Pneumatosis Intestinalis (PI) is a rare post lung transplant complication which is characterized by the presence of gas within the wall of the small and large intestine. PI in lung transplant recipients has been previously described and is associated with disease of varying clinical trajectories. Here we describe a case of PI in a lung transplant recipient that was attributed to immunosuppression with Mycophenolate Mofetil (MMF) as evidenced by the presence of increased crypt epithelial apoptosis on biopsy of the duodenum and clinical improvement with withdrawal of the offending agent. This case demonstrates that medication adverse effects are an important differential to consider in lung transplant recipients with PI.
Pneumatosis intestinalis, Lung transplantation, Immunosuppression, Mycophenolate mofetil, Medication adverse effects
Pneumatosis Intestinalis (PI) is defined as the presence of gas within the bowel wall [1]. This imaging finding is associated with various clinical phenotypes, ranging from benign to life-threatening [2]. It occurs in approximately 0.03% of the general population [3]. PI is often a clinical sign of an underlying pathology and is divided into primary and secondary types. In the primary type, PI is often benign and is associated with gas collection in a cystic pattern. Secondary PI is due to an underlying pathology and is associated with linear collections of gas within the bowel wall [4,5]. The etiologies of PI are numerous and include pulmonary diseases, autoimmune conditions, organ transplantation, intestinal ischemia, and medication adverse effects [1,6]. PI has been previously reported in lung and other solid organ recipients and has been postulated to be associated with immunosuppressive medications such a corticosteroid [7-10]. However, there were no previous reports that directly implicate Mycophenolate Mofetil (MMF) with PI. In this paper, we hope to describe a case of PI in a lung transplant recipient that has been associated with MMF.
A 59-year-old man with a history of Scleroderma related pulmonary fibrosis who underwent bilateral orthotopic lung transplantation presented three months post-transplant with progressively worsening diffuse abdominal pain, nausea, and bloating of 3-day duration. The patient's past medical history was notable for hypertension, hyperlipidemia, Gastroesophageal Reflux Disease (GERD) which was treated with Nissen fundoplication, esophageal dysmotility and Raynaud's phenomenon. At presentation, the patient was on Mycophenolate (Cellcept) 1000 mg suspension twice daily, Tacrolimus 2 mg suspension once daily (trough level > 12 ng/ml on admission), and Prednisone 15 mg suspension once daily for immunosuppression. Computer Tomography (CT) of the abdomen showed large amount of pneumoperitoneum with colonic pneumatosis (Figure 1). The patient underwent emergent exploratory laparotomy which showed extensive pneumatosis of ascending and transverse colon without bowel ischemia or areas of perforation. Symptom as well as radiological findings improved within one week of bowel rest, intravenous fluid administration, temporary cessation of anti-proliferative immunosuppression (MMF), as well as serial abdominal exams. He was discharged and presented, three weeks later, with recurrent abdominal pain, weight loss, and loose dark tarry stools. At the time, he was only taking oxycodone and acetaminophen for pain and was avoiding Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). He was on appropriate post-transplant antimicrobial prophylaxis as well as gastric acid suppression medications. He did not smoke, use illicit substances, or drink alcohol. On arrival to the ED, the patient was afebrile with a heart rate of 64 beats per minute and a blood pressure of 120/87 mmHg, a respiratory rate of 18, and an oxygen saturation of a 100% on room air. He was alert, oriented, and non-toxic. His cardiac examination was notable for a holosystolic murmur at the left upper sternal border. His chest was clear to auscultation. His abdominal examination was notable for distention, increased tympany to percussion, and tenderness at the site of previous exploratory laparotomy. He had a clean, dry incision, without drainage. His Gastro-Jejunal (GJ) tube was in appropriate position. The remainder of his physical exam was normal. On laboratory evaluation, his hemoglobin level was 9.8 mg/dL, WBC count 7500 cells/mcL, and platelets were 247000/mcL. Sodium was 136 mmol/L, potassium 4.5 mmol/L, bicarbonate 31 mmol/L, BUN 30 mg/dL, and creatinine 1.02 mg/dL. Total bilirubin was 0.8 mg/dL, aspartate aminotransferase was 53 u/L, alanine aminotransferase was 43 u/L, alkaline phosphatase was 99 u/L. Serum lactate was 1.3 mmol/L. Tacrolimus level was 6.9 ng/mL. Clostridium difficile toxin assay was negative. EBV and CMV DNA PCR were negative. Abdominal imaging showed progression of previously noted PI and pneumoperitoneum (Figure 2). Gastroenterology colleagues were consulted and performed EGD with duodenal biopsy. The pathology is shown in the images below (Figure 3).
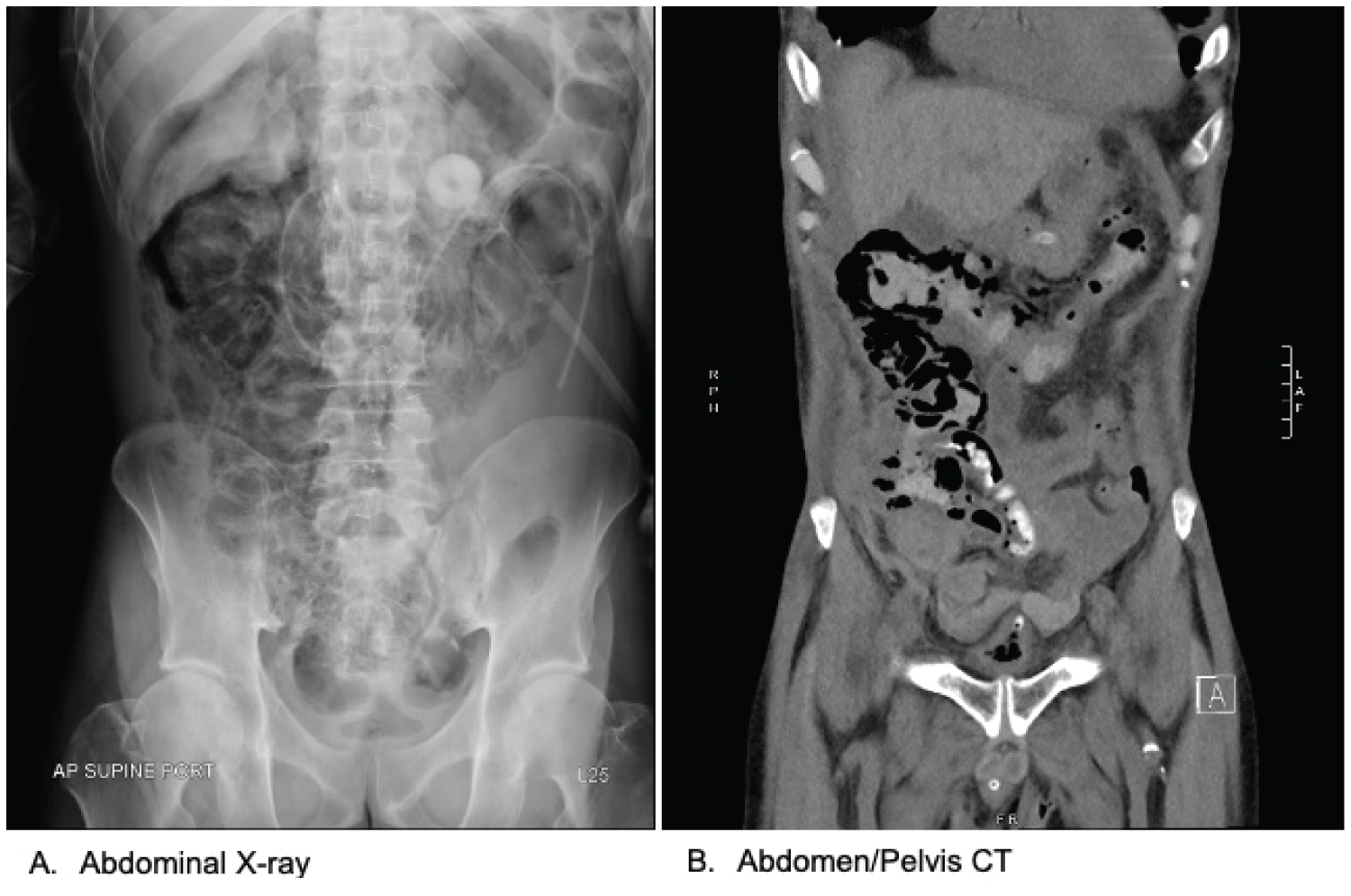 Figure 1: A) Abdominal film demonstrating curvilinear lucencies suggestive of pneumatosis intestinalis; B) Coronal view of CT Abdomen/Pelvis demonstrating pneumatosis intestinalis involving the ascending and transverse colon.
View Figure 1
Figure 1: A) Abdominal film demonstrating curvilinear lucencies suggestive of pneumatosis intestinalis; B) Coronal view of CT Abdomen/Pelvis demonstrating pneumatosis intestinalis involving the ascending and transverse colon.
View Figure 1
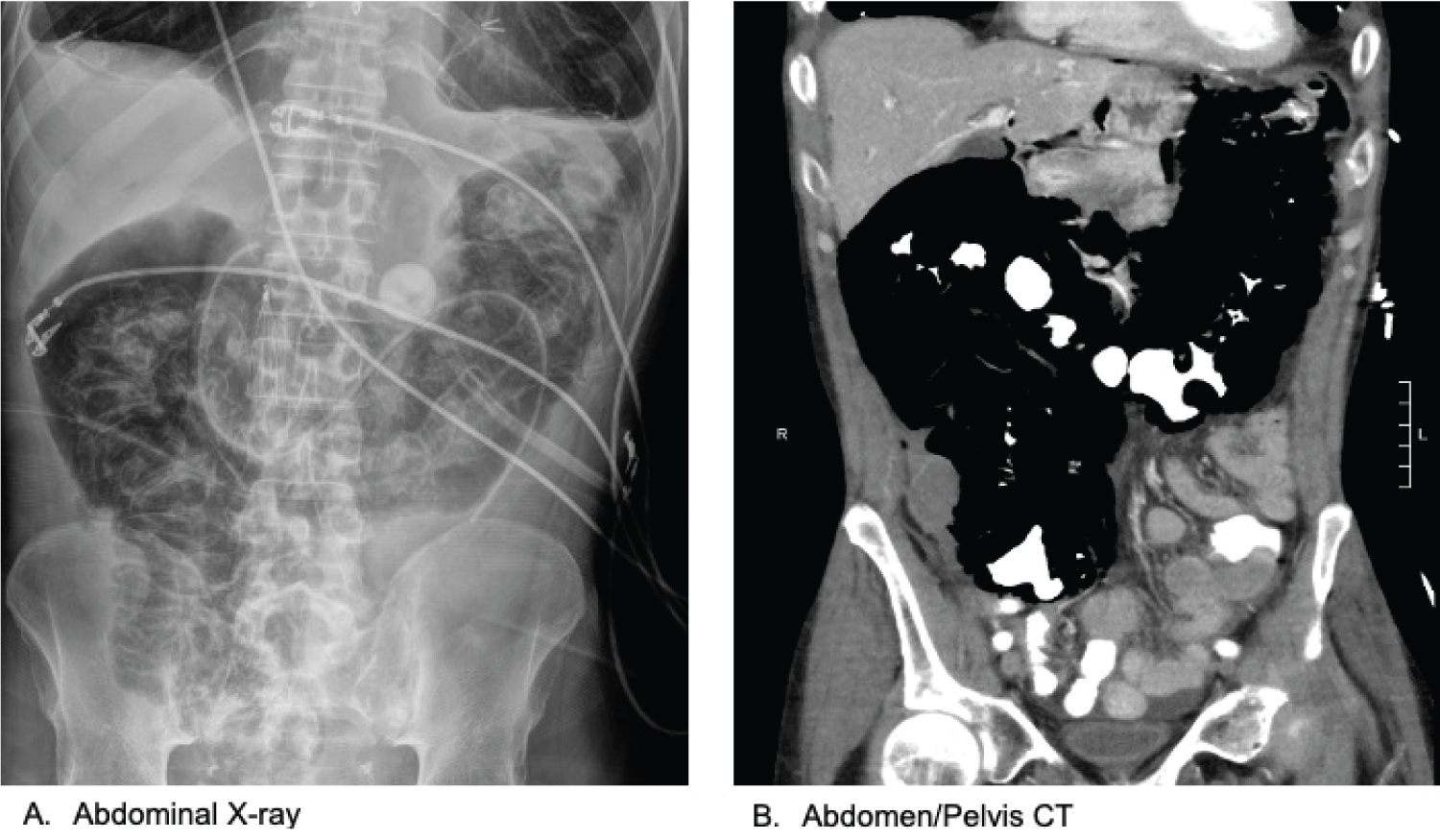 Figure 2: A) Abdominal film demonstrating evidence of significant pneumoperitoneum; B) Coronal view of CT Abdomen/Pelvis demonstrating marked pneumatosis intestinalis now involving the distal ileum and proximal colon and extending into the splenic flexure as well as pneumoperitoneum. There was no extravasation of oral contrast to suggest perforation of viscous.
View Figure 2
Figure 2: A) Abdominal film demonstrating evidence of significant pneumoperitoneum; B) Coronal view of CT Abdomen/Pelvis demonstrating marked pneumatosis intestinalis now involving the distal ileum and proximal colon and extending into the splenic flexure as well as pneumoperitoneum. There was no extravasation of oral contrast to suggest perforation of viscous.
View Figure 2
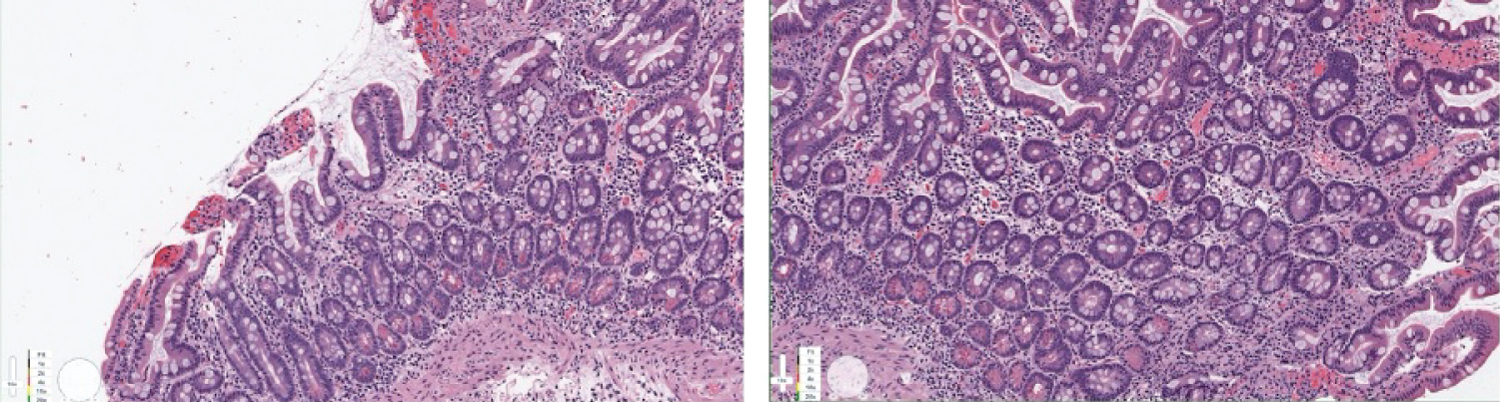 Figure 3: Endoscopic biopsy of duodenal mucosa showing increased crypt epithelial apoptosis indicative of Mycophenolate Mofetil induced PI.
View Figure 3
Figure 3: Endoscopic biopsy of duodenal mucosa showing increased crypt epithelial apoptosis indicative of Mycophenolate Mofetil induced PI.
View Figure 3
PI is defined as the presence of gas within the wall of the small or large intestine [1]. PI in lung transplant recipients has been previously described in the literature and is postulated to be associated with numerous causes with varying clinical trajectories [7-10]. The pathophysiology of PI is not well elucidated in the literature, but it has been hypothesized to be associated with three main theories in solid organ transplant recipients including the mechanical, bacterial or infectious, and the atrophy of Gastrointestinal (GI) lymphoid aggregates theories [10]. The mechanical theory is routed around infiltration of bowel gas through the GI mucosa. This may be due to alveolar rupture in patients with obstructive lung disease or those that are mechanically ventilated with gas dissection into the mediastinum and the intra-abdominal vasculature. The infectious theory is related to gas formation by GI flora or infection which may subsequently diffuse into the GI mucosa. The last theory was thought to be due to atrophy of GI lymphoid aggregates which causes compromised areas in GI mucosa that are more vulnerable to penetration of bowel gas. Often, PI in lung transplant recipients is localized to the large intestine, namely the ascending and transverse colon as seen in our patient [7-9]. Patients can be completely asymptomatic or have GI symptoms which include abdominal pain, distention, bloating, diarrhea, and gastrointestinal bleeding. CT of the abdomen is the most common method of diagnosis [11,12]. In the majority of reported cases, PI was benign and managed conservatively. Features that may be associated with poor prognosis or need for invasive surgical management include an amalgam of clinical, laboratory, and radiological characteristics. These include increased age (> 60 years), a toxic appearing patient with a rigid abdomen, elevated lactate (> 2 mm/L), elevated WBC count (> 12000 cells/mcL), and the presence of portal venous gas on imaging. Therefore, the management of these patients should be dictated by a thoughtful multidisciplinary team of transplant pulmonologist and surgeons who are committed to observing a patient closely for clinical deterioration. Classically, patients are managed with bowel rest, intravenous hydration, possible use of parenteral nutrition, antimicrobials, and the withdrawal of offending medications if suspected. A variety of associations between PI and medications have been documented in the literature and those include corticosteroids, chemotherapeutic agents, tyrosine kinase inhibitors, and certain laxatives [13-15]. MMF is an important maintenance anti-proliferative immunosuppressive medication used in lung transplantation [16]. It is a well-known cause of gastrointestinal adverse effects in solid organ transplant recipients which usually manifests as diarrhea, abdominal pain, nausea, and/or vomiting. In a retrospective review of solid organ transplant recipients on MMF who underwent colonic biopsy, 9% were diagnosed with MMF colitis and 47% had histological evidence of it but no endoscopic findings [17]. These histological features included architectural distortion, apoptosis, and inflammatory infiltration of crypt epithelium; findings that were also isolated in our patient's duodenal biopsy. Although PI was previously described in lung transplant patients, to our knowledge this is first paper that describes a possible association with MMF use.
Upon follow-up, the patient was noted to have improved with a conservative management strategy which included bowel rest, intravenous hydration, and the permanent cessation of MMF. Thereafter, the patient was discharged in a stable condition without recurrence of symptoms. He currently continues to do well two years after incident with subsequent imaging of his abdomen demonstrating resolution of PI.
In summary, PI and pneumoperitoneum are infrequent complications following thoracic organ transplantation. Resolution of symptoms and radiological findings can be protracted. Careful observation and patient selection should be done prior to invasive surgical intervention given that the majority of cases are associated with benign PI. In patients with PI on MMF, this drug should be considered as a causative agent due to its histological features including architectural distortion, apoptosis, and inflammatory infiltration of crypt epithelium. Medication adverse effect should remain on the differential diagnosis of post-transplant complications.
This case report received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.