There is limited information describing features and outcomes of patients requiring Intermediate Respiratory Care Unit (IRCU) hospitalization for COVID19 disease and as of yet, no mechanical or medical treatments have clearly demonstrated efficacy in IRCU.
Demographics and clinical variables on admission, as well as medical and mechanical therapeutic interventions, were extracted from Electronic Clinical Records in 274 SARS-CoV-2 infected patients attending a third level hospital IRCU. Using multivariate logistic regression analysis, variables that best discriminated mortality were obtained. Principal components analysis and a Neural Network (NN) algorithm were applied.
In relation to respiratory support, high-flow oxygen therapy and weaning procedures were associated with survival as were CPAP and non-invasive ventilation with low levels of support among the most severely affected. The IRCU achieved a survival rate of 87.6%, avoided 178 ICU admissions, successfully referred 35% to the ICU, and of these, 94% later survived the weaning phase. Higher mortality incidence was associated with cardiac and respiratory diseases and fever, heart rhythm and blood pressure disturbances. Following analysis of specific therapeutic options Corticoids and Anticoagulants were associated with better outcomes.
The IRCU prevented the collapse of the ICU, allowed for recovered ICU patients to be quickly released from their unit, thus freeing up critical care beds and permitting them to function more effectively and in terms of mortality, achieved good results that did not worsen due to a possible delay in intubation. In addition, we have generated an open-access NN capable of identifying severity predictors of SARS-CoV-2.
SARS-CoV-2 pandemic, Neural network, Artificial intelligence, CPAP, NIMV, High flow oxygen, Cyclosporine, Tocilizumab, Corticosteroids
During the current pandemic, the Intermediate Respiratory Care Unit (IRCU) dealt directly with patients suffering from very severe respiratory failure due to viral pneumonia caused by SARS-CoV-2. Four lines of respiratory therapies were applied: Continuous Positive Airway Pressure (CPAP), Non Invasive Mechanical Ventilation (NIMV) and High Flow Oxygen (HFO) in the acute phase and Invasive Mechanical Ventilation (IMV) during weaning.
We describe the IRCU's experience and results, which was able to quadruple its capacity in less than a week, while being attended by three 24-hour on-duty pulmonologists and supported by six more physicians from other specialties. Regulations and recommendations established through national consensus were followed when treating this entity [1].
Regarding HFO systems, air mixing was quickly eliminated due to hypoxemia. But strict control of oxygen partial pressure was necessary to avoid hyperoxia [2] and to timely identify the existence of refractory shunts that would not respond to oxygen therapy [3]. New procedures were acquired from the ICU to help solve such extreme hypoxia, such as the prone position [4] combined with HFO therapy or NIMV. Lower pressurization rates and pressure supports were used to avoid over ventilation and volutrauma [5] injury (VILI).
Throughout this pandemic, weaning of some patients during the recovery phase has overlapped the hyper acute peak flow phase of acutely ill patients, and the IRCU had to quickly adapt to successfully face these two synchronous situations, implementing two different protocols of action at the same time, which required a high level of organization and excess demand upon medical and nursing staff.
The object of this study is to analyze mortality using direct statistical analysis based on logistic regression and predictive NN analysis. This article also illustrates patients' flow between the ICU and our highly specialized IRCU with more than 20 years of experience [6-8].
A total of 274 patients from January 1st 2020 to May 5th 2020 admitted to a third-level hospital IRCU were included. All patients had confirmed SARS-CoV-2 infection by a positive result upon polymerase chain reaction testing. The local clinical practice guide (Supplementary Table 1) was followed which includes the admission criteria in IRCU or ICU. De-identified medical records were confidentially collected from Microsoft Sql Integration Services (SSIS) reporting database and analyzed using R version 3.5.2 (R Project for Statistical Computing; R Foundation). Approval from the Institutional Ethics Committee was obtained. All methods were carried out in accordance with relevant guidelines and regulations.
Table 1: Comorbidities: Exitus vs. rest 274 patients. Univariate, multivariate logistic regression analysis and Hosmer and Lemeshow test. View Table 1
Demographics, baseline comorbidities, vital signs, use of treatments, either mechanical or medical, and outcomes were extracted from clinical charts and manually revised.
Patients and parameters with a high percentage (> 50%) of missing values were excluded from the analysis. In the remaining patients, missing data was imputed using KNN method (K = 5).
The Neural Network (NN) model was built using the caret R package (https://CRAN.R-project.org/package = caret). We used the "nnet" method, and implemented an automatic selection of: i) The optimal number of units per hidden layer (1 to 5), and ii) The optimal value for the regularization parameter to avoid over-fitting (0.1 to 0.5 in increments of 0.1 units). We used the "two Class Summary" method to compute sensitivity, specificity and the area under the ROC curve. This NN algorithm was performed 10 times over a matrix of re-sampled patients. Re-sampled matrices were composed of patients with severe status (outcome = 1) and a random selection of the same number of patients with less severe status (outcome = 0). For each re-sampled matrix, we performed a 10-fold cross validation where 90% of patients we randomly used as the training dataset and the remaining (10%) as the testing dataset. A total of 100 NN models were performed and a ROC curve was calculated for the set of predictions and real values using 10-fold cross-validation. The final AUC, accuracy, sensitivity and specificity were calculated as the mean of the 100 NN models performed in total. Our R script is available at: https://github.com/pminguez/MachineLearning4UnbalancedData/blob/master/nn4covid19.R
Principal Component Analysis (PCA) was performed on the matrices with missing data imputation, centered and scaled, using the prcomp and factoextra R libraries. Elipses represent 95% of the data. PCA analyses were performed over the same scenarios chosen to perform the NN.
For binary type of variables, which included comorbidities, treatments and gender, odds ratios for each of the two severity classes in each comparison were calculated, contingency 2 × 2 tables were obtained and significance was calculated with 2-tailed Fisher test. P values were adjusted using FDR and adjusted p values < 0.05 were taken as significant. Graphics were built sorting variables by relative odds ratios (1/odds ratio when < 1).
For non-binary variables comprising vital signs, a non-parametric Wilcoxon rank sum test was performed over the set of values of each variable for the two severity states. P-values were adjusted by FDR and FDR < 0.05 were considered as significant. Means of the two sets of values for each variable was calculated to assign a direction of the test.
First, the univariate logistic regression models were obtained. Then, the best multivariable model was sought. For this, all possible models were adjusted, and the one with the lowest AIC value (Akaike Criterion) was chosen. Finally, the goodness of fit of the model was assessed (Hosmer and Lemeshow Test), and the ROC curve was obtained using the Leave One Out (LOO) cross-validation technique.
Fisher's exact test was performed to investigate the association between the type of respiratory support and mortality. To compare the parameters used with the different techniques and their association with mortality, the student's t test was used. In all cases it was considered that there was statistical significance when p < 0.05. We applied our NN based algorithm to detect treatments predicting success or failure. The Fisher test was applied to binary data while Wilcoxon was used with continuous data.
Supplementary Figure 1 shows epidemiologic data, drugs used and comorbidities of the patients, while Figure 1 describes the dataset, with a global mortality rate of 12.4%, 178 ICU admissions were avoided, 35% of IRCU patients required transfer to the ICU and of these, 96% of them later survived at weaning. Of the patients included, 20.8% were < 50 years, 44.5% were between 50 and 70 and 34.3% were over 70-years-old.
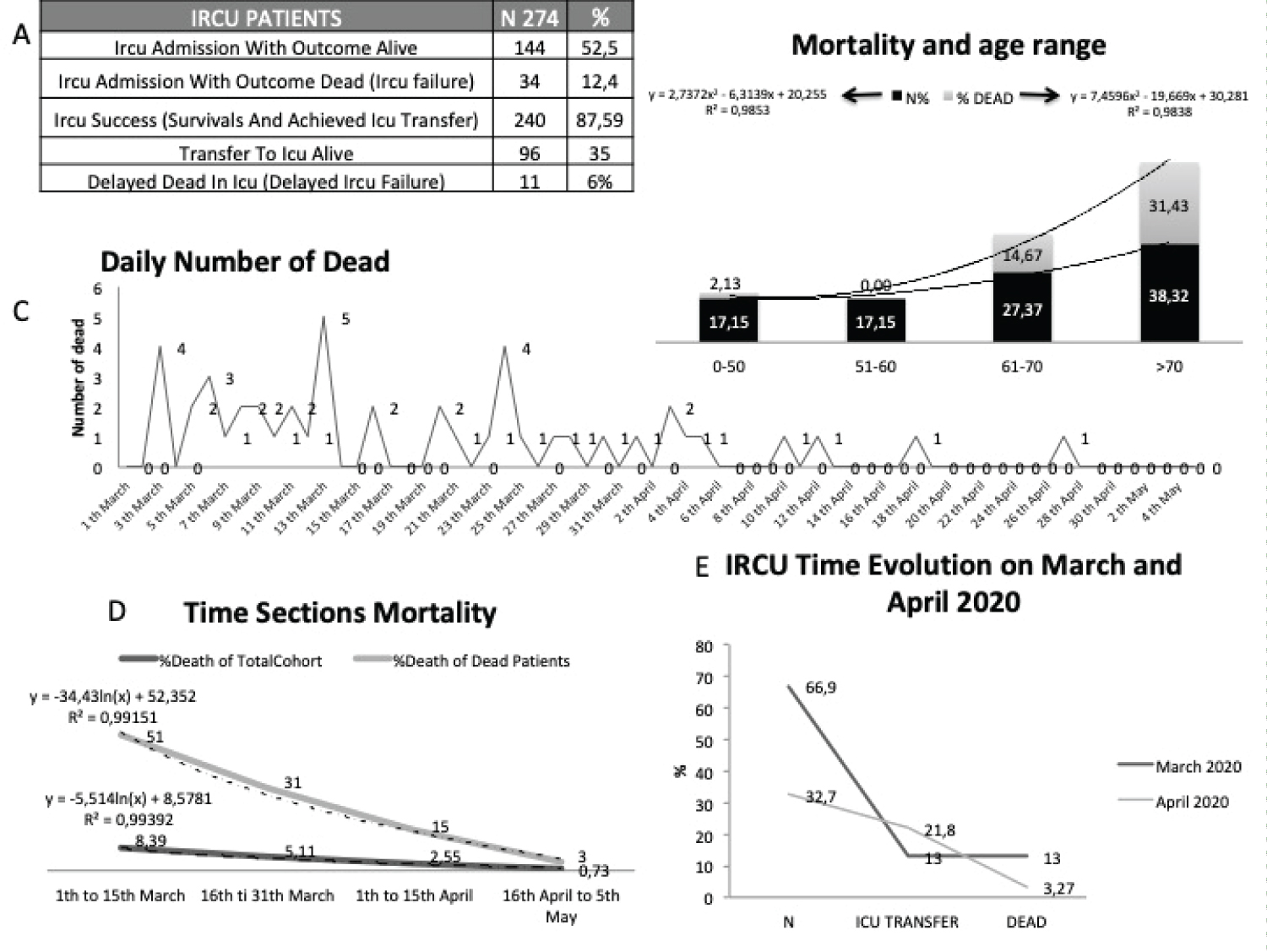 Figure 1: Outcomes: Analysis of 274 patients; A) Outcomes; B) Mortality related to age; C) Daily number of dead in IRCU; D) Time sections mortality from march to may 2020; E) Admissions, ICU transfer and mortality over time.
View Figure 1
Figure 1: Outcomes: Analysis of 274 patients; A) Outcomes; B) Mortality related to age; C) Daily number of dead in IRCU; D) Time sections mortality from march to may 2020; E) Admissions, ICU transfer and mortality over time.
View Figure 1
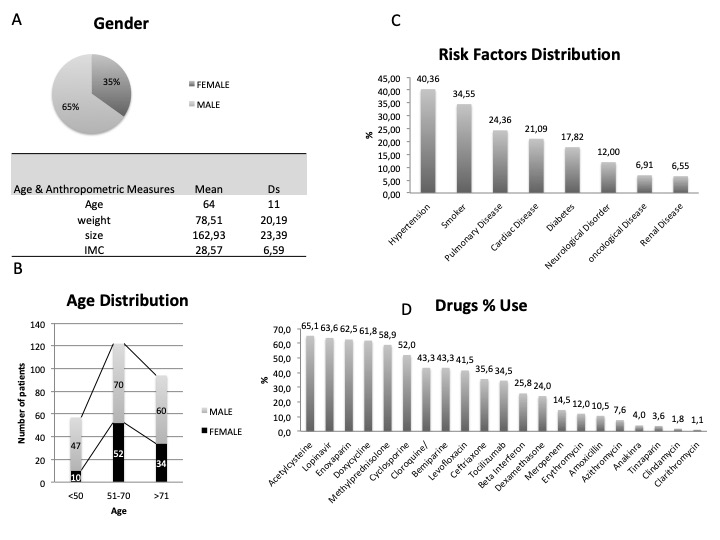 Supplementary Figure 1: Demographics analysis of 274 patients. A) Gender and anthropometrics; B) Age distribution; C) Comorbidities; D) Drugs frequency used.
View Supplementary Figure 1
Supplementary Figure 1: Demographics analysis of 274 patients. A) Gender and anthropometrics; B) Age distribution; C) Comorbidities; D) Drugs frequency used.
View Supplementary Figure 1
Mortality increased with age, reaching 32% in patients older than 70 years (Figure 1B). A day-to-day report of deaths (Figure 1C) and fortnite mortality (Figure 1D and Figure 1E) illustrate a higher mortality rate at the beginning of the pandemic. Mortality reached its maximum in early March amongst the elderly, and then progressively decreased, being clearly contained by early April. Supplementary Figure 2 presents the Helmet cohort, showing Comorbidities, Biomarkers and global and Helmet vital signs.
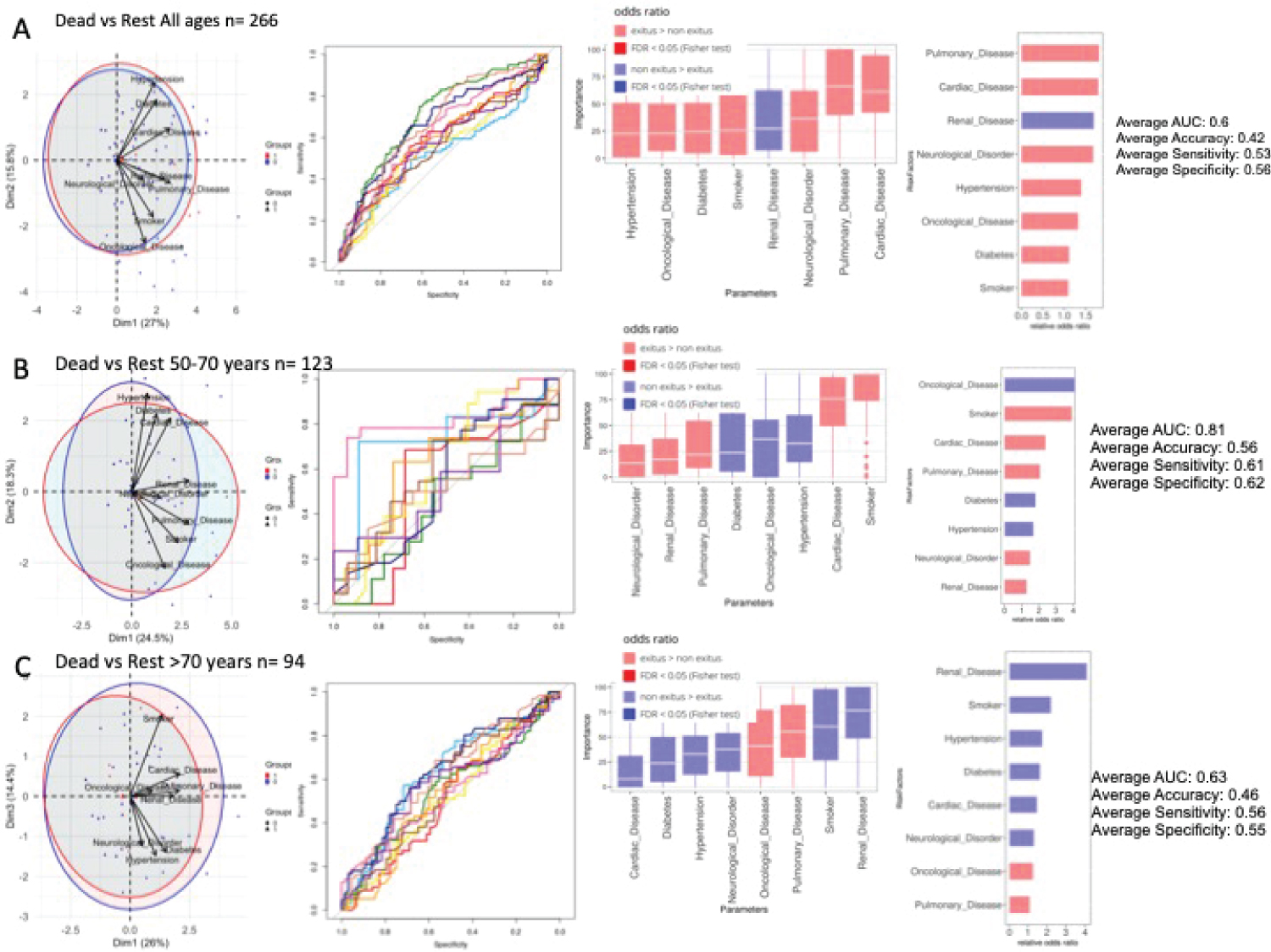 Figure 2: Comorbidities in dead and non-dead admitted patients. Analysis of 274 patients. A) All ages. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05; B) 50-70 years-old. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05; C) > 70-years-old. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05.
View Figure 2
Figure 2: Comorbidities in dead and non-dead admitted patients. Analysis of 274 patients. A) All ages. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05; B) 50-70 years-old. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05; C) > 70-years-old. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05.
View Figure 2
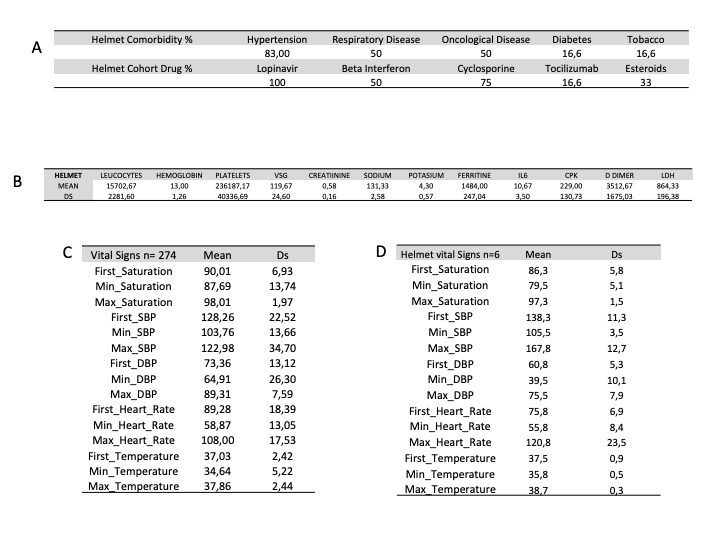 Supplementary Figure 2: Patients cohort using helmet. A) Comorbidities and drug consumption; B) Biomarkers; C) Vital signs of the global cohort (274 patients); D) Vital signs of the helmet cohort (6 patients).
View Supplementary Figure 2
Supplementary Figure 2: Patients cohort using helmet. A) Comorbidities and drug consumption; B) Biomarkers; C) Vital signs of the global cohort (274 patients); D) Vital signs of the helmet cohort (6 patients).
View Supplementary Figure 2
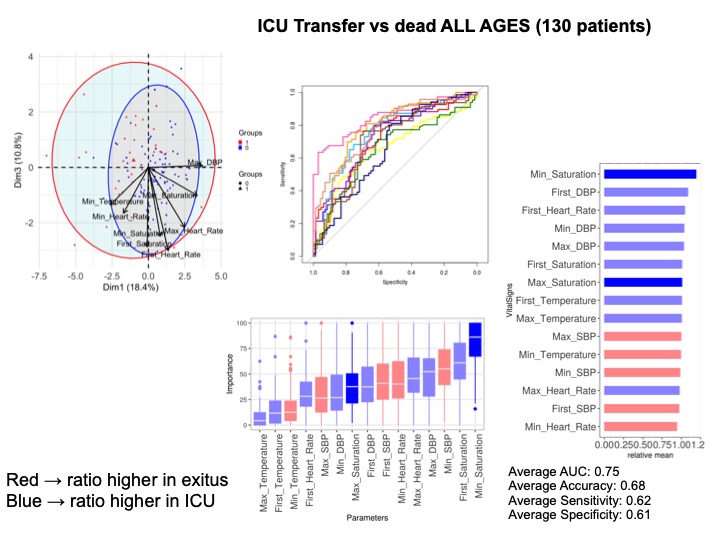
We performed PCA and NN analyses over the 274 patients, classifying them into two states-deceased vs. alive or transferred to ICU- at the time of data collection. Figure 2A shows principal component (PC or dimension 1) against PC2. Cardiovascular affections and respiratory disorders were the conditions which most contributed to separation between states. Odds ratios of the different comorbidities further revealed that seven disease families were associated with fatal outcomes without enrichment. Figure 2B show results for Ages between 50-70 years (123 patients) where tobacco consumption, cardiac and respiratory disease were most relevant, while for those older than 70 years (94 patients) Figure 2C respiratory and oncological disease seemed to be the most important comorbidities related to death. For the most severe patients (n = 130) transferred to ICU or dead, Hypertension, cardiac, respiratory or oncological disease show a trend contributing to separation between states with an AUC = 0.66 (Supplementary Figure 3).
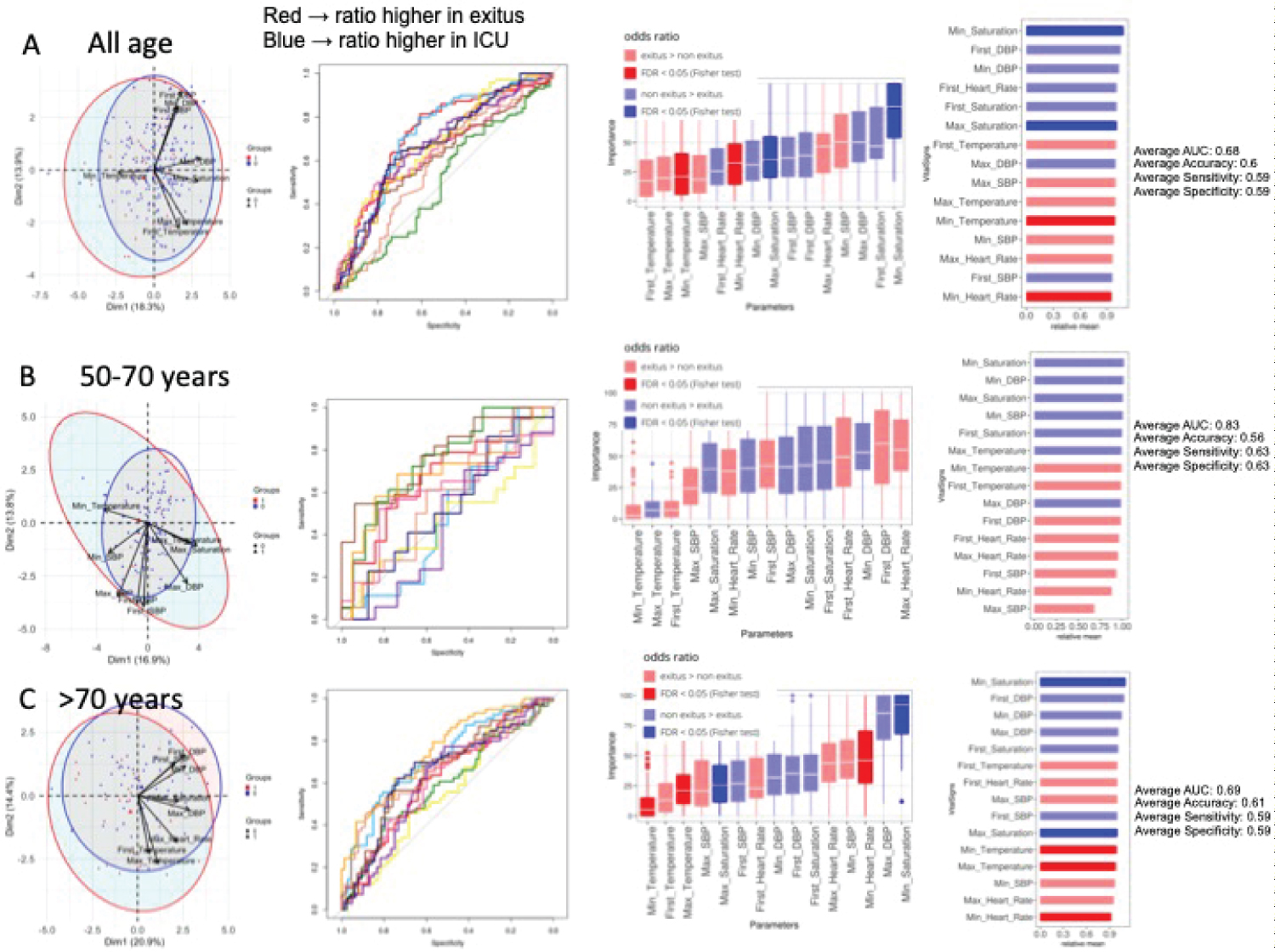 Figure 3: Vital signs in dead and non-dead admitted patients. Analysis of 274 patients. A) All ages. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05; B) 50-70 years-old. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05; C) > 70-years-old. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05.
View Figure 3
Figure 3: Vital signs in dead and non-dead admitted patients. Analysis of 274 patients. A) All ages. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05; B) 50-70 years-old. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05; C) > 70-years-old. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05.
View Figure 3
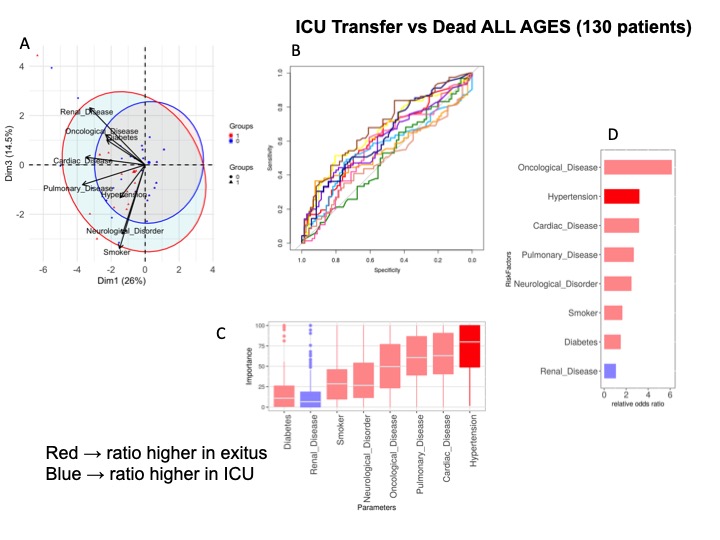 Supplementary Figure 3: Comorbidities in dead and ICU transferred patients. Analysis of 130 patients. A) All ages. PCA analysis; B) NN ROCs; C) Importance in NN model, and D) Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05.
View Supplementary Figure 3
Supplementary Figure 3: Comorbidities in dead and ICU transferred patients. Analysis of 130 patients. A) All ages. PCA analysis; B) NN ROCs; C) Importance in NN model, and D) Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05.
View Supplementary Figure 3
Univariate and multivariate regression analysis confirms the results obtained by the NN in the overall cohort (Table 1) and in the more severe patients (Table 2). The LOO-CV AUC for the logistic model was 0.73 (0.64, 1) for the overall cohort and the Hosmer-Lemeshow test reached p = 1. The LOO-CV AUC for the logistic model in the more severe was 0.53 (0.68, 0.62) and the Hosmer-Lemeshow test reached p = 1 for the more severe patients (Supplementary Figure 4A and Supplementary Figure 4B). We note that all comorbidities were collected from electronic medical records without data associated with them in relation to the severity of the disease or the syndromic type.
Table 2: Comorbidities: Exitus vs. ICU 130 patients. Univariate, multivariate logistic regression analysis and Hosmer and Lemeshow test. View Table 2
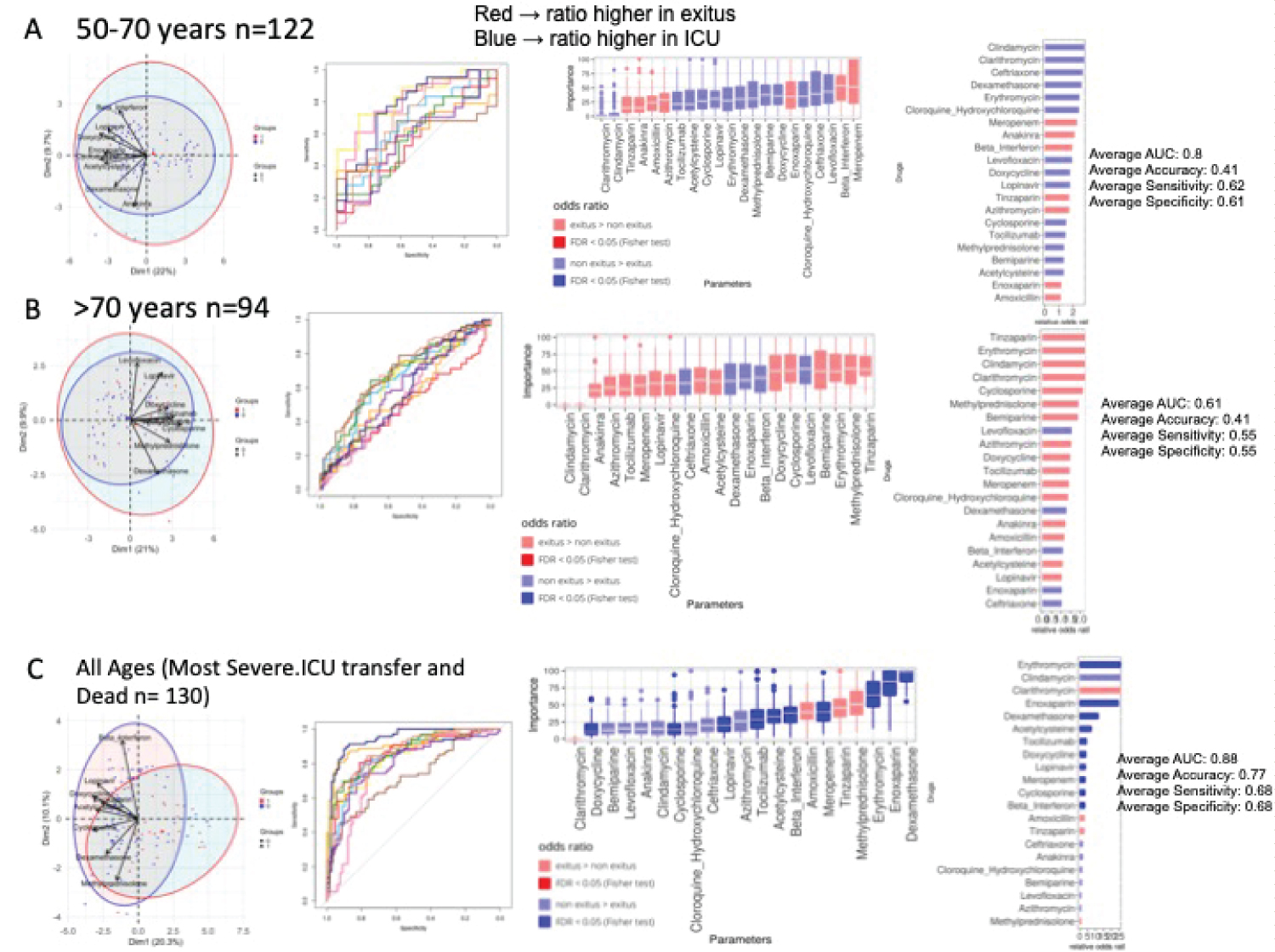 Figure 4: Drugs consumption in dead and non-dead admitted patients. A) 50-70 years-old. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05; B) > 70-years-old. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05; C) All ages ICU transferred vs. dead. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05.
View Figure 4
Figure 4: Drugs consumption in dead and non-dead admitted patients. A) 50-70 years-old. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05; B) > 70-years-old. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05; C) All ages ICU transferred vs. dead. PCA analysis, NN ROCs, importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05.
View Figure 4
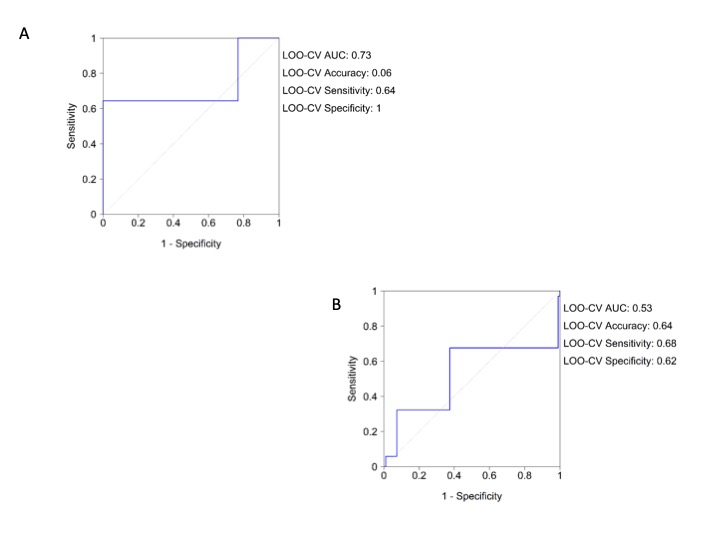 Supplementary Figure 4: Logistic regression analysis roc curve for comorbidities. A) Overall population; B) More severe patients (dead and ICU transferred patients).
View Supplementary Figure 4
Supplementary Figure 4: Logistic regression analysis roc curve for comorbidities. A) Overall population; B) More severe patients (dead and ICU transferred patients).
View Supplementary Figure 4
In relation to abnormal vital signs in the Emergency Department, heart rate and temperature were found to be the variables which best discriminated between deceased patients and survivors, according to PCA and NN analysis for all patients (Figure 3A). The NN was able to classify patients with an AUC = 0.68, achieving a value of 0.83 when the classification was made in the 50-70 year range, where temperature, heart rate and systolic blood pressure (Figure 3B) were selected. Similar results were obtained in patients > 70 years (Figure 3C). Results between deceased and ICU-admitted patients show relevance of bradycardia, hypothermia and arterial blood pressure (Supplementary Figure 5).
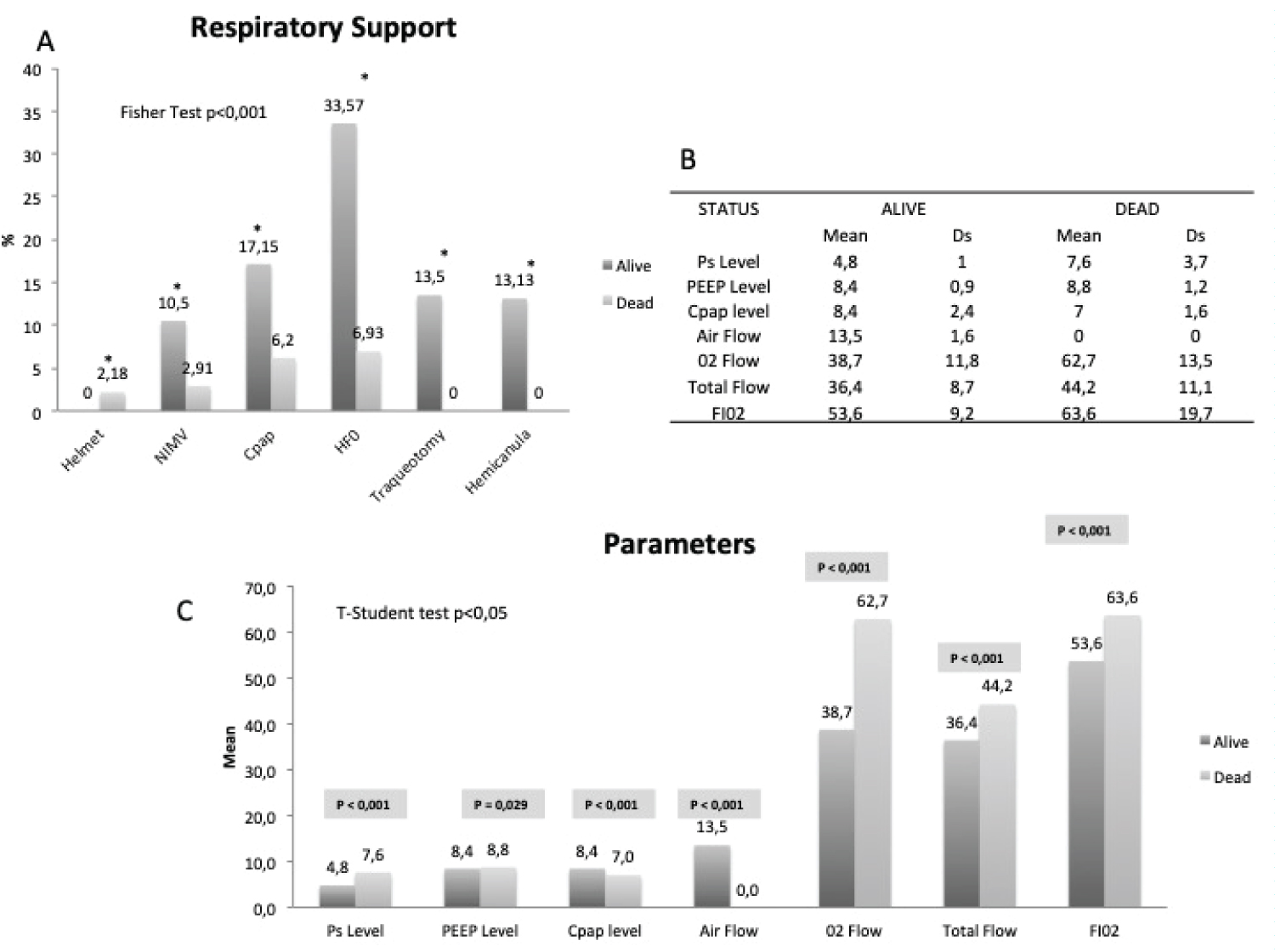 Figure 5: Respiratory support demographics. A) Respiratory support in alive and dead. Fisher test; B) Parameters (mean and sd) used with every technique; C) Comparison between parameters related to respiratory support (t-student test).
View Figure 5
Figure 5: Respiratory support demographics. A) Respiratory support in alive and dead. Fisher test; B) Parameters (mean and sd) used with every technique; C) Comparison between parameters related to respiratory support (t-student test).
View Figure 5
 Supplementary Figure 5: Vital signs in dead and ICU transferred patients. Analysis of 130 patients; A) All ages. PCA analysis; B) NN ROCs; C) Importance in NN model, and D) Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05.
View Supplementary Figure 5
Supplementary Figure 5: Vital signs in dead and ICU transferred patients. Analysis of 130 patients; A) All ages. PCA analysis; B) NN ROCs; C) Importance in NN model, and D) Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05.
View Supplementary Figure 5
Univariate and multivariate regression analysis confirmed the results obtained by the NN with oxygen saturation being significant in the overall cohort (Table 3), as in more severe patients (Table 4). The LOO-CV AUC for the logistic model was 0.76 (0.76, 0.69) for the overall cohort and the Hosmer-Lemeshow test reached p = 0.773. The LOO-CV AUC for the logistic model in the more severe was 0.83 (0.88, 0.67) and the Hosmer-Lemeshow test reached p = 0.114 for the more severe patients, (Supplementary Figure 6A and Supplementary Figure 6B).
Table 3: Vital signs: Exitus vs. rest 274 patients. Univariate, multivariate logistic regression analysis and Hosmer and Lemeshow test. View Table 3
Table 4: Vital signs: Exitus vs. ICU 130 patients. Univariate, multivariate logistic regression analysis and Hosmer and Lemeshow test. View Table 4
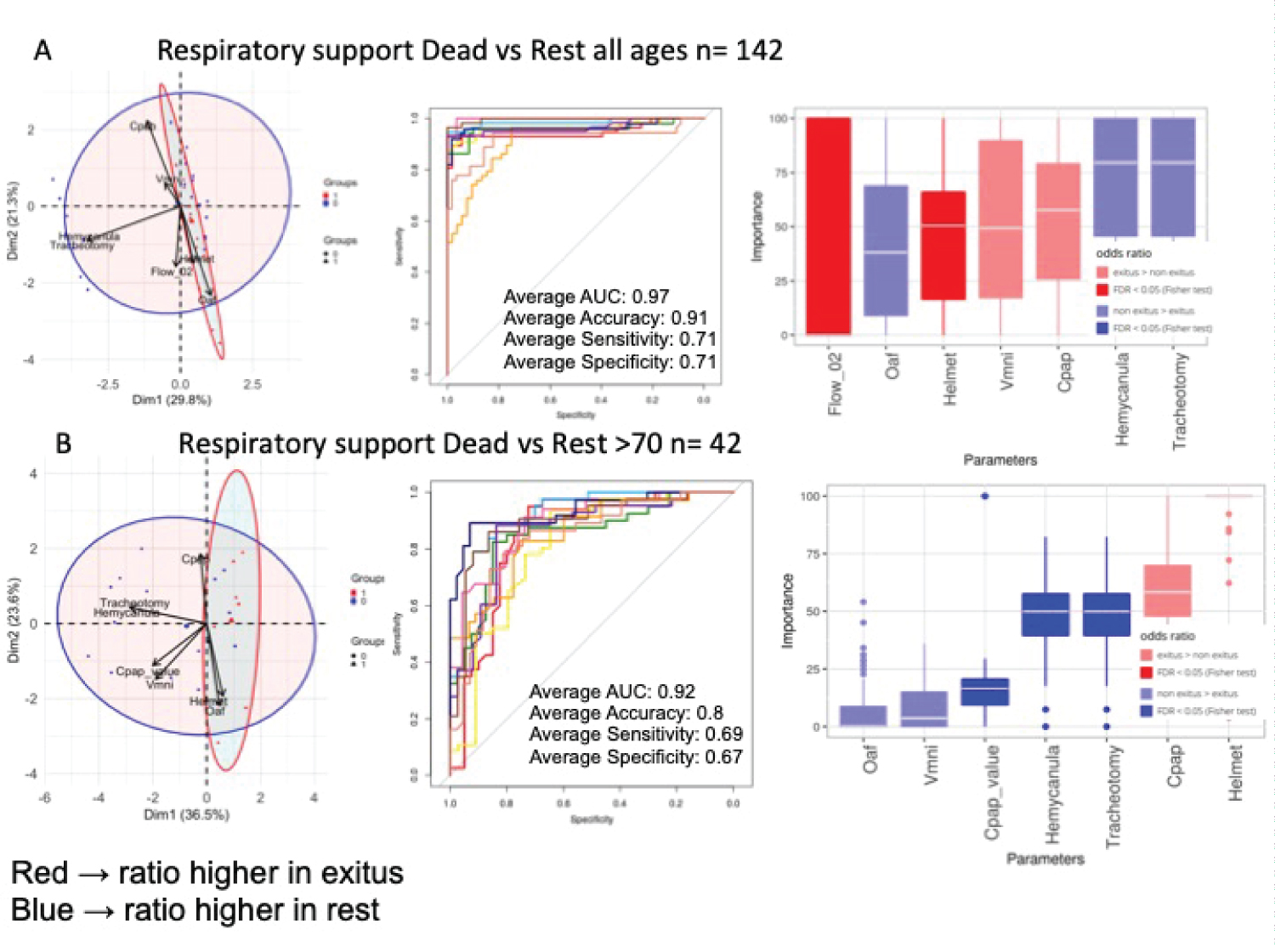 Figure 6: Respiratory support in dead and non-dead admitted patients. A) All ages. PCA analysis; B) NN ROCs; C) Importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05; B) > 70-years-old. PCA analysis; B) NN ROCs; C) Importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05.
View Figure 6
Figure 6: Respiratory support in dead and non-dead admitted patients. A) All ages. PCA analysis; B) NN ROCs; C) Importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05; B) > 70-years-old. PCA analysis; B) NN ROCs; C) Importance in NN model, and Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05.
View Figure 6
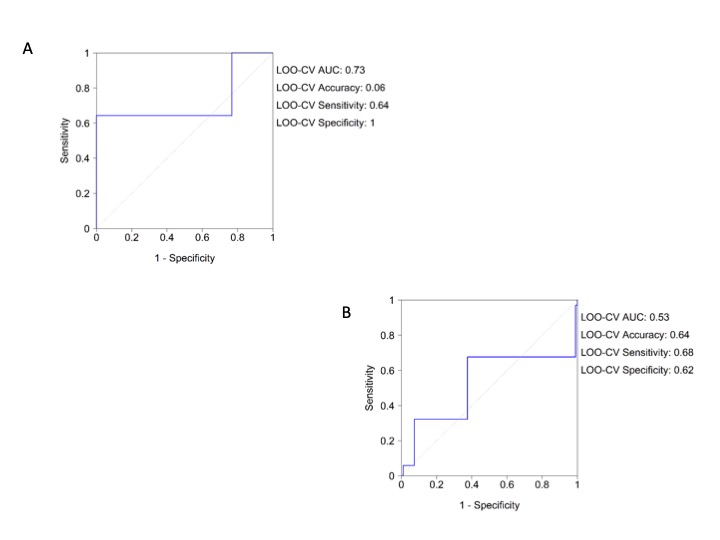 Supplementary Figure 6: Logistic regression analysis roc curve for vital signs. A) Overall population; B) More severe patients (dead and ICU transferred patients).
View Supplementary Figure 6
Supplementary Figure 6: Logistic regression analysis roc curve for vital signs. A) Overall population; B) More severe patients (dead and ICU transferred patients).
View Supplementary Figure 6
Medical treatment: In the 50-70 year range, drugs associated with survival were Corticoids, Chloroquine, Lopinavir and Cyclosporine (Figure 4A). Regarding those > 70 years (Figure 4B), Corticoids, Betaferon and Anticoagulants show a trend associated with survival. We further explored the effect of drugs on survival in the most severe group of patients. Interestingly, PCA showed a clear separation between these categories and revealed a positive contribution of all specific drugs to survival. And the NN in this cohort show (Figure 4C), with an AUC = 0.88, that Corticoids, Enoxaparin, Acetylcisteine, Beta interferon, Tocilizumab, Lopinavir, and Cyclosporine were the compounds with greater capacity to classify patients. Enrichment analysis showed the benefit of these drugs, with high odds ratios and FDR < 0.05, along with Betaferon. NN in severe patients older than 70 years attain an AUC of 0.83 with similar results and a contribution of Anakinra to survival without enrichment (Supplementary Figure 7).
 Supplementary Figure 7: Drug consumption in dead and ICU transferred patients > 70 years. Analysis of 37 patients. A) PCA analysis; B) NN ROCs, C) Importance in NN model, and D) Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05.
View Supplementary Figure 7
Supplementary Figure 7: Drug consumption in dead and ICU transferred patients > 70 years. Analysis of 37 patients. A) PCA analysis; B) NN ROCs, C) Importance in NN model, and D) Fisher test results, sorted by relative odd ratios. Significance taken as FDR < 0.05.
View Supplementary Figure 7
It is important to highlight that both univariate and multivariate regression analysis confirm the results obtained by the NN in the overall cohort (Table 5) and in the more severe patients (Table 6). The LOO-CV AUC for the logistic model was 0.65 (0.82, 0.76) for the overall cohort and the Hosmer-Lemeshow test reached p = 1. The LOO-CV AUC for the logistic model in the more severe was 0.91 (0.79, 0.92) and the Hosmer-Lemeshow test reached p = 0.998 for the more severe patients (Supplementary Figure 8A and Supplementary Figure 8B).
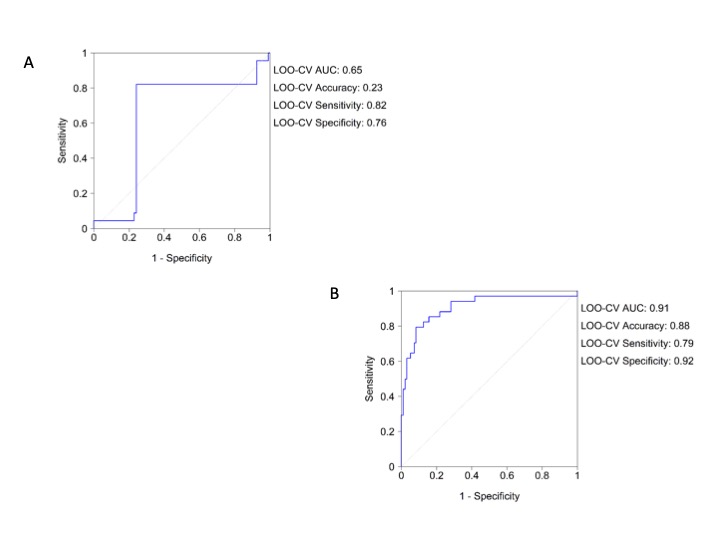 Supplementary Figure 8: Logistic regression analysis roc curve for vital signs. A) Overall population; B) More severe patients (dead and ICU transferred patients).
View Supplementary Figure 8
Supplementary Figure 8: Logistic regression analysis roc curve for vital signs. A) Overall population; B) More severe patients (dead and ICU transferred patients).
View Supplementary Figure 8
Table 5: Drugs: Exitus vs. rest 274 patients. Univariate, multivariate logistic regression analysis and Hosmer and Lemeshow test. View Table 5
Table 6: Drugs: Exitus vs. ICU 130 patients. Univariate, multivariate logistic regression analysis and Hosmer and Lemeshow test. View Table 6
Respiratory support therapies: Regarding respiratory support, Figure 5A shows the use of support techniques, including Helmet, which was only used in 2.18% of the subjects. NIMV was used in 13.41%, CPAP in 23.7%, HFO in 40.5% and IMV, only for weaning, in 13.5%. The rest of the patients were on Oxygen alone either by nasal cannula or mask. In general, given the high minute volumes of the patients, HFO was chosen as the initial therapy. When hypoxemia was refractory, a CPAP test was performed and maintained if recruitment improved hypoxemia. In case of a history of COPD, hypercapnia or previous use, NIMV with low levels of support pressure was used. Helmet was relegated to extreme situations. There is an association between the type of respiratory support and mortality. Figure 5B shows the parameters used. Pressure Support and PEEP levels refer to NIMV through Helmet or full-face mask, CPAP levels refer to equipment capable of administering this mode of ventilation except Helmet. We must consider that two devices were used with the HFO: The OptiflowTM system (Fisher & Payckel Healthcare) where the air and oxygen flow was selected separately and Precision FlowR (Vapotherm Inc) in which the total flow and FiO2 were fixed. The Student's t-test Figure 5C shows that the choice of parameters is related to survival. NN Figure 6A shows that HFO and the tracheotomy weaning procedure that incorporated the use of a hemi-cannula with an AUC of 0.97 are associated with survival. In the cohort of severe patients older than 70 years Figure 6B, all techniques improved survival, being CPAP especially robust once its programming value is included and weaning. NIMV and HFO were also associated with survival and the only technique in this group of patients associated with mortality is Helmet ventilation, although it was only used in 6 patients. Oxygen alone, either through nasal cannula or mask weren't related to survival.
The study demonstrates that IRCU admission did not worsen mortality and limited the demand for ICU resources. The IRCU prevented a collapse of the ICU, allowed it to quickly release patients so as to effectively return to normal, and achieved, considering the severity of lung injury, good results in terms of mortality, which did not increase because of a possible delay in intubation. Also, the present study illustrates the performance of a NN capable of identifying and classifying mortality predictors in SARS-CoV-2 infected patients that can be used in future mono or multicenter studies. Different statistical approaches were used in the treatment of data. The principal component analysis shows a photograph of the distribution of the variables, the logistic regression extracts those associated with survival, and the neural network selects those associated with survival with the greatest predictive power in a model designed to achieve self-learning and improve predictive capacity when increasing the size of the sample.
In the cohort, male subjects, as previously described [9], were more frequently affected. Among those admitted, 12.4% died, considering survival (52.5%) and the possibility of transfer to the ICU (35%) as success. Normally, transfer to the ICU is a criterion of IRCU failure, but in this pandemic keeping the patient alive and well oxygenated until achieving an ICU bed was decisive. In fact, the patients referred to this unit returned for the most part to wean (80%) and 94% survived. 6% died in the ICU, so we can conclude that intubation was not ultimately delayed. In our ICU, all beds were finally able to dedicate to invasive support since the ICRU absorbed the non-invasive support in its entirety. If this had not been the case, it would have collapsed since the number of subjects treated at the UCRI far exceeded the available ICU beds. The reduction in mortality by early April may be likely due to better access to the ICU (Figure 1E), the use of weaning procedures without mortality, better organization, better use of the therapeutic guide and perhaps drugs associated with survival.
Regarding comorbidities, we detect that underlying diseases, including hypertension, respiratory, cardiovascular and oncological disease, may be risk factors for severe patients compared with non-severe patients [10,11]. In general cohorts [12-14], the discriminating risk factors are hypertension, diabetes and neurological disorders. Although renal dysfunction seems to be protective according to the analysis of neural networks, the logistic regression rules it out (Table 1 and Table 2).
In regard to vital signs, arterial blood pressure alterations, heart rate and temperature are better predictors of poor prognosis, as previously documented [13,15]. The multivariate regression model selects hypoxemia and hyperoxia also. This underlines the importance of containing extreme saturations in this type of patient [2].
Regarding treatment, the institution was guided by a unified protocol. In almost all cases either with NN or Logistic Regression, with the limitations of this type of study, it is possible to glimpse a signal in favour of steroids, and anticoagulants were associated with survival, as indicated by other authors [15-20] and among the youngest and most seriously ill, other compounds such as Lopinavir, Tocilizumab and Cyclosporine stood out, which has already been described in broader cohorts considering cytokine storm syndrome and immunosuppression [15,21]. Corticosteroids are controversial in COVID-19 pneumonia in accordance with current WHO guidance [22]. However, in agreement with previous observations [23], we found that their combination with other drugs was consistently associated to a higher survival. Tocilizumab is currently undergoing a clinical trial, but data from a pilot study [24] suggests that it could have a beneficial effect. In severe cases, the role of Acetylcysteine stands out, probably improving the dense secretions and possibly having an antioxidant effect [25]. The use of Cyclosporine A has been recently discussed [26]. Our institution set up a randomized clinical trial assessing the efficacy of Cyclosporine as an add-on therapy to standard of care, which is now ongoing [27]. All these conclusions regarding the advantages of one or the other treatments must be confirmed in randomized clinical trials in order to validate them.
During the pandemic, mortality in the IRCU has doubled its usual annual rate of around 6% while at tending approximately 250 patients per year [8]. During this current crisis, the IRCU treated nearly 300 patients during a month and a half period.
With respect to Helmet, it was only used in 6 patients with a mortality of 100%. This was probably related to the decision of relegating this mask to the final stage of refractory extreme hypoxia. In Supplementary Figure 2, there are evident severity features of these patients compared to the general cohort. However, during this pandemic Helmet has been recommended as the first choice interface [1]. It has been described to improve the prognosis in patients with ARDS [28] minimize aerosolization and dispersion of particles from the respiratory tract [29], as well as decrease the incidence of complications usually associated with other masks [30-32]. In the institution, personnel have already been trained for a possible future wave in which this technique will be in the front line.
HFO has shown remarkable superiority in the cohort. In randomized controlled trials [33] HFO has been shown to improve survival in non-intubated patients by comparison with simple oxygen therapy or noninvasive ventilation. In this cohort, a poor evolution is clearly seen in subjects treated with 02 only (02 Mask or reservoir bag) Figure 6A. As it has been described [34,35], it reduces the ventilatory demand, the severity of dyspnea and the respiratory rate. In consequence it decreases risk of (P-SILI).
Regarding CPAP, the current data shows an association with survival in the most severe patients. In COVID-19 patients, the recommendation of NIMV use is still being debated [36]. It is included in the WHO [37], the Italian Thoracic Society [38] and the Respiratory Care Committee of the Chinese Thoracic Society [39] recommendations, but it is advised against in the European Society of Intensive Care Medicine and the Society of Critical Care Medicine [40]. In a recent French retrospective observational study, the use of CPAP was associated with lower intubation rate, particularly in patients with a do-not-intubate (DNI) decision [41]. Some studies even begin to recommend non-invasive support due to excess mortality from invasive ventilation [42].
Increased respiratory drive observed in these patients often conduced to spontaneous tidal volumes above 9 ml/kg, which was associated with mortality [43], which in turn lead to a need to reduce pressure support and even restrict its use to COPD patients or ventilatory failure [1]. The reduction of breathing rate and work of breathing after the introduction of NIMV, in order to avoid patient self-inflicted lung injury (P-SILI) [44-46], was closely monitored, and determined if sedation/neuromuscular blockade and invasive mechanical ventilation was needed.
Remarkably higher lung compliance in COVID-19 patients was observed, distinguishing it from other forms of ARDS [47]. Lower support pressure levels were found to be protective, probably in relation to lower minute volume ventilation in the context of the aforementioned P-SILI.
Regarding weaning and decannulation of tracheostomized patients, the IRCU follows a specific protocol [48], in which a tracheal hemi-cannula is placed in most patients. Both extubated and tracheostomized patients were admitted following ICU discharge, allowing to quickly freeing up ICU beds and resources. All of these patients survived. Open tracheostomy was performed in all cases following COVID-19 recommendations [49]. Delirium was a limitation during weaning [50], managed by progressively decreasing sedation as required, and once overcome, we followed the previously mentioned protocol, including a brief spontaneous breathing trial, progressive downsizing of the tracheostomy tube and increasingly longer periods of cuff deflation. A hemi-cannula was always employed to maintain access in case recannulation was required. After decannulation, 2 patients required recannulation, which was easily done thanks to the hemi-cannula. The average time until the hemi-cannula was removed was 4 +/- 2 days. Time till total decannulation was shorter than previously published by other groups [51].
Regarding the limitations of this study, we should point out that the results of the neural network are not superior to those of the logistic regression, since this tool needs a larger cohort in order to learn and thus improve its predictive capacity. Despite this, the results it offers are confirmed by the logistical analysis. Additionally, it is a very useful tool in case it is used in multicenter cohorts. Also the study has no control braches, and use retrospectively observational data. This is a descriptive study, performed under a common clinical protocol of the entire hospital, in which it tries to identify variables that may have a greater relationship with a worse patient prognosis. Given the descriptive, retrospective, single-center nature of the study, no definitive conclusions can be drawn. These are indicative, inconclusive results that should be established under randomized clinical trials.
In conclusion, the IRCU prevented the collapse of the ICU without increasing mortality. IRCU risk factors associated with mortality were cardiorespiratory, hemodynamic alterations and fever, indicating a poorer prognosis. The therapeutic options, which offered the best results, were related to corticoids, anticoagulants and some antirheumatics. In our series, HFO and weaning are the procedures most related to survival, while CPAP and NIMV with a protective profile were able to contain mortality in the most severe cases. Mortality did not increase with the evident delay in intubation in those cases where it was not avoidable.
This survey is a retrospective study using de-identified medical record data. No patient management protocols have been altered due to the study. The study was approved by the Institutional Ethics Committee, certificate 09/20 with n° EO085-20_FJD.
The datasets analyzed during the current study are available from the corresponding author upon request.
The authors declare that they have no competing interests.
The study has no specific funding. LF is supported by ISCIII (CA18/00017). PM has a Miguel Servet contract funded by the ISCIII (CP16/00116).