Candidiasis is a type of fungal infection that often spreads to the skin, mouth, throat, esophagus, and vagina. It is caused by the build-up of yeast called Candida [1]. There are multiple types of Candida that can be infective agents, with C. albicans being the most prevalent. When it spreads to the mouth and throat, it is called thrush or otherwise known as Oropharyngeal Candidiasis [1,2]. In this retrospective study, the frequency of candidiasis in the "throat" (pharyngeal), was examined exclusively in 1255 patients who underwent bronchoscopy. Most of the findings of pharyngeal candidiasis were incidental.
The objective of this retrospective study was to investigate whether or not age was a factor in the occurrence of pharyngeal candidiasis in patients undergoing bronchoscopy. The age of the sample ranged from 18-95 years. It was found that the lowest incidence of pharyngeal candidiasis was at age's ≤ 25 (0.2%). Moreover, at ages 26 to 50 years there was a moderate chance of having the condition (9.3%). As well between ages 76-100 years, there was slightly more incidence (16.1%). The highest incidence of presenting pharyngeal candidiasis was between ages 51-75 years (39.2%). Furthermore, additional study is required before confirming a link between age and pharyngeal candidiasis.
Candidiasis otherwise known as moniliasis is a fungal infection caused by yeasts that belong to the genus Candida. The most prevalent of the Candida family, is C. albicans (Monilia albicans). Some lesser known types include C. glabrata, C. krusei, and C. tropicalis [2]. These yeasts normally reside in the gastrointestinal tract and may appear on mucous membranes without inducing an infection [3]. As well they are regulated by the body's own native bacteria and immune defenses.
If these mechanisms are disrupted, an overgrowth of these yeast can occur, and symptoms may develop [2]. Local factors predisposing for candidiasis may include smoking, decreased saliva production, and altered or immature mucosal flora [4]. Some systemic factors may include malnutrition, congenital conditions and malignancies [5,6]. Furthermore other risk factors may include surgery, burns, long ICU (intensive care unit) stays and an abnormality in local flora, triggered by broad spectrum antibiotic use or chemotherapy. As well, health conditions such as immune deficiency (immunocompromise) [7], pregnancy or diabetes mellitus may also make the patient prone to a Candida infection [3].
The symptoms one may experience depends on the localization of the infection. These infections may occur in the oral cavity, pharynx, esophagus, genitourinary tract and skin. Oropharyngeal Candidiasis or thrush affects moist surfaces around the lips, cheeks, tongue and throat [2]. Candida infections of the mouth can spread to the esophagus, causing esophagitis. Consequently, this may make swallowing problematic or painful [2]. Candidal vaginitis often occurs in pregnant or diabetic women [1]. As a result, patients may present with white or yellow vaginal discharge (leukorrhea), burning and itching of genitalia [7,8]. Cutaneous Candidiasis causes patches of red, moist and sensitive skin [2]. The most severe is systemic candidiasis (invasive). This is when Candida spreads to the bloodstream (candidemia) and may lead to potentially fatal conditions like septic shock [9]. Furthermore, pharyngeal candidiasis is usually diagnosed by physical examination and culture samples [2]. It may be treated by using topical antifungal agents such as clotrimazole and nystatin for a few weeks [1,3]. This is similar to the treatments for cutaneous and vaginal candidiasis. Treatment for systemic candidiasis is typically indicated in immunocompromised patients. The usual treatment is the use of an intravenous anti-fungal drug such as fluconazole or caspofungin [1,3,10].
A retrospective study was conducted on patients at the Galway Clinic. The sample size was 1255 patients. The data was gathered from a database of patient charts from 2012-2019. The statistical software used for data analysis was IBM SPSS Statistics v25. The level of significance used on the basis of rejecting or retaining the null hypotheses (Ho) was 0.05. The null hypothesis was that there "Is no difference between age and the incidence of pharyngeal candidiasis". If the p-value achieved in the study was less than 0.05, Ho would be rejected. In contrast if Ho was greater than 0.05, Ho would be retained. A 95% confidence interval was deployed. As well, statistical methods used for analysis included an independent sample t-test, binary logistic regression, histogram, boxplot and a frequency table.
There were 1255 patients who participated in the study. In Table 1, it was seen there were 5 patients aged ≤ 25 years. Ages 26-50 had about 157 (12.4%) patients. Ages 51-75 had 736 (58.6%) patients. Finally, the oldest group had about 357 patients. Furthermore, out of those presenting pharyngeal candidiasis, 3 (0.2%) were patients aged ≤ 25 years. About 117 (9.3%) patients aged 26-50 had pharyngeal candidiasis, whereas 203 (16.1%) patients between ages 76-100 had pharyngeal candidiasis. Age group 51-75 had the highest number of patients presenting pharyngeal candidiasis at 494 (39.2%).
Table 1: Patient characteristics. View Table 1
The data displayed a small lack of uniformity (Figure 1). The distribution also appears to be slightly skewed to the left. The average mean of those with pharyngeal candidiasis was 65.4 years with a standard deviation of about 13.0.
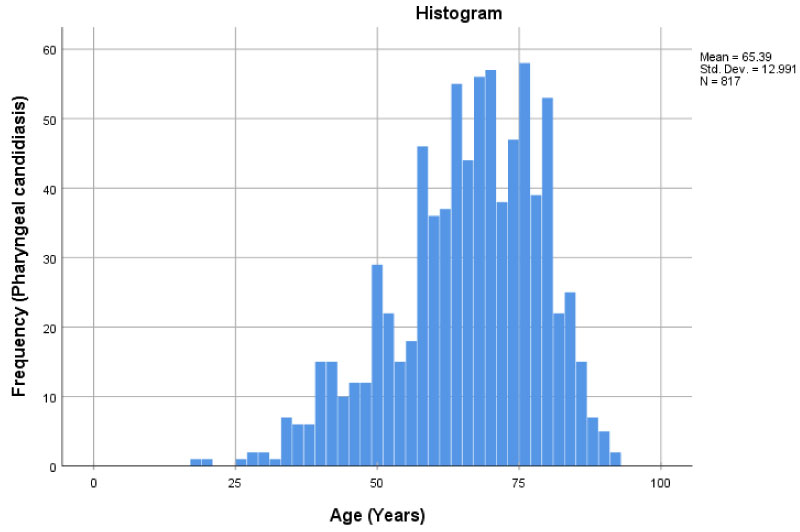 Figure 1: Histogram displaying the frequency of pharyngeal candidiasis at different ages.
Figure 1: Histogram displaying the frequency of pharyngeal candidiasis at different ages.
*Age… (Years) is independent variable, frequency is dependent variable.
View Figure 1
Once again, using the visualization of the boxplot it was seen that the distribution was skewed to the left (Figure 2). Those that did have pharyngeal candidiasis, had a lower median of 67 years. The youngest individual having pharyngeal candidiasis was 18 and the oldest was 92-years-old. There was also an interquartile range of 17 years for those with pharyngeal candidiasis.
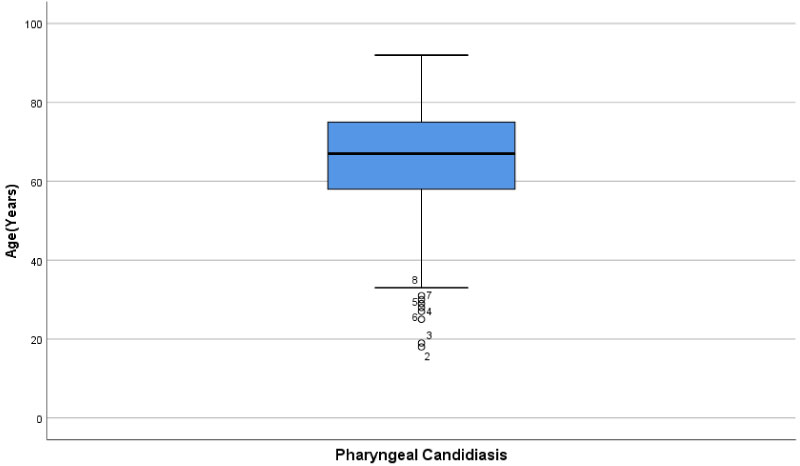 Figure 2: Boxplot of patient age and incidence of pharyngeal candidiasis.
Figure 2: Boxplot of patient age and incidence of pharyngeal candidiasis.
*Candidiasis is categorical variable, age (years) is scale variable.
View Figure 2
An independent sample t-test was conducted (Table 2), and it was seen that the mean age for those with pharyngeal candidiasis was 65.39 years, with a standard deviation of 12.99 years. Whereas for those that without the condition was 68.79 years, with a standard deviation of 13.10 years. The difference in means between those with and without candidiasis was -3.397 years. The p-value was lower than the set level of 0.05, being less than 0.0001. As well the confidence interval had a lower limit of [-4.915] and an upper limit of [-1.879].
Table 2: T-test of patient age and incidence of candidiasis. View Table 2
A logistic regression analysis was carried (Table 3) out to predict pharyngeal candidiasis using the different age groups. Ages 76-100 (not visible), was used a reference point for comparing with other ages. It was also seen that there was an odds ratio of 1.138 for those ≤ 25. A slightly higher odds ratio of 1.549 was seen for age group 51-75. The highest ratio was seen between in age group 26-50 at 2.219. Both age groups 51-75 and 26-50 had significant P-values, of 0.000 and 0.001 respectively.
Table 3: Binary logistic regression. View Table 3
When examining the patient characteristics table (Table 1), it was seen that most of the patients in the study displayed pharyngeal candidiasis. Those in age group 51-75 had the highest incidence of candidiasis (39.2%), whereas those ≤ 25 had the least incidence (0.2%). As well a majority of the patients in the study, were between 51-75 years of age. The least amount of participants were found in the youngest age groups less than/equal to 25 years. When examining patient comorbidity (Table 4), diabetic patients aged 51-75 had the most pharyngeal candidiasis (7.3%) out of all the age groups. Diabetic age group 76-100 had 37 (2.9%) patients with pharyngeal candidiasis. In contrast to the other age groups, there were no diabetic patients in age groups ≤ 25 and 26-50. There were the most immunosuppressed patients (those on long term immunosuppressants), in the age group 51-75 who presented with pharyngeal candidiasis (1.67%). Furthermore, there were no immunosuppressed patients ≤ 25. Out of the age groups that did have immunosuppressed patients, age group 26-50 had the least number of patients with pharyngeal candidiasis (0.6%). When examining the histogram and the boxplot (Figure 1 and Figure 2), the distribution of individuals with pharyngeal candidiasis was skewed to the left. The boxplot also appeared to have a few outliers. This was mainly seen from about 35 years to about 18 years (min, but also major outlier). The independent sample T-test had a p-value of < 0.0001 and was statistically significant, suggesting there was in fact a difference between age and the incidence of pharyngeal candidiasis (Table 2). Thus, the null hypothesis that there is no difference between age and the incidence of pharyngeal candidiasis was rejected. The mean age of those with Candidiasis was 65.39 (12.99) years, which showed some discrepancies, to those who did not present the condition with a mean age of 68.79 (13.10) years. The difference in means was 3.397 years lower for those with candidiasis and had an interval between -4.195 and -1.879. This means that those with candidiasis tend to be 3.4 years younger than those without the condition. The binary logistic regression (Table 3) showed that those who were in ages groups 26-50 were more than 2.219 times more likely to have candidiasis compared to those between 76-100. Furthermore, those that are between ages 51-75 and ≤ 25, were also slightly more likely at 1.549 and 1.138 respectively, to have the condition compared to ages 76-100.
Table 4: Patient comorbidity. View Table 4
The impact of this study may indicate, that since there is a difference between age and one's susceptibility to candidiasis, some age groups may be at a greater risk than others.
Some limitations in the study include the population size. Ideally it would be beneficial to have a larger sample size to increase accuracy and reduce the possibility of errors. Furthermore, a majority of the patients, were somewhere in the range of 60 to 80 years of age. This could have heavily influenced the data. The data may have been perhaps more balanced if younger individuals were examined (for example, those younger or equal to 25). As well this study was also limited to patients at the Galway Clinic. In the future, inclusion of other sample population sources (other hospitals, clinics), may also be worthwhile. Alternatively, it may also be useful to follow and study the patients throughout time. For example, if a cohort study had been performed it would allow for better analysis on the effects of age as a factor leading to candidiasis. As well different age groups could be selected, and then followed over a long period of time.
Being aware of the potential risk factors for pharyngeal candidiasis, is not only important knowledge for a physician, but for the health of their patient. Pharyngeal candidiasis may lead to burning or painful sensations, bleeding, loss of taste and impediment of eating or swallowing. It may also lead to fungal pneumonia and rarely systemic fungal infection [1]. Being able to identify an association between possible factors and causes would be of great benefit to the patient. Once again, the aim of the study was to investigate whether or not age was a factor in the occurrence of pharyngeal candidiasis in patients undergoing bronchoscopy. It was seen that there was a statistically significant difference in age and the incidence of candidiasis. This was suggested through the p-value < 0.0001, being less than 0.05 upon statistical analysis (independent sample T-test) and the null hypothesis being rejected. As well those with pharyngeal candidiasis tended to be about 3.4 years younger than those without the condition. The lowest incidence of pharyngeal candidiasis once again was at ages ≤ 25 (0.2%). Moreover, at ages 26 to 50 years there was a moderate chance of having the condition (9.3%). As well between ages 76-100 years, there was slightly more incidence (16.1%). The highest incidence of presenting pharyngeal candidiasis was between ages 51-75 years (39.2%). Considering only patient comorbidity, diabetic patients aged 51-75 had the most pharyngeal candidiasis (7.3%) out of all the age groups. As well, in immunosuppressed patients, age group 51-75 presented with the most pharyngeal candidiasis (1.67%). Despite these findings, all other age groups have a higher likelihood for pharyngeal candidiasis compared to those between ages 76-100 years (binary logistic regression). Furthermore, supplementary studies will be required for more conclusive results.
Special thanks to the Galway Clinic for providing the data reported.