeneration System"; Quintech Life Science; Singapore) filled with 700 ml of α-MEM Running conditions for the TisXell Regneration System bioreactor were determined with a biaxial rotating of 5 × rpm and a perfusion flow rate of 10 × rpm [31]. Cell culture part was repeated three times.
Living cell count was performed with the CASY1 cell counter (Schärfe System, Germany) according to manufacture protocol. Proliferation rate were estimated with an in-house MTT assay. The conversion of the yellow thiazolyl blue tetrazolium bromit (0.5 mg/ml; Sigma-Aldrich, Germany) to the purple formazan was measured at λ 570 nm. All assays were performed according to the manufacture protocols and done in triplicates.
The analysis of the mRNA-expression of osteogenic and endothelial markers was performed by RT-PCR. Total RNA was extracted and purified by the "miRNeasy Mini Kit" (Qiagen, Germany). Purity and concentration of the isolated total RNA was determined by a spectrophotometric reading (NanoDrop™ 2000; ThermoFisher Scientific, Germany). Genomic DNA contamination was reduced by a DNase I treatment (ZeroBase™ DNase I; epicenter, Germany) followed by a cDNA synthesis (1 µg RNA; MMLV Reverse Transcriptase 1st strand cDNA synthesis kit; epicenter, Germany). cDNA was amplified on Mastercycler® RealPlex S4 (Eppendorf, Germany) using commercially available primers in triplicates (Eurofins Genomics GmbH, Germany) and the DyNAmo Color Flash SYBR Green qPCR Kit (Biozym; Germany). Primers are listed in Table 1. PCR conditions were: Initial denaturation at 95 ℃ during 7 minutes, followed by 45 cycles of denaturation at 95 ℃ for 10 s, annealing at 54 ℃ for 15 s and extension at 72 ℃ for 20 s. Analysis was performed using the Mastercycler® software (Eppendorf, Germany).
Table 1: Primers used for RT-qPCR. View Table 1
In order to determine the protein expression of specific stem cell markers, osteogenic markers, and endothelial markers, an immunohistochemistry was performed. hADSC collagen scaffolds were fixed in 4% of buffered formalin (Fisher Scientific UK limited, UK) for 1 h and embedded immediately in HistoGel (Thermo Scientific, Germany). Samples were watered and dehydrated through an increasing grades ethanol solution. After, the samples were transferred to cedar wood oil (Merck, Germany) during 24 h and into warm 1:1 paraffin-cedar wood oil mixture for 48 h, followed by warm paraffin (Paraplast Plus; Tyco Healthcare Group LP, USA) for 72 h. After cooling down for 24 h at -20 ℃, samples were embedded into fresh paraffin and sectioned with a microtome (Leica Microsystems GmbH, Germany). Sections were mounted onto slides one day before staining. Afterwards, they were deparaffinized in xylene and rehydrated through decreasing grades ethanol solution.
The following primary monoclonal antibodies from mouse were tested: CD13 (clone WM 15, dilution 1:100, Thermo Fischer Scientific, USA), CD31 (clone JC70A; dilution 1:20; Dako, Germany), CD34 (clone QBEnd/10, dilution 1:100; EMD Millipore, USA), CD44 (clone A3D8, dilution 1:100; Sigma Aldrich, Germany), CD90 (clone AF-9; dilution 1:50; Thermo Fischer Scientific, USA), and osteocalcin (clone OC4-30; dilution 1:50; TaKaRa, Japan). In addition, primary polyclonal antibodies acquired from rabbit were evaluated: osteonectin (dilution 1:200; Merck, Germany), osteopontin (dilution 1:100; Merck, Germany), and vWF (#F3520, dilution 1:1600, Sigma Aldrich, Germany). Dako "REAL™" Detection Kit was used for secondary antibody detection (Dako, Germany). Haematoxylin was used for counter-staining (Sigma-Aldrich, Germany). Negative as well as positive controls were implemented according to manufacturer's protocols. Staining results were summarized in a semi quantitative score (IRS) defined as: 0 = no staining, 1 = staining in less than 20% of cells, 2 = staining in 21 to 50% of cells, 3 = staining in 51 to 90% of cells, and 4 = staining in more than 90% of cells. Six slices were analyzed for each sample and for each antibody. Samples were analyzed by three well-trained professionals with experience in histochemical techniques and analysis.
The expression factors and IRS score were evaluated by one-way ANOVA with a modified Levene test. PostHoc analysis was performed with Bonferroni-Holm test. Statistical significance was set at p < 0.05. The statistical analyses were performed using the software Daniel's XL Toolbox version 6.53, available at http://xltoolbox.sourceforge.net.
Figure 1 shows the results for the living cell count and proliferation (MTT). The growth rate is two times higher in dynamic culturing conditions, whereas the functional activity is 1.2 to 1.5 times higher under dynamic conditions in comparison to static conditions. The dynamic condition showed a statistically significant difference between dynamic and static condition in living cell count (p = 0.034) and proliferation rate (p = 0.013). Furthermore, the cells migrated deeper into scaffold matrix under dynamic conditions, as shown on Figure 2. Conversely, under static conditions, cells were arranged mostly at the matrix surface.
 Figure 1: Living cell count (p = 0.034) and MTT (p = 0.013) [light bar = static cultivation; dark bar = dynamic cultivation; standard abbreviation as error marks; at day 4 only information for static cultivation existing]. View Figure 1
Figure 1: Living cell count (p = 0.034) and MTT (p = 0.013) [light bar = static cultivation; dark bar = dynamic cultivation; standard abbreviation as error marks; at day 4 only information for static cultivation existing]. View Figure 1
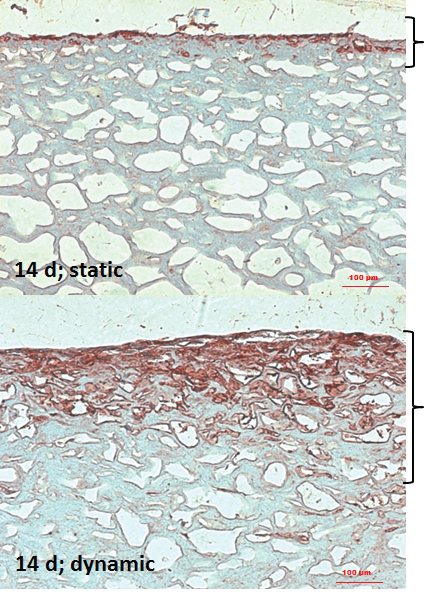 Figure 2: IHC of osteopontin in static and dynamic culturing conditions at day 14 (bold bracket = growth area; 100-fold magnification; size bar 100 µm). View Figure 2
Figure 2: IHC of osteopontin in static and dynamic culturing conditions at day 14 (bold bracket = growth area; 100-fold magnification; size bar 100 µm). View Figure 2
The gene expressions of stem cell, osteogenic and endothelial markers are summarized in Figure 3. The statistical analysis showed a significance for stem cell markers (p = 0.021) and endothelial markers (p = 0.043). A Posthoc test was accomplished for gene expression and showed statistical significance among each other with at least p = 0.05 (asterisks in Figure 3). For osteogenic marker, there was no significant in one-way ANOVA. Nevertheless, gene expression of stem cell and osteogenic markers increased along the time under dynamic culturing conditions and decreased under static culturing conditions (Figure 3A and Figure 3B). The alteration in gene expression for all analysed genes referring to incubation time were significant with p = 0.000078 and alteration at day 14 under dynamic conditions were significantly higher compared to day 10 and day 4 under static and dynamic condition with at least p = 0.008. The gene expression of BGLAP (osteocalcin) and PECAM 1 (CD31) was not observed, neither under static as under dynamic culturing conditions. Expression of CD34 and vWF showed slightly effects between static and dynamic culturing conditions (Figure 3C).
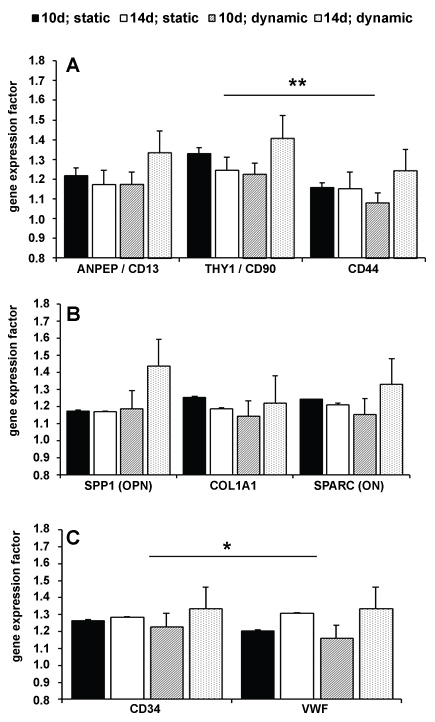 Figure 3: Gene expression factors of stem cell markers [A, p = 0.021], osteogenic markers [B], and endothelial markers [C, p = 0.043] (black & white bars = static cultivation at day 10 and day 14; dark & light spots = dynamic cultivation at day 10 and day 14; error marks = standard abbreviation). View Figure 3
Figure 3: Gene expression factors of stem cell markers [A, p = 0.021], osteogenic markers [B], and endothelial markers [C, p = 0.043] (black & white bars = static cultivation at day 10 and day 14; dark & light spots = dynamic cultivation at day 10 and day 14; error marks = standard abbreviation). View Figure 3
The results of the immunohistochemistry are summarized in Figure 4. The statistical analysis showed a statistical significance for stem cell markers (p = 0.000019), osteogenic (p = 0.013) and endothelial markers (p = 0.029). A Posthoc test was accomplished for protein expression and showed statistical significance among each other with at least p = 0.03 (asterisk in Figure 4). IHC results mostly affirmed results of gene expression analysis. Protein expression of stem cell and osteogenic markers remained constant or decrease under static conditions and increased under dynamic conditions (Table 2). Collagen 1 was not analyzed due to the collagen origin of used scaffolds. As well as in gene expression analysis, osteocalcin was not expressed. Protein expression of endothelial markers also affirmed in parts results of gene expression analysis (Table 2). Expression of CD34 was weak and remained constant or increased. As well as no gene expression of PECAM 1 was observable, expression of CD31 was not detectable. In contrast, expression of vWF was under dynamic condition very weak and it decreased during incubation.
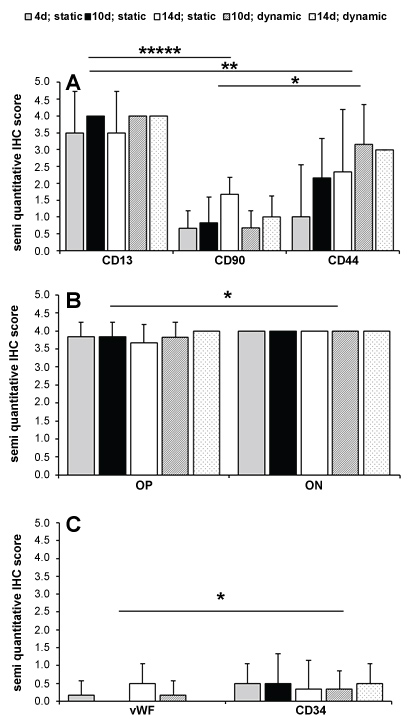 Figure 4: Semi quantitative IHC score of stem cell markers [A, p = 0.000019], osteogenic markers [B, p = 0.013], and endothelial markers [C, p = 0.029] (grey, black & white bars = static cultivation at day 4, 10 and 14; dark & light spots = dynamic cultivation at day 10 and day 14; error marks = standard abbreviations). View Figure 4
Figure 4: Semi quantitative IHC score of stem cell markers [A, p = 0.000019], osteogenic markers [B, p = 0.013], and endothelial markers [C, p = 0.029] (grey, black & white bars = static cultivation at day 4, 10 and 14; dark & light spots = dynamic cultivation at day 10 and day 14; error marks = standard abbreviations). View Figure 4
Table 2: Semi-quantitative IHC score of stem cell markers, osteogenic markers, and endothelial markers (Standard abbreviation in brackets; p = p-value). View Table 2
The basic principle of a dynamic culture system is a change on the cell cytoskeleton [29], improving nutrient transport [28] and enhancing the mechanical signals related with cell proliferation pathways [32]. The perfusion bioreactor used in this study facilitates the fluid of the medium into the scaffold, creating an optimal microenvironment for cell differentiation [28]. However, as it is usually associated with an osteogenic medium, its role on the osteogenesis process is still unknown. The present study aimed to evaluate whether the mechanical vibration by a perfusion reactor is able to induce alone the proliferation and differentiation of hADSCs.
The growth and proliferation rates of stem cells, such as the gene expression and presence of osteogenic markers, were stimulated under dynamic conditions, leading to a better response in comparison with static cultures. In this respect, the use of mechanical vibration seems to increase the effectiveness of cells on the treatment of large bone defects. Interestingly, the current study showed that the mechanical vibration alone was responsible for a higher expression of collagen I, osteopontin and osteonectin. These results corroborate with previous studies, which showed that the use of mechanical vibration alone is able to induce osteogenic differentiation, although this seems to be greater when an osteogenic medium is used [25,29,33]. Pre, et al. claimed that the use of mechanical vibration alone induces osteogenic differentiation, although a culture medium is favorable to induce bone formation. The authors evaluated the effect of high-frequency vibration on hADSCs in osteogenic and proliferative medium [33]. After 28 days, the highest calcium deposition was showed for cells cultured in osteogenic medium under mechanical stimulation. The statement is supported by Woloszyk, et al. who showed that a higher mineralization process was shown in human dental pulp stem cells under dynamic conditions, and that was even more abundant in cultures submitted to both mechanical vibration and osteogenic medium [25]. However, based on findings of the present study, it is not possible to claim whether the use of an osteogenic medium would favor the bone formation, since none comparison was performed considering different medium.
Nonetheless, the hypothesis that the mechanical vibration induces an early differentiation is supported on this study, which showed higher levels of protein expression for the dynamic group after 14 days of culture. According to Pre, et al. the osteogenic differentiation seems to be stimulated on an early stage, decreasing along time after 21 weeks of culture [33]. Considering the short period of analysis from the present study, none prediction could be made regarding the late response of hADSCs.
Likewise, the absence of the expression of osteocalcin in this study is possibly related with the fact that the analysis was performed in 14 days of culture. As osteocalcin is a marker of mature osteogenesis which represents the calcium deposition, it is assumed that its expression could occur on a late-stage differentiation. This statement is supported by previous studies, which showed that the expression of mineralization markers occurs after 14 days of treatment, following the decrease of the proliferation activity [25,33]. According to these studies, the mineralization process seems to be higher for stem cells under dynamic conditions, being even more abundant in cultures submitted to both mechanical vibration and osteogenic medium [25,33]. In a previous study, the osteogenic differentiation and mineralization of hADSC using stimulating factors as dexamethasone, vitamin c and glycerophosphate were shown [11]. In this study, these inducing factors were not added to the culture medium.
Additionally, the low expression of mineralization markers may be related with the absence of differentiation of stem cells into endothelial lineage, which was not evident neither for dynamic conditions nor for the static group. Similar findings were shown for human cells by Jung, et al. on which endothelial lineage on human cells obtained from different types of adipose tissue were weakly expressed [11]. Nonetheless, it is considered that the vascularization of the tissue has an important role on the bone formation. In previous studies, it was showed that the angiogenic differentiation occurs when the stem cells are associated with a culture medium composed with angiogenic factors [34,35]. The bone regeneration requires vascularization and osteogenic factors to initiate bone formation, such as a microenvironment composed by proteins, hormones and growth factors plays an important role on this process [10]. In the study presented by Zhang, et al. the level of mineralization was accompanied by angiogenesis in human adipose-derived stem cells [26].
Adipose-derived stem cells were chosen due to their proliferation capability and osteogenic differentiation potential, such as the acquisition facility by minimally invasive surgical approaches [10,11,36]. Although their potential is improved by under dynamic conditions, the association of mechanical vibration with intrinsic factors may be useful to stimulate the angiogenesis and bone formation.
The mechanical stimulation by means of a bioreactor increased the proliferation and differentiation of hADSCs in comparison to static cultures.
This study was supported by Professor Senninger, the head of the department of Visceral Surgery of the University Hospital Muenster. We also thank QuinXell international GmbH (Muenster, Germany) for providing the TisXell Regeneration System for this study.