Background: To review local chest drain insertions, the indications, use of fibrinolytics, and the success rate of talc pleurodesis in a tertiary hospital in New Zealand.
Methods: This is an observational study of all patients requiring chest drain insertion in the respiratory unit in Christchurch Hospital from January 2015 to December 2016. We analysed patient characteristics, type of drain inserted, and the nature of pleural fluid. We report the success of fibrinolytic therapy for empyema and pleurodesis for malignant pleural effusions.
Results: A total of 486 chest drains were inserted for 333 patients, with a median age of 69 years and the majority of patients were male (60%). The main indications for chest drain insertion were malignant pleural effusions (50.5%), non-malignant pleural fluid (33%) and pneumothorax (17.1%). Most drains inserted were of small caliber; they were 12 French (Fr) (63.4%), central venous catheters to drain the pleural space (14.2%), and indwelling pleural catheters (10.9%). Talc slurry pleurodesis for malignant pleural effusions was used in 40 cases with a success rate of 75%. 22 patients required surgical intervention; 14 had a pneumothorax, 4 had a CPE/empyema and 4 had other indications. In the 41 cases of empyema and complicated parapneumonic effusions (CPE), 29 organisms were cultured. The majority of organisms identified were Streptococcus and Staphylococcus species. Fibrinolytics were used in 26 cases (63.4%) of empyema and CPE.
Conclusion: Small bore catheters were used in Christchurch in keeping with international guidelines. The success rate of talc slurry pleurodesis and fibrinolytic therapy was in keeping with international experience.
Chest drain, Pleural effusion, Parapneumonic, Pneumothorax, Empyema, Pleurodesis, Fibrinolytic therapy
UK: United Kingdom; IPC: Indwelling Pleural Catheters; CPE: Complicated Pleural Effusion; COPD: Chronic Obstructive Pulmonary Disease; ILD: Interstitial Lung Disease; CVC: Central Venous Catheter
Pleural disease and the use of chest drains with adjuvant procedures are increasing worldwide [1]. Pleural disease affects approximately 3,000 per million people in the United Kingdom (UK) [2]. The common indications for chest drain insertion are malignant effusions, pneumothoraces and empyema. Point of care ultrasound guidance has improved safety, diagnostic accuracy and efficacy [3,4].
Pleural procedures are becoming increasingly complex and require a well-supported environment. It is thought that these interventions should be delivered via a dedicated pleural service which our centre has not yet established. Prominent leaders in pleural disease have published an aspirational statement to ensure safe and effective pleural medical services [5]. There is evidence that a dedicated pleural service improves diagnostic efficiency and reduces pleural complications [6]. Many services are led by respiratory physicians with support from the cardio-thoracic surgical department. Therapeutic interventions such as indwelling pleural catheters (IPC), talc pleurodesis and fibrinolytic therapy have become standard techniques and have improved patient symptoms, reduced hospital stay, and decreased the need for surgical interventions.
The aims of this study were twofold. The primary intention was to audit the indications for chest drain insertions in a tertiary centre. The secondary aim was to compare the performance to data from other hospital centres. This study is to ascertain the local need for a dedicated pleural service.
This study was performed in the Respiratory Services at the Canterbury District Health Board. This service provides secondary and tertiary care for approximately 540,000 people in the upper half of the South Island of New Zealand. In Christchurch hospital, chest drains can only be inserted by the Respiratory and Cardio-thoracic surgical services. Generally, the Cardio-thoracic surgical department would insert chest drains for trauma or post cardiac surgery and all other drains would be performed by the Respiratory services.
Only medical chest drains inserted under the Respiratory service were included in this study. This was a retrospective study and according to the Ministry of Health guidelines, did not need ethical review. However, we underwent an internal approval process via discussion with the physician group and the service manager who were supportive of the audit.
Adult patients who had chest drains inserted and admitted to the Respiratory Services from January 2015 to December 2016 were included in this study. Records for chest drain insertion are kept by the Respiratory department. Patient electronic records were reviewed to determine smoking history, immunosuppression, diabetes mellitus, underlying lung disease, type of drain inserted, pleural fluid biochemistry, culture, cytology, pleurodesis, fibrinolytic therapy and surgical intervention. Data was collected through the electronic health care system (Health Connect South, Orion Health Care) which included discharge summaries, clinic letters and community details (Health one). Descriptive statistics were used to present the results.
Empyema was defined by frank pus, culture positive, or gram stain positive. Complicated pleural effusion (CPE) was defined by Lights criteria as an exudate and pH ≤ 7.2. Those with pleural infection secondary to trauma or cardiothoracic surgery were excluded. Those who required surgical intervention was defined as failure of resolution of empyema or CPE despite drain insertion and/or fibrinolytic therapy. Recurrence of malignant pleural fluid was defined as requiring a further chest drain insertion more than 10 days from the previous chest drain. Talc slurry pleurodesis was considered successful when no further chest drain insertion was required on the same side for 10 months or until patient’s death.
A total of 486 chest drains were inserted for 333 patients over two years. The patients were aged between 16-95 years with a median age of 69 years. Most patients were either ex-smokers (46.3%) or current smokers (17.4%).
About a quarter of all patients had background lung diseases including chronic obstructive pulmonary disease (COPD) n = 68 (20.4%), interstitial lung disease (ILD)/restrictive n = 15 (4.5%) and prior pulmonary embolus n = 11 (3.3%).
All pleural effusion drains were inserted under bedside ultrasound guidance. The most commonly used drains were 12 French (Fr) n = 308 (63.4%), central venous catheters (CVC) n = 69 (14.2%), and indwelling pleural catheters (IPC) n = 53 (10.9%) (Figure 1). CVCs are 16Ga which is approximately 5 Fr in caliber. They were used for diagnostic and symptomatic drainage of pleural effusions. Eighteen patients (3.7%) had a drain placed by interventional radiology and unfortunately 2 of these drains were of unknown size. The 24 Fr and 28 Fr drains were inserted with blunt dissection often post thoracoscopy by Respiratory physicians. The main indications for chest drain insertion were malignancy n = 168 (50.5%), non-malignant pleural fluid n = 110 (33%) and pneumothorax n = 57 (17.1%).
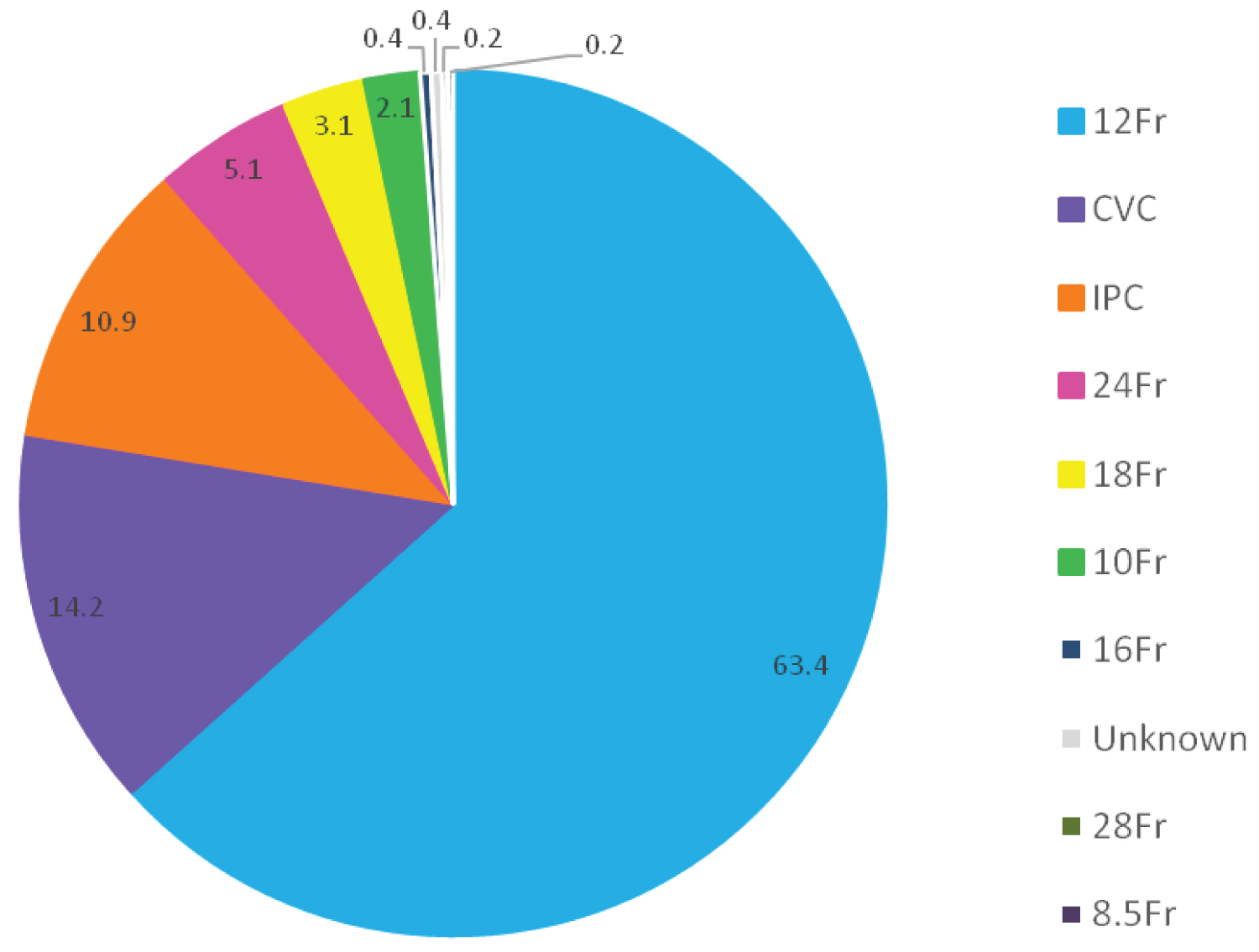 Figure 1: Size of chest drain insertion. CVC: Central venous catheter used as a small-bore chest drain; IPC: Indwelling pleural catheters were mainly used for malignant pleural effusion. All chest drains for pleural effusions were inserted in the clinical space under ultra-sound guidance with the exceptions of the surgical and radiological placed drains.
View Figure 1
Figure 1: Size of chest drain insertion. CVC: Central venous catheter used as a small-bore chest drain; IPC: Indwelling pleural catheters were mainly used for malignant pleural effusion. All chest drains for pleural effusions were inserted in the clinical space under ultra-sound guidance with the exceptions of the surgical and radiological placed drains.
View Figure 1
Malignant effusions occurred in 168 patients. The majority were male n = 88 (52.4%) with a median age of 71 years (Table 1). More than half of the patients were current n = 24 (14.3%) or ex smokers n = 80 (47.6%). The main cancers in our cohort were lung cancer n = 67 (39.9%), breast cancer n = 29 (17.3%), mesothelioma n = 15 (8.9%), and malignancy of unknown origin n = 15 (8.9%) (Figure 2). Talc slurry pleurodesis for malignant pleural effusion was used in 40 (23.8%) cases; the effusion recurred in 10 patients. The success rate was 82.5% at one month and 75% at six and ten months. At the time of the audit, IPCs were only inserted for recurrent pleural effusion, on patients’ request or for trapped lung. A total of 55 patients had recurrence of pleural fluid requiring more than one chest drain.
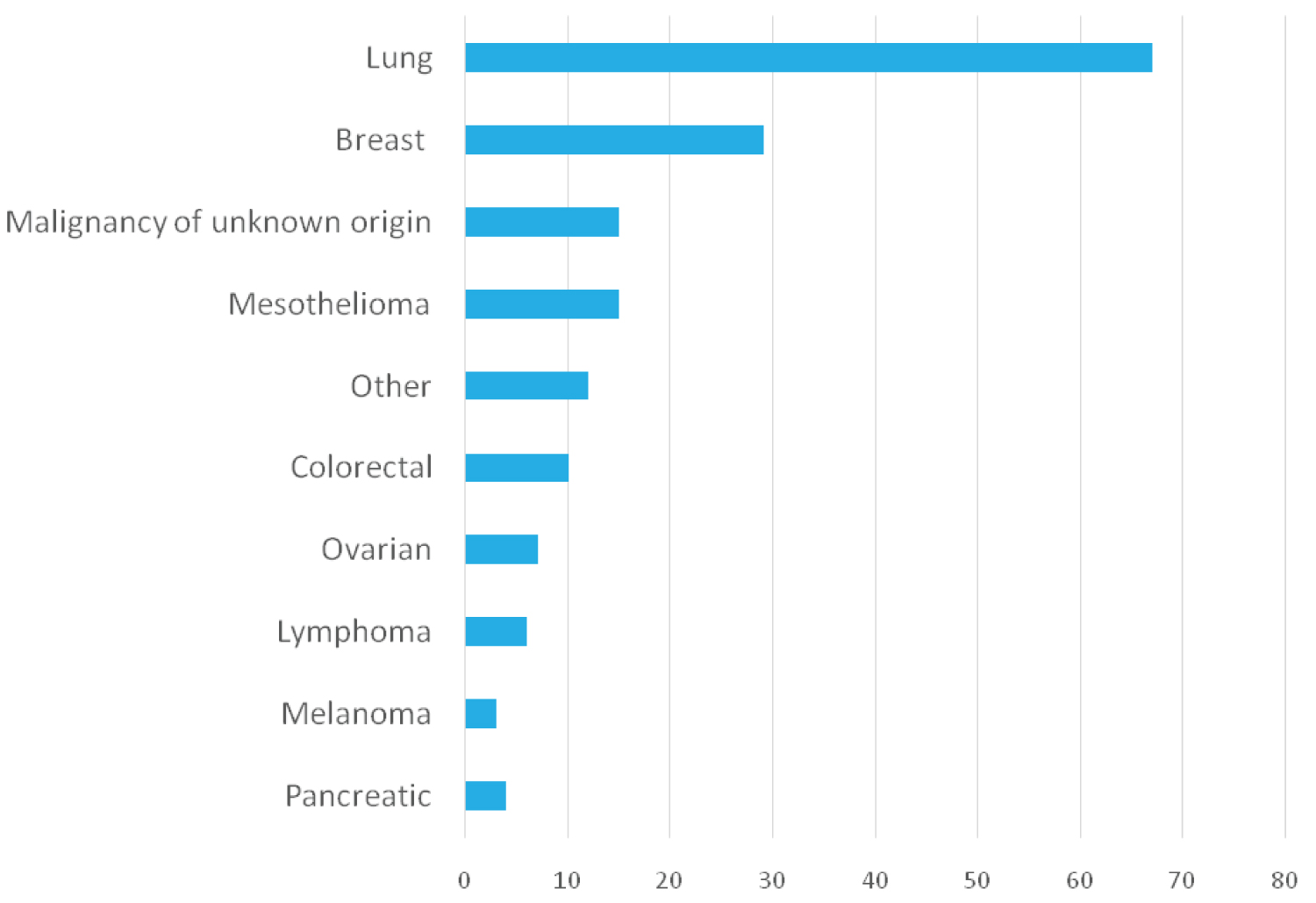 Figure 2: Underlying malignancy causing pleural effusions. Malignant causes of pleural effusion based on 168 patients. Other malignancies included gastric, bladder, cholangiocarcinoma, endometrial, leiomyosarcoma, prostate, renal, tongue, hemangioendothelioma and oesophageal. View Figure 2
Figure 2: Underlying malignancy causing pleural effusions. Malignant causes of pleural effusion based on 168 patients. Other malignancies included gastric, bladder, cholangiocarcinoma, endometrial, leiomyosarcoma, prostate, renal, tongue, hemangioendothelioma and oesophageal. View Figure 2
Table 1: Patients characteristics and medical background. View Table 1
Fifty seven patients had a pneumothorax with a total of 71 chest drains inserted. The majority were male n = 41 (71.9%) and median age was 60 years (Table 1). Most patients were ex smokers n = 24 (42.1%) or current smokers n = 19 (33.3%). Many patients had a history of COPD n = 19 (33.3%) or prior pneumothoraces n = 6 (10.5%). There were two individuals who had a pneumothorax which developed into an empyema/CPE and two patients also had an underlying malignancy. Fourteen patients (24.6%) with pneumothorax subsequently underwent surgical intervention.
Forty one patients had an empyema or CPE with a total of 50 chest drains inserted, of whom 27 patients were male with a median age of 68 years (Table 1). Twenty seven cases had more than 50% polymorphic white cells and gram stain was positive in 17 cases (35.4%). Organisms were cultured in 20 patients (49%) with 29 organisms cultured (Table 2), however antibacterial activity was detected in 17 out of 26 samples (65.4%). The main organisms cultured were Streptococcus intermedius n = 4, Staphylococcus aureus n = 3, and mixed anaerobic flora n = 3 (Table 2). Most samples cultured two or more organisms and seven patients were excluded from this cohort as the organisms cultured were considered to be contaminants. Fibrinolytic therapy was used in 26 out of 41 patients (63.4%).
Table 2: Organisms cultured in complex parapneumonic effusions and empyema. View Table 2
Immunosuppression is an important risk factor for developing an empyema or CPE. A total of thirteen individuals were immunosuppressed with underlying diabetes mellitus n = 3 (7.3%), prednisone n = 3 (7.3%), steroid sparring immunosuppressant n = 1 (2.4%), malignancy n = 2 (4.9%), diabetes mellitus with malignancy n = 3 (7.3%) and prednisone with malignancy n = 1 (2.4%).
Four patients (9.8%) with empyema/CPE required surgical intervention. Two of these patients had prior fibrinolytic therapy. However, two patients went forward to surgery without an attempt at fibrinolysis. One patient had a hydropneumothorax that developed into an empyema on the opposite side. The other patient had a simple parapneumonic effusion biochemically, which later developed into an empyema. He underwent medical thoracoscopy which revealed a multiloculated pleural space thought unlikely to resolve with fibrinolytic therapy alone.
Other effusions (Non-malignant, non empyema/CPE) were detected in 81 patients. The median age was 73 years and patients were predominantly male n = 54 (66.7%) (Table 1). Most chest drains were inserted for simple parapneumonic effusion n = 48 (59.3%), cardiac failure n = 9 (11.1%), and hydropneumothorax n = 8 (9.9%) (Figure 3). As CVC drains were inserted for diagnostic and symptomatic management instead of simple needle aspirates; there were more patients with simple parapneumonic effusions in this study. Four patients with other pleural effusions underwent surgery (two with hydropneumothorax, one extramedullary haematopoiesis, and one rheumatoid arthritis). All patients were inpatient for their pleural interventions.
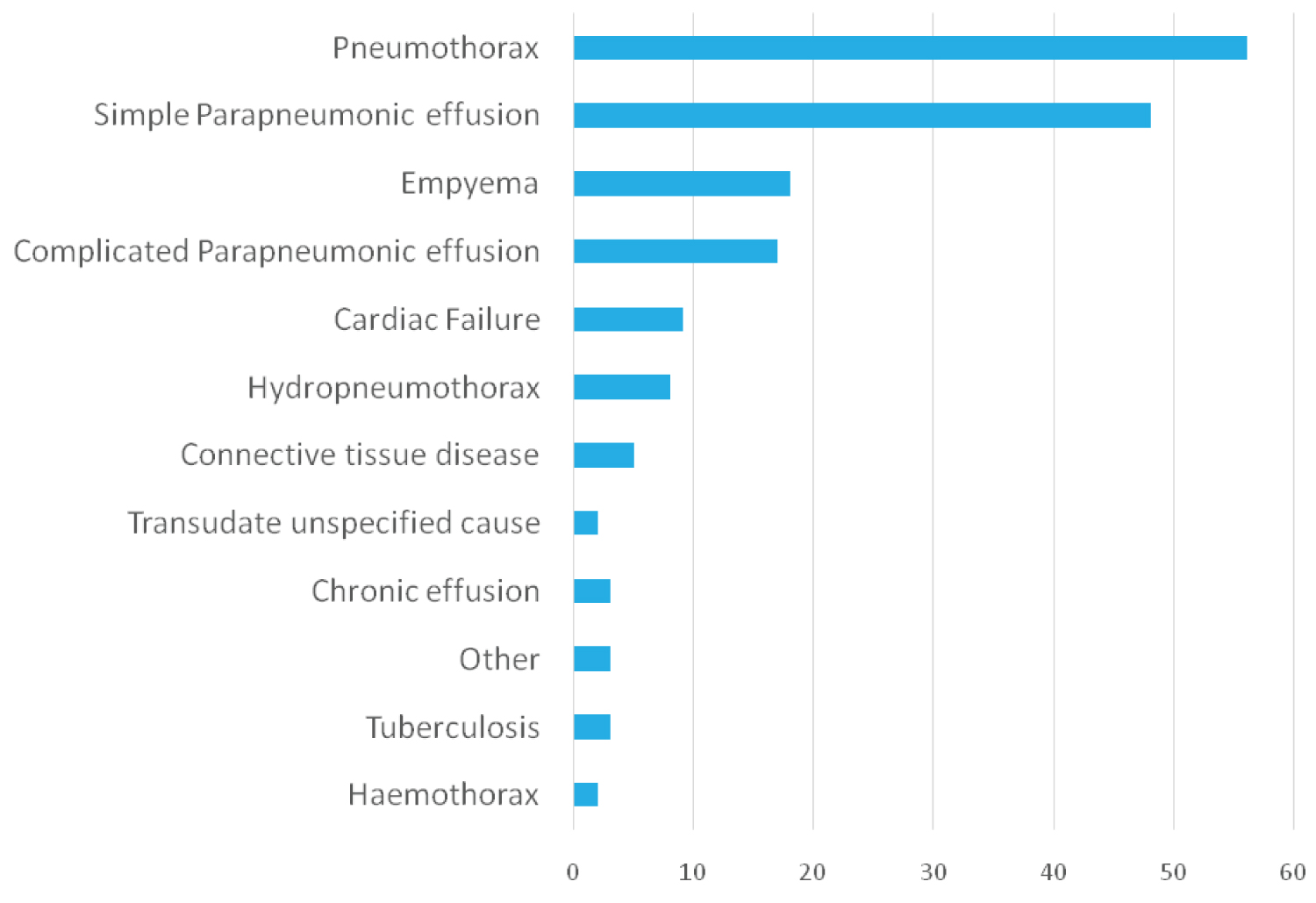 Figure 3: Underlying non-malignant indications for chest drain insertions. Non-malignant indications for chest drain insertion. Other indications included peritoneal dialysis, asbestos related lung disease and reactive pleural effusion. View Figure 3
Figure 3: Underlying non-malignant indications for chest drain insertions. Non-malignant indications for chest drain insertion. Other indications included peritoneal dialysis, asbestos related lung disease and reactive pleural effusion. View Figure 3
This study described the experience of pleural disease management in a single tertiary centre in New Zealand, of 240 chest drains per year. Of these, more than 50% of total chest drain insertions were for malignancy and the majority of drains inserted was 18 Fr or smaller. Lung cancer was the most common malignant cause of pleural fluid in males, while breast cancer was the most common cause for females.
Based on reports from the United States and United Kingdom, a growing number of patients with pleural infection and malignancy can be expected [6]. This may be due to the aging population and associated increased risk of malignancy. To improve efficiency and manage increasing demand for pleural investigations, a dedicated pleural service would be beneficial in Christchurch Hospital. This would require a specialist respiratory physician in pleural disease with a dedicated team including nurses, radiologists, cardiothoracic and pathologists [6]. Access to resources such as a specialist respiratory ward, thoracic ultrasound and thoracoscopy would be required [6]. At the time of the audit Christchurch Hospital did not have a dedicated pleural service.
At the time of this study, talc pleurodesis was generally offered as the initial modality of treatment for recurrent malignant effusion. Our success rate of 82.5% at one month and 75% at six months is comparable to international data which showed talc pleurodesis was successful for 70-75% at one month and less than 50% at six months [7,8].
Indwelling pleural catheter was used after pleurodesis had failed, on patient request or if pleurodesis was judged to have a low chance of success. This could account for the lower numbers of individuals who underwent talc pleurodesis. At the time of the study talc pleurodesis was not used through CVC or IPCs.
Recently, IPC placement has been proposed as the primary treatment modality with the option of pleurodesis as an outpatient via the IPC [9]. Although the rates of infection post drain were not assessed in this study, the international evidence suggests that the risk of infection may be less than 5% [10]. Evidence is also emerging that patients with IPC insertion have a shorter stay in hospital and fewer pleural procedures compared to pleurodesis [7]. It may be possible to reduce the number of chest drain insertions, by offering pleurodesis via an upfront IPC.
Pleural infection still has high morbidity and mortality, often dependent on patient characteristics and the causative organism [11]. Organism identification is important to guide antibiotic treatment. At the time of our study, our centre was not culturing pleural fluid in blood culture bottles to aid microbiological yield. The use of blood culture bottles can increase organism identification by 20 percent [11]. There has since been a change in clinical practice in our centre.
Fibrinolytic therapy was only used in Christchurch if there was a failure to drain. This can be a time-consuming process with demand on nursing staff and doctors being present on the ward for the first installment of fibrinolytics in case of complications. At the time of the study, the standard fibrinolytic therapy used was alteplase (10 mg twice daily) followed by dornase alfa (5 mg twice daily) for a maximum of six doses.
Other New Zealand studies were also reviewing the management of empyema/CPE. Between 1993 and 1995, Lindstorm and Kolbe observed 47 cases of empyema, 59% had an organism identified, fibrinolytic therapy was used in 56% and a surgical intervention was only needed in one case (0.5%) [12]. Epstein and colleagues reported on 65 chest tube placements in 49 patients over a one-year period in 2008-2009 with 27 cases of empyema [13]. No fibrinolysis therapy use was reported and about half of all patients, who had a drain placed for an empyema needed surgical intervention. Wong and Yap reported on 108 patients with a CPE/empyema over a three-year period between Jan 2010 to December 2012 [14]. Their number of empyema patients per year was similar to our number observed (36 vs. 21); so was their yield of identifying an infective organism and their rates of needing surgical interventions was 13% (Table 3) [14].
Table 3: Grouped data of New Zealand patients with chest drain insertion due to empyema/CPE for comparison. View Table 3
The limitations of this study include the retrospective nature of the study and this is a single centre study which may not be representative of New Zealand. The primary aim was to assess the indications for chest drain insertions in Christchurch hospital, so complications of chest drain insertions (mortality and morbidity) was not formally assessed. Therefore, to further assess the benefits of a dedicated pleural service, chest drain complications, length of hospital admission and financial implications will need to be reviewed. When comparing cases in different centres one needs to be mindful of different populations, different services and different time points of the interventions.
This study has reviewed a large number of patients requiring pleural interventions. Fibrinolytics were used for CPE and empyema and only a small number of patients have subsequently required surgical intervention. Our data suggests similar findings in comparison to other centres. Approximately half of the chest drains were inserted for patients with malignancy; with the intention to improve quality of life and decrease length of hospital stay, there may be room for improvement to effectively manage pleural diseases by reducing the number of recurrent effusions. A dedicated pleural service may further improve delivery of care with a focus on outpatient services which is not currently available.
Not applicable.
The authors have no financial or non-financial competing interests.
Y-TL and LB had the initial idea, Y-TL and ZWG performed the data collection and analysis. Y-TL prepared the first draft of the manuscript. All authors read, reviewed and approved the final draft.